Abstract
Systemic inflammatory status has been reported to impact survival of prostate cancer (PCa) patients; however, evidence is lacking on whether the inflammatory potential of diet can influence prognosis of PCa patients. To investigate the association between a dietary inflammatory index (DII) and PCa survival, we conducted a retrospective cohort study including 726 men with PCa originally enrolled, between 1995 and 2002, in an Italian case–control study. Information on diet and Gleason score was collected at PCa diagnosis. DII was derived from a food frequency questionnaire using a validated algorithm. Adjusted hazard ratios (HRs) of death with 95% confidence intervals (CIs) were estimated using a Fine-Gray model. DII scores were not significantly associated with all-cause mortality of PCa patients (HR highest vs. lowest DII tertile = 1.25; 95% CI: 0.86–1.83). However, considerable heterogeneity emerged according to Gleason score (p < 0.01): no associations emerged among men with Gleason score 2–6 PCa; whereas, among patients with Gleason score 7–10 PCa, DII was directly associated with both all-cause and PCa-specific mortality (HR highest vs. lowest DII tertile: 2.78; 95% CI: 1.41–5.48; and 4.01; 95% CI: 1.25–12.86; respectively). Among patients with Gleason score 7–10 PCa, ten-year all-cause survival probabilities were 58% (95% CI: 47–67%) for highest and 78% (95% CI: 67–86%) for lowest DII tertile. Study findings support the hypothesis that diet, through its inflammatory potential, may influence the prognosis of patients with more aggressive PCa. Dietary interventions aimed at decreasing inflammation may be considered to improve survival of men with PCa.
Keywords: prostate cancer, survival, diet, inflammation, inflammatory index
Prostate cancer (PCa) has become the most commonly diagnosed cancer among men in high-income countries.1 However, because of relatively low virulence, PCa represents the third cause of cancer death among men.1 Reasons for increased survival2 include the diagnosis of latent, early-stage and nonlethal tumors identified through prostate-specific antigen (PSA) testing. Therefore, great attention should be devoted to identifying modifiable lifestyle factors potentially affecting prognosis of men with PCa, especially those diagnosed at later stages or with advanced histopathological grade, or both.
Systemic inflammatory status, measured through C-reactive protein (CRP), has been associated with poorer all-cause survival after PCa.3 Similar inverse associations also have been observed for all-cause and cancer-specific survival among colorectal4 and breast cancer patients.5 The Glasgow prognostic score (GPS), which employed an inflammatory index based on CRP and albumin levels, has been associated to worse prognosis in several cancers,4,6 including PCa.6,7
Current evidence indicates that dietary factors play a central role in modulating the inflammatory process through a number of bioactive dietary components interacting in defined inflammatory pathways.8 Specific foods and nutrients also have been identified as pro- or anti-inflammatory agents, according to their association with increased or decreased levels of inflammatory markers—such as CRP, interleukin (IL)–6 and tumor necrosis factor (TNF)-α.9–11
Using information available in the extensive literature on the subject, a dietary inflammatory index (DII) has been developed by our study group.12 The DII has been shown to be strongly associated with inflammatory status,11 and elevated DII scores have been associated with increased all-cause and digestive cancer-specific mortality in two cohorts of healthy women.13,14
Although the DII has been shown to be associated with PCa incidence,15 no evidence exists on whether the inflammatory potential of diet has any influence on PCa prognosis. Therefore, to evaluate long-term effects of DII on all-cause and PCa-specific survival, we analyzed data from an Italian cohort of men diagnosed with PCa.
Material and Methods
A retrospective cohort study was conducted on men with PCa originally enrolled as cases in a multicentre hospital-based, case–control study carried out in Italy to explore the associations between lifestyle factors and PCa risk.16,17 Briefly, 780 patients with histologically confirmed PCa diagnosed between 1995 and 2002 at the age of 46–74 years (median age: 66 years, inter-quartile range, IQR: 61–69) had been enrolled in the Pordenone area, Northeastern Italy. PCa cases were consecutive patients newly diagnosed with PCa in major local hospitals (where most of the patients with PCa are referred to for diagnosis and treatment). All eligible cases had no previous cancer diagnosis at any site and were enrolled before receiving cancer treatment. All cases signed an informed consent, according to the rules of the internal Board of Ethics, which approved the original study protocol and the prospective extension. Gleason scores were retrieved from medical records and centrally reviewed by a Pathologist. Patients with missing information on Gleason scores (n = 94) reported a risk of death similar to that of patients with Gleason score 7–10.17
Fifty PCa patients who had reported regular use of aspirin at the time of cancer diagnosis were excluded because anti-inflammatory medications can confound the association between DII and inflammatory status. Additionally, four cases were excluded because they lacked comprehensive dietary information necessary for the DII computation, thus, leaving 726 eligible patients for the present analysis.
