Abstract
The endoplasmic reticulum (ER) is an energy-sensing organelle with intimate ties to programming cell activation and metabolic fate. T-cell receptor (TCR) activation represents a form of acute cell stress and induces mobilization of ER Ca2+ stores. The role of the ER in programming T-cell activation and metabolic fate remains largely undefined. Gp96 is an ER protein with functions as a molecular chaperone and Ca2+ buffering protein. We hypothesized that the ER stress response may be important for CD4+ T-cell activation and that gp96 may be integral to this process. To test our hypothesis we utilized genetic deletion of gp96 gene Hsp90b1 in a CD4+ T cell–specific manner. We show that gp96-deficient CD4+ T cells cannot undergo activation-induced glycolysis due to defective Ca2+ mobilization upon TCR engagement. We found that activating naïve CD4+ T cells while inhibiting ER Ca2+ exchange, through pharmacological blockade of the ER Ca2+ channel inositol trisphosphate receptor (IP3R), led to a reduction in cytosolic Ca2+ content and generated a pool of CD62Lhigh/CD44low CD4+ T cells compared to wild-type (WT) matched controls. In vivo IP3R-inhibited CD4+ T cells exhibited elevated tumor control above WT T cells. Together these data show that ER-modulated cytosolic Ca2+ plays a role in defining CD4+ T-cell phenotype and function. Factors associated with the ER stress response are suitable targets for T-cell based immunotherapies.
Keywords: T cell, Calcium, ER Stress, Metabolism, Adoptive Cellular Therapy
INTRODUCTION
Upon antigen recognition in the proper immunogenic context, T cells undergo multiple rounds of proliferation (1). To fulfill the bioenergetic demands of activation and expansion naive T cells undergo a metabolic shift from oxidative phosphorylation (OXPHOS) toward aerobic glycolysis (2). During the activation process, driven by T-cell receptor (TCR) engagement, a rapid rise in cytosolic Ca2+ occurs that intimately controls subsequent programming of T-cell differentiation and effector function (3) (4) (5). The endoplasmic reticulum (ER) maintains cell Ca2+ stores, and release of Ca2+ into the cytosol promotes T-cell activation (6) (7) (8) . In activated T cells, metabolites from the glycolytic pathway work to inhibit Ca2+ re-uptake by the ER to potentiate the effector T-cell metabolic cycle (9). The role of initial Ca2+ rise and the channels that drive this process has not been well studied in the context of the T-cell metabolic programs.
The ER houses molecular chaperones with numerous functions in the cell stress response, protein folding, and Ca2+ control (10) (11,12) (13). In the face of acute perturbations in cellular homeostasis, characterized by an imbalance in cellular Ca2+, ER stress chaperones are mobilized at the gene and protein levels in order to restore protein homeostasis via increased protein folding and chaperoning (14). Gp96 (glucose-regulated protein, grp94) is a major ER stress protein that is a molecular chaperone and Ca2+ buffering protein and is responsive to changes in glucose availability (12) (15). The specific role of gp96 in metabolism and activation and the general involvement of ER stress in response to TCR engagement are unknown.
We show that acute ER stress was tied to activation, which resulted in upregulation of gp96. CD4+ T cells required gp96 for activation and induction of glycolysis, in association with modulation of Ca2+ mobilization. We elucidated a role for Ca2+ in programming CD4+ activation and differentiation and demonstrated that the ER-Ca2+ axis can be targeted to modulate CD4+ T-cell phenotype and metabolic dependence. Direct inhibition of the inositol triphosphate receptor (IP3R), the ER-mitochondrial Ca2+ channel, resulted in a pool of 62Lhigh/CD44low CD4+ T cells with increased spare respiratory capacity compared to untreated control T cells that produced better antitumor activity in vivo.
MATERIALS & METHODS
Mice
T cell–specific deletion of gp96 on a C57BL/6J background was accomplished by crossing Hsp90b1flox/flox (16) mice with Cd4-Cre (Tg(Cd4-cre)1Cwi/BfluJ) transgenic mice (The Jackson Laboratory). C57BL/6J, Rag1−/− (B6.129S7-Rag1tm1Mom/J), OT-1 (C57BL/6-Tg(TcraTcrb)1100Mjb/J), TRP-1 (B6.Cg-Rag1tm1Mom Tyrp1B-w Tg(Tcra,Tcrb)9Rest/J), or BALB/cJ mice were purchased from Jackson Laboratories. Ly5.2 (B6.SJL-Ptprca Pepcb/BoyJ) mice were a kind gift of Dr. Xue-Zhong Yu. All animal experiments were approved Medical University of South Carolina (MUSC) Institutional Animal Care and Use Committee and the Division of Laboratory Animal Resources at MUSC maintained all mice.