PCa cases were interviewed during their hospital stay by trained nurses, using a structured questionnaire to assess information on socio-demographic characteristics and lifestyle factors (e.g., education, tobacco smoking and alcohol drinking habits, lifetime anthropometric measures, anamnesis). Usual diet during the two years prior to PCa diagnosis was assessed using an interviewer-administered food frequency questionnaire (FFQ), including 78 foods and beverages, as well as a range of the most common Italian recipes. Men were asked to indicate the average weekly frequency of consumption of each dietary item; intakes lower than once a week, but at least once a month, were coded as 0.5 per week. Nutrient and total energy intake was determined using the Italian food composition database.18 The FFQ showed satisfactory validity19 and reproducibility20 with Spearman correlation coefficients ranging between 0.50 and 0.60 for validity and between 0.60 and 0.70 for reproducibility.
The DII score was calculated for each PCa patient by linking individual dietary data with a comprehensive database (the database collected data from 11 countries), which provided a robust estimate of a mean and standard deviation for each parameter.12,15 This was achieved by subtracting the “standard global mean” from the intake reported via the FFQ and dividing this value by the standard deviation (both calculated from the world database) to get ‘z’ scores. To minimize the effect of “right skewing”, ‘z’ scores were converted to normal percentiles, then these percentiles were centered (on zero) by doubling each value and subtracting 1. The centered percentile score for each food parameter for each individual was then multiplied by the respective food parameter effect score (inflammatory potential for each food parameter), which was derived through a review of the literature, in order to obtain a food parameter-specific DII score for an individual. The overall DII score was calculated, for each participant, as a linear combination of all of the food parameter-specific DII scores,12 as follows: DII=b1×n1 + b2×n2……….b31×n31, where b refers to the literature-derived inflammatory effects score for each of the evaluable food parameters and n refers to the food parameter-specific centered percentiles, which were derived from the current case–control study dietary data. A description of the validation work, including both dietary recalls and a structured questionnaire similar to a FFQ, also is available.11 The methodology is depicted in Supporting Information Appendix.
The food parameters used for DII computation were carbohydrate, protein, fat, alcohol, fiber, cholesterol, saturated fat, mono-unsaturated fat, poly-unsaturated fat, omega-3, omega-6, niacin, thiamine, riboflavin, vitamin-B6, iron, zinc, vitamin A, vitamin C, vitamin D, vitamin E, folic acid, beta carotene, anthocyanidins, flavan-3-ol, flavonol, flavonones, flavones, isoflavones, caffeine and tea. A higher DII score indicates a more pro-inflammatory diet and a lower DII score indicates a more anti-inflammatory diet. Body mass index (BMI, kg/m2) was calculated as weight (kg) divided by height (m) squared (median = 26.4 kg/m2, IQR: 24.4–28.7 kg/m2). The interviewers measured waist circumference as the abdominal circumference 2 cm above the umbilicus (median = 100 cm, IQR: 94–106 cm). Abdominal obesity was defined as waist circumference >102 cm. A total of 56 PCa patients (7.7%) lacked information on waist circumference. For these men abdominal obesity was approximated by BMI > 27.7 kg/m2 as suggested by a linear regression model predicting waist circumference through BMI (the Pearson correlation = 0.71).
Information on vital status and, in case of death, the date and the underlying cause were obtained through a linkage with regional health system databases, available through population-based Cancer Registries in the study areas (i.e., Friuli Venezia Giulia and Veneto).17 Each patient accumulated person-time at risk from the date of PCa diagnosis up to the date of death, the date of last follow-up or to December 31, 2013, whichever came first.
To account for competing causes of mortality, the cumulative incidence method21 was applied to both PCa-specific and nonPCa-specific mortality. Cumulative mortality rates between groups were compared by means of the Gray’s test.22 To estimate the hazard ratio (HR) of death and the corresponding 95% confidence intervals (CI), the Cox proportional hazard regression model23 was used for all-cause mortality, and the Fine and Gray’s regression model24 was used for PCa-specific and nonPCa-specific mortality. HRs were adjusted for area of residence at diagnosis (Friuli Venezia Giulia or Veneto region), calendar period of cancer diagnosis (1995–97, 1998–00, 2001–02), age at diagnosis (46–59, 60–64, 65–69, 70–74 years), education (<7, 7–11, 12 years), Gleason score (2–6, 7–10, unknown), smoking habits (never, former, current <15 cigarettes/day, current 15 cigarettes/ day), abdominal obesity (no, yes), alcohol intake (drinks/ week) and energy intake (kcal/day). Heterogeneity between strata of Gleason score was tested using a Wald χ2 test.25
Results
Among 726 PCa patients, median length of follow-up was 12.7 years (interquartile range: 9.3–14.8 years) for 8514 total person-years of observation. Overall, 244 (33.6%) deaths were recorded (median follow-up 7.5 years): 76 (31.1%, median follow-up 6.3 years) were due to PCa and 168 (68.9%, median follow-up 7.9 years) were due to other causes. The proportions of men alive after 10 and 15 years from PCa diagnosis were 74% (95% CI: 71–77%) and 65% (95% CI: 61–68%), respectively. Ten-year overall survival was 81% (95% CI: 77–85%) in those with Gleason score 2–6 and 68% (95% CI: 62–73%) in those with Gleason score 7–10 (data not shown).
Among the enrolled 726 PCa patients, no significant associations emerged between DII and education, smoking habit and Gleason score. However, the association with abdominal obesity was of borderline statistical significance (p values = 0.05; Table 1).
Table 1.
Distribution of 726 men with prostate cancer according to dietary inflammatory index and selected variables at diagnosis. Northeastern Italy, 1995–2002
| Dietary inflammatory index (tertiles) | p-value1 | |||
|---|---|---|---|---|
| I—low, no. (%) | II—medium, no. (%) | III—high, no. (%) | ||
| Age at diagnosis (yrs) | ||||
| 46–59 | 50 (37.9) | 36 (27.3) | 46 (34.9) | 0.41 |
| 60–64 | 54 (31.6) | 52 (30.4) | 65 (38.0) | |
| 65–69 | 77 (33.1) | 86 (36.9) | 70 (30.0) | |
| 70–74 | 59 (31.1) | 66 (34.7) | 65 (34.2) | |
| Education (yrs) | ||||
| <7 | 130 (35.1) | 109 (29.5) | 131 (35.4) | 0.13 |
| 7–11 | 61 (28.1) | 86 (39.6) | 70 (32.3) | |
| ≥12 | 49 (35.3) | 45 (32.4) | 45 (32.4) | |
| Smoking habit | ||||
| Never smoker | 76 (35.2) | 68 (31.5) | 72 (33.3) | 0.34 |
| Former smoker | 126 (34.2) | 127 (34.5) | 115 (31.3) | |
| Current smoker | ||||
| <15 cig./day | 22 (30.1) | 21 (28.8) | 30 (41.1) | |
| ≥15 cig./day | 16 (23.2) | 24 (34.8) | 29 (42.0) | |
| Abdominal obesity2 | ||||
| No | 138 (29.8) | 160 (34.6) | 165 (35.6) | 0.05 |
| Yes | 102 (38.8) | 80 (30.4) | 81 (30.8) | |
| Gleason score | ||||
| 2–6 | 120 (32.5) | 127 (34.4) | 122 (33.1) | 0.60 |
| 7–10 | 84 (31.9) | 82 (31.2) | 97 (36.9) | |
| Unknown | 36 (38.3) | 31 (33.0) | 27 (28.7) | |
Pearson χ2 test.
Defined as waist circumference >102 cm (or BMI >27.7 kg/m2, when information on waist was missing).
Overall, PCa patients with elevated DII were not at elevated risk of death for all-cause (HR = 1.25; 95% CI: 0.86–1.83; Table 2). However, substantial heterogeneity according to Gleason score was evident in the association between DII and all-cause mortality (pheterogeneity < 0.01). There was no association in PCa patients with Gleason score 2–6 (HRDIItertile3vs1: 0.75; 95% CI: 0.40–1.41); whereas a strong direct relationship was observed in patients with Gleason score 7–10 (HR: 2.78; 95% CI: 1.41–5.48; ptrend < 0.01). The same pattern emerged from survival analysis: no differences emerged in Kaplan–Meier estimates according to DII in PCa patients with Gleason score 2–6 (p = 0.66—Fig. 1); conversely, among patients with Gleason score 7–10, the all-cause survival probabilities at 10 years were 78% (95% CI: 67–86%) for low DII score and 58% (95% CI: 47–67%) for high DII (p = 0.02; Fig. 1).
Table 2.
Hazard ratio (HR) for all-cause death, prostate cancer (PCa) death, and nonPCa death, with corresponding 95% confidence intervals (CI), in 726 men with PCa, according to tertiles of dietary inflammatory index in strata of Gleason score. Northeastern Italy, 1995–2002
| Dietary inflammatory index (tertiles) | χ2 trend | ||||||
|---|---|---|---|---|---|---|---|
| I—low | II—medium | III—high | |||||
| Cases (deaths) |
HR1 (95%CI) |
Cases (deaths) |
HR1 (95%CI) |
Cases (deaths) |
HR1 (95%CI) |
||
| All–cause mortality2 | 240 (77) | 13 | 240 (77) | 1.06 (0.75–1.50) | 246 (90) | 1.25 (0.86–1.83) | 1.44, p = 0.23 |
| Gleason score 2–6 | 120 (31) | 13 | 127 (36) | 0.89 (0.52–1.52) | 122 (29) | 0.75 (0.40–1.41) | 0.81, p = 0.37 |
| Gleason score 7–10 | 84 (23) | 13 | 82 (30) | 1.90 (1.00–3.60) | 97 (46) | 2.78 (1.41–5.48) | 9.23, p < 0.01 |
| χ2 heterogeneity: 7.59; p < 0.01 | |||||||
| PCa mortality2 | 240 (26) | 13 | 240 (21) | 0.96 (0.49–1.85) | 246 (29) | 1.42 (0.73–2.76) | 1.18, p = 0.28 |
| Gleason score 2–6 | 120 (9) | 13 | 127 (5) | 0.50 (0.11–2.30) | 122 (4) | 0.41 (0.08–2.06) | 1.13, p = 0.29 |
| Gleason score 7–10 | 84 (7) | 13 | 82 (12) | 2.39 (0.73–7.78) | 97 (22) | 4.01 (1.25–12.86) | 6.19, p = 0.01 |
| χ2 heterogeneity: 5.30; p = 0.02 | |||||||
| NonPCa mortality2 | 240 (51) | 13 | 240 (56) | 1.07 (0.70–1.64) | 246 (61) | 1.07 (0.68–1.69) | 0.16, p = 0.77 |
| Gleason score 2–6 | 120 (22) | 13 | 127 (31) | 1.04 (0.55–1.98) | 122 (25) | 0.88 (0.43–1.78) | 0.005, p = 0.69 |
| Gleason score 7–10 | 84 (16) | 13 | 82 (18) | 1.38 (0.62–3.10) | 97 (24) | 1.59 (0.67–3.81) | 1.07, p = 0.30 |
| χ2 heterogeneity: 1.13; p = 0.29 | |||||||
Estimated from proportional hazard model using the Fine-Gray method for competing risks, adjusting for area of residence, calendar period of diagnosis, age at diagnosis, education, smoking habits, abdominal obesity, alcohol intake, energy intake.
Further adjusted for Gleason score.
Reference category.
Figure 1.
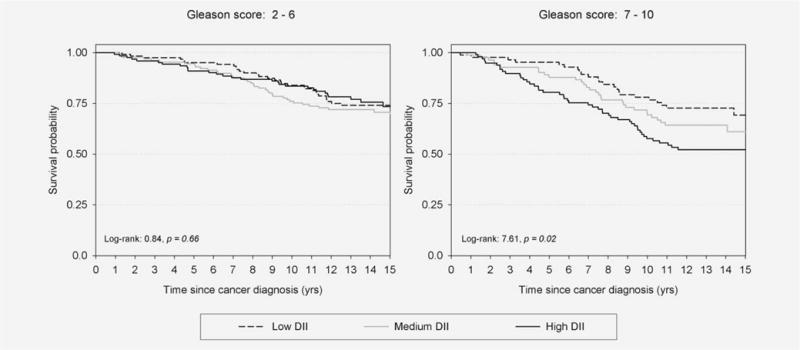
All-cause survival curves among 726 men with prostate cancer, according to tertiles (low, medium, high) of dietary inflammation index (DII) in strata of Gleason score. Northeastern Italy, 1995–2002.
Among PCa patients with Gleason 7–10 (Table 2), DII was positively associated with PCa-specific mortality (HR for the highest tertile = 4.01; 95% CI: 1.25–12.86; ptrend = 0.01) but not with nonPCa mortality (HR for the highest tertile = 1.59; 95% CI: 0.67–3.81; ptrend = 0.30). Indeed, the cumulative 10-year PCa-specific mortality in PCa patients with Gleason score 7–10 increased from 7.4% in those with low DII to 21.7% (Fig. 2, p = 0.03) in patients with high DII whereas no heterogeneity was found among PCa patients with Gleason 2–6. NonPCa mortality was similar across DII levels in both Gleason sub-groups (Fig. 2).
Figure 2.
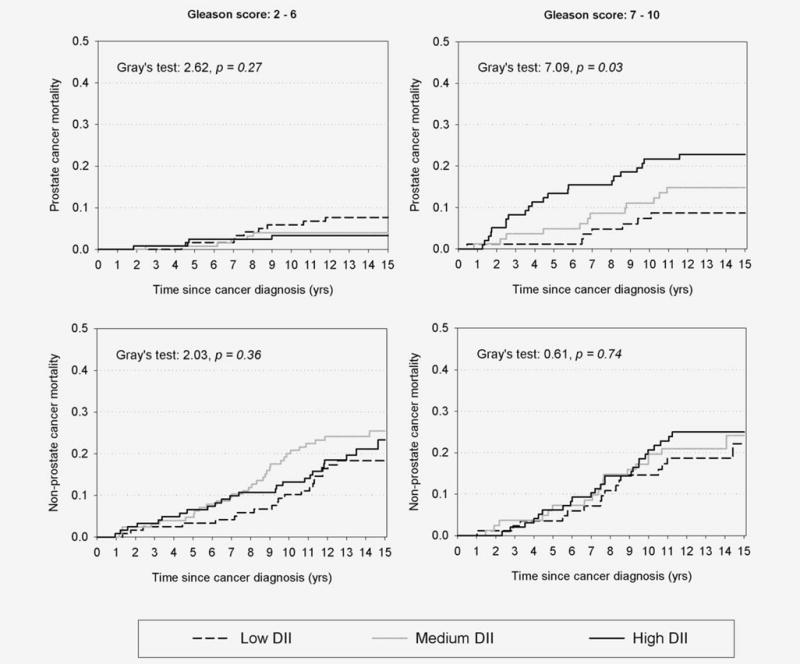
Cumulative prostate cancer (PCa) and nonPCa mortality among 726 men with PCa, according to tertiles (low, medium, high) of dietary inflammation index (DII) in strata of Gleason score. Northeastern Italy, 1995–2002.
Discussion
These study findings suggest that diet, through its inflammatory potential, may play a role in survival after PCa diagnosis among men with more aggressive, poor-prognosis cancers. Moreover, the association was more evident for PCa-specific mortality rather than for nonPCa-specific one. However, despite the strong statistical significance of heterogeneity across Gleason scores, caution is warranted as sub-group analyses were based on a limited sample size, and the interactions found in this exploratory analysis need to be confirmed by other studies.
Different indicators of inflammatory status have been consistently related to worse prognosis in several cancers. CRP levels were significantly associated with reduced all-cause survival (HR of death = 1.83) in a pooled analysis of PCa patients, regardless of tumor stage.3 Concerning other neoplasms, elevated levels of CRP and serum amyloid A have been related to reduced overall and disease-free survival of women with breast cancer.5 Furthermore, a recent meta-analysis4 found that colorectal cancer patients with elevated CRP levels had a two-fold higher risk of all-cause death, increasing up to 4-fold for colorectal cancer-specific mortality. Another inflammation score, the mGPS, based on CRP and albumin levels, was found to be associated with PCa prognosis, reporting 35% gap in 5-year relative survival between PCa patients with the highest vs. the lowest mGPS levels, corresponding to a 2-fold excess risk of death.7 Similar associations between mGPS and cancer prognosis emerged in another study analyzing several cancers—including PCa, regardless of cancer site.6
Increasing evidence supports a role of diet in regulating the inflammatory milieu.8 Specific foods and nutrients have been associated with increased levels of inflammatory markers, such as CRP, IL-6 and TNF-α. A diet rich in fats and processed meat may have a pro-inflammatory effect by increasing CRP and IL-6 levels.9,10,26 Conversely, consumption of fruit, vegetables and whole grain products may exert anti-inflammatory effects.9,10,26 Instead of focusing on specific foods or nutrients, the DII evaluates the inflammatory potential of the whole diet, weighting 32 diet parameters11 in the present study. By its design, DII correlates with inflammatory markers such as CRP, IL-1β, IL-6, IL-10 and TNF-α.11,12
The mechanisms through which chronic inflammatory status is related to PCa cancer survival are still unclear. Chronic inflammation can promote cancer progression through the production of reactive oxygen species and nitric oxide.27 These may reduce apoptosis and induce angiogenesis, mutations, and increased cell proliferation by damaging DNA and other cellular macromolecules. Inflammatory processes also may induce the vascular endothelial growth factors and other, angiogenesis-enhancing growth factors.27 Furthermore, pro-inflammatory diet may increase circulating levels of insulin, causing insulin resistance.28 Finally, in advanced diseases, inflammatory status may interfere with therapy, causing treatment resistance.3
The lack of information on diet modification after PCa diagnosis was a major weakness of the present study. Diet modifications have been reported by Avery and colleagues29 among patients diagnosed with PCa after 2002, mainly referring to the consumption of selected food items possibly associated to PCa onset (i.e., more tomatoes, protein and fruit/ vegetable juice). Nonetheless, no significant changes in the mean intake of these food items were detected in that study.29 Similarly, in the health professionals follow-up study, few men reported extreme changes in their diet after PCa onset.30 Thus, diet modifications were unlikely to have occurred in our study population, also considering that in Italy, at the time the study was conducted (i.e., between 1995 and 2002): (i) the general population was unaware of a putative association between diet and PCa risk and (ii) no guidelines for dietary intervention in patients with PCa were in force. In addition, the lack of information on PCa characteristics (e.g., PSA at diagnosis, stage) and treatments ought to be acknowledged as a potential bias. Although some studies have reported lower PSA levels among nonsteroidal anti-inflammatory drug users,31,32 there is no direct evidence on the association of inflammatory status and PSA level; therefore, it is unlikely that a bias has occurred. Further, to rule out the possible bias due to the association between inflammation and disease severity, analyses were stratified by Gleason score.
Among the strengths of our study is the long follow-up of PCa cases, which allowed assessing the role of metabolic disorders in long-term survival. Men with PCa included in this study, although enrolled as cases in a previous hospital-based case–control study, can be considered as representative of the population with PCa living in the study areas. Finally, accurate evaluation of mortality outcomes was made possible by the local availability of high-quality population-based cancer registries.33 However, some misclassification on the specific cause of death cannot be totally excluded.
The results of the present study show that diet may influence the prognosis of PCa patients through its inflammatory potential. Therefore, diet modification to diminish the consumption of pro-inflammatory foods may be a feasible and cheap intervention to reduce mortality in the increasing number of men living after a PCa diagnosis, which in Italy amounts to approximately 300,000 men.33
Supplementary Material
What’s new?
Systemic inflammatory status has been reported to impact survival of prostate cancer (PCa) patients; however, evidence is lacking on whether the inflammatory potential of diet can influence prognosis. Here the authors used, for the first time, a novel, validated dietary indicator of the inflammatory potential of diet to investigate the prognosis of PCa patients. Among those with more aggressive disease, dietary inflammatory index (DII) scores were strongly associated with both all-cause and PCa-specific mortality in a retrospective cohort study. A diet modification to diminish the consumption of pro-inflammatory foods may represent an effective intervention to reduce mortality among PCa patients.
Acknowledgments
The authors wish to thank Mrs. Luigina Mei for editorial assistance.
Grant sponsor: Italian Association for Research on Cancer; Grant number: AIRC IG No. 1468; Grant sponsor: United States National Institute for Diabetes, Digestive and Kidney Diseases (Shivappa and H ebert); Grant number: R44DK103377
Abbreviations
- BMI
body mass index
- CIs
confidence intervals
- CRP
C-reactive protein
- DII
dietary inflammatory index
- FFQ
food frequency questionnaire
- GPS
Glasgow prognostic score
- HR
hazard ratios
- IL
interleukin
- PCa
prostate cancer
- PSA
prostate-specific antigen
- TNF
tumor necrosis factor
Footnotes
Additional Supporting Information may be found in the online version of this article.
Disclosure
JRH owns controlling interest in Connecting Health Innovations LLC (CHI), a company planning to license the right to his invention of the dietary inflammatory index (DII) from the University of South Carolina in order to develop computer and smart phone applications for patient counseling and dietary intervention in clinical settings. NS is an employee of CHI. The subject matter of this paper will not have any direct bearing on that work, nor has that activity exerted any influence on this project.
References
- 1.Ferlay J, Soerjomataram I, Ervik M, et al. GLO-BOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC cancer base No. 11. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. [last access: November 31, 2015] [Google Scholar]
- 2.Trama A, Foschi R, Larrañaga N, EUROCARE-5 Working Group et al. Survival of male genital cancers (prostate, testis and penis) in Europe 1999–2007: results from the EUROCARE-5 study. Eur J Cancer. 2015;51:2206–16. doi: 10.1016/j.ejca.2015.07.027. [DOI] [PubMed] [Google Scholar]
- 3.Graff JN, Beer TM, Liu B, et al. Pooled analysis of C-reactive protein levels and mortality in prostate cancer patients. Clin Genitourin Cancer. 2015;13:e217–21. doi: 10.1016/j.clgc.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 4.Woo HD, Kim K, Kim J. Association between preoperative C-reactive protein level and colorectal cancer survival: a meta-analysis. Cancer Causes Control. 2015;26:1661–70. doi: 10.1007/s10552-015-0663-8. [DOI] [PubMed] [Google Scholar]
- 5.Pierce BL, Ballard-Barbash R, Bernstein L, et al. Elevated biomarkers of inflammation are associated with reduced survival among breast cancer patients. J Clin Oncol. 2009;27:3437–44. doi: 10.1200/JCO.2008.18.9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proctor MJ, Morrison DS, Talwar D, et al. An inflammation-based prognostic score (mGPS) predicts cancer survival independent of tumour site: a Glasgow inflammation outcome study. Br J Cancer. 2011;104:726–34. doi: 10.1038/sj.bjc.6606087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shafique K, Proctor MJ, McMillan DC, et al. Systemic inflammation and survival of patients with prostate cancer: evidence from the Glasgow inflammation outcome study. Prostate Cancer Prostatic Dis. 2012;15:195–201. doi: 10.1038/pcan.2011.60. [DOI] [PubMed] [Google Scholar]
- 8.Ricordi C, Garcia-Contreras M, Farnetti S. Diet and inflammation: possible effects on immunity, chronic diseases, and life span. J Am Coll Nutr. 2015;34:20–23. doi: 10.1080/07315724.2015.1080101. [DOI] [PubMed] [Google Scholar]
- 9.Nettleton JA, Steffen LM, Mayer-Davis EJ, et al. Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the multi-ethnic study of atherosclerosis (MESA) Am J Clin Nutr. 2006;83:1369–79. doi: 10.1093/ajcn/83.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esmaillzadeh A, Kimiagar M, Mehrabi Y, et al. Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr. 2007;137:992–8. doi: 10.1093/jn/137.4.992. [DOI] [PubMed] [Google Scholar]
- 11.Shivappa N, Steck SE, Hurley TG, et al. A population-based dietary inflammatory index predicts levels of C-reactive protein in the seasonal variation of blood cholesterol study (SEASONS) Public Health Nutr. 2014;17:1825–33. doi: 10.1017/S1368980013002565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shivappa N, Steck SE, Hurley TG, et al. Designing and developing a literature-derived, population-based dietary inflammatory index. Public Health Nutr. 2014;17:1689–96. doi: 10.1017/S1368980013002115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shivappa N, Blair CK, Prizment AE, et al. Association between inflammatory potential of diet and mortality in the Iowa Women’s Health study. Eur J Nutr. 2015 doi: 10.1007/s00394-015-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shivappa N, Harris H, Wolk A, et al. Association of inflammatory potential of diet and mortality among women in the Swedish mammography cohort. Eur J Nutr. 2015 doi: 10.1007/s00394-015-1005z. [DOI] [PubMed] [Google Scholar]
- 15.Shivappa N, Bosetti C, Zucchetto A, et al. Association between dietary inflammatory index and prostate cancer among Italian men. Br J Nutr. 2014;113:278–83. doi: 10.1017/S0007114514003572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dal Maso L, Zucchetto A, La Vecchia C, et al. Prostate cancer and body size at different ages: an Italian multicentre case-control study. Br J Cancer. 2004;90:2176–80. doi: 10.1038/sj.bjc.6601859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polesel J, Gini A, Dal Maso L, et al. The negative impact of tobacco smoking on survival after prostate cancer diagnosis. Cancer Causes Control. 2015;26:1299–305. doi: 10.1007/s10552-015-0624-2. [DOI] [PubMed] [Google Scholar]
- 18.Gnagnarella P, Parpinel M, Salvini S, et al. The update of the Italian food composition database. J Food Comp Anal. 2004;17:509–22. [Google Scholar]
- 19.Decarli A, Franceschi S, Ferraroni M, et al. Validation of a food-frequency questionnaire to assess dietary intakes in cancer studies in Italy. Results for specific nutrients. Ann Epidemiol. 1996;6:110–18. doi: 10.1016/1047-2797(95)00129-8. [DOI] [PubMed] [Google Scholar]
- 20.Franceschi S, Barbone F, Negri E, et al. Reproducibility of an Italian food frequency questionnaire for cancer studies. Results for specific nutrients. Ann Epidemiol. 1995;5:69–75. doi: 10.1016/1047-2797(95)92893-d. [DOI] [PubMed] [Google Scholar]
- 21.Kalbfleish J, Prentice R. The statistical analyses of failure time data. 2nd. New York, USA: John Wiley & Sons; 2002. [Google Scholar]
- 22.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;161:1141–54. [Google Scholar]
- 23.Cox DR. Regression models and life tables. J R Stat Soc. 1972;34:187–220. [Google Scholar]
- 24.Fine JP, Gray RJ. A proportional hazard model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 25.Rothman KJ, Greenland S, Lash TL. Modern epidemiology. 3rd. Philadelphia, USA: Lippincott, Williams & Wilkins; 2008. [Google Scholar]
- 26.Schulze MB, Hoffmann K, Manson JE, et al. Dietary pattern, inflammation, and incidence of type 2 diabetes in women. Am J Clin Nutr. 2005;82:675–84. doi: 10.1093/ajcn.82.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–40. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 28.Festa A, D’Agostino R, Howard G, et al. Chronic subclinical inflammation as part of the insulin resistance syndrome: the insulin resistance atherosclerosis study (IRAS) Circulation. 2000;102:42–7. doi: 10.1161/01.cir.102.1.42. [DOI] [PubMed] [Google Scholar]
- 29.Avery KNL, Donovan JL, Gilbert R, et al. Men with prostate cancer make positive dietary changes following diagnosis and treatment. Cancer Causes Control. 2013;24:1119–28. doi: 10.1007/s10552-013-0189-x. [DOI] [PubMed] [Google Scholar]
- 30.Kenfield SA, DuPre N, Richman EL, et al. Mediterranean diet and prostate cancer risk and mortality in the health professionals follow-up study. Eur Urol. 2014;65:887–94. doi: 10.1016/j.eururo.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray M, Delahunt B, Fowles JR, et al. Demographic and clinical factors as determinants of serum levels of prostate specific antigen and its derivates. Anticancer Res. 2004;24:2069–72. [PubMed] [Google Scholar]
- 32.Singer EA, Palapattu GS, van Wijngaarden E. Prostate-specific antigen levels in relation to consumption of nonsteroidal anti-inflammatory drugs and acetominophen. Results from the 2001–2002 National Health and Nutrition Examination Survey. Cancer. 2008;113:2053–7. doi: 10.1002/cncr.23806. [DOI] [PubMed] [Google Scholar]
- 33.AIRTum Working Group. Italian cancer figures, report 2014: Prevalence and cure of cancer in Italy. Epidemiol Prev. 2014;38:1–122. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