Human Samples
This work was determined by MUSC Institutional Review Board to be exempt under protocol PRO55960. Normal donor patients undergoing routine non-cancer associated surgery were consented under MUSC Biorepository surgical consent forms and samples were de-identified. Studies were conducted in accordance with the Declaration of Helsinki, International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS), Belmont Report, or U.S. Common Rule. Blood (5mL) was collected in EDTA coated tubes and PBMC were isolated via Histopaque centrifugation (Sigma).
RT-PCR and Immunoblot Analysis
RNA was isolated with RNeasy Mini Kit (QIAGEN) and single-strand cDNA was made with High Capacity RNA-to-cDNA Kit (Applied Biosystems, Thermo Fisher). Taqman gene expression assays (Applied Biosystems, Thermo Fisher) were used to perform real-time PCR using the StepOnePlus Real-Time PCR system (Applied Biosystems, Thermo Fisher). mRNA expression for grp94 and grp78 was normalized to GAPDH. For immunoblot cell lysates of naïve CD4+ T cells bead sorted from thymus or spleen (Miltenyi) were prepared. Lysates were probed with gp96 antibody (Enzo Life Sciences) or β-actin control (Sigma).
Flow Cytometry
The following fluorochrome-conjugated monoclonal antibodies were purchased from BD Pharmingen (FITC-vβ14 (14-2), FITC-BrDU (Bu20)) and eBioscience (FITC or APC-CD4 (GK1.5), APC-CD8 (53-6.7), FITC, PE, or Percp-Cy5.5-CD62L (MEL-14), PE-CD18 (M18/2), PE-Cy7-CD44 (IM7), APC-Cy7-CD45.1 (A20), V450-CD45.2 (ID4), PE-CD45RO (UCHLI), PE-Cy7-CD62L (DREG-56), APC-CD4 (OKT4). PE-Gp96 (9G10) intracellular monoclonal antibody and respective isotype control were purchased from AbCAM. Extracellular stains were performed in PBS supplemented with 2% FBS. All intracellular stains, unless otherwise noted below, were performed with Foxp3/Transcription Factor intracellular staining buffer (eBioscience). For BrDU stain BrDU FACS kit and protocol were used (BD Bioscience). Cytosolic Ca2+ (Fluo-4, 0.5 µM), mitochondrial Ca2+ (Rhod-2, 2 µM), and mitochondrial membrane potential (TMRM, 10 nM) dyes (Molecular Probes) were loaded at 37°C for 30 minutes in warm PBS and extracellular stains were added post dye incubation. Samples were run directly on flow cytometer.
In vitro plate-bound and peptide activation
For primary T cell activations of WT or 96KO splenocytes, CD4+ or CD8+ T cells were isolated with naïve CD4+ T cell or CD8+ T cell isolation kits (Miltenyi) and cultured for 18 h with plate-bound aCD3 and soluble CD28 (ebioscience) with IL2 (NCI). Splenocytes from TRP-1 TCR transgenic mice were cultured with cognate peptide-loaded irradiated (10Gy) WT splenic feeder cells and plated. Total splenocytes from OT-1 TCR transgenic mice were pre-incubated with OVA peptide and plated.
Microscopy
Naïve bead-isolated CD4+ T cells (Miltenyi) were counted and loaded with Fluo-4 (0.5 µM, Molecular Probes) in T cell media for 30 min at 37°C. Cells were labeled with CD4-APC and spun onto poly-lysine coated plates (Tissue-Tec) and fresh media was added. Imaging was performed using the Olympus FV10 for 148 frames at 11s intervals directly after addition of CD3/28 beads (DynaBeads). Fiji Image J software was used to quantify Fluo-4 fluorescence over time on a single cell basis.
Metabolic Assays
Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured in non-buffered RS media supplemented with HEPES under basal conditions and in response to 1µM oligomycin, 1.5 µM FCCP, and 100 nM rotenone + 1 µM Antimycin A (OCR) or 10 µM glucose, 1 µM oligomycin, and 10 µM 2-deoxyglucose (ECAR) with XF-96 Extracellular Flux Analyzer (Seahorse Bioscience). Cell Taq was used for T cell adherence. For in vivo 2-NBDG (Cayman Chemical) uptake, 100 µg/mouse was injected via tail vein and mice were bled 15 minutes post injection. Ex vivo 2-NBDG uptake was assessed 18 h post T-cell activation after glucose-free medium treatment. Lactate in cell culture media was measured with Lactic Acid kit (Sigma).
Adoptive Cellular Therapy (ACT)
B16F10 cells were obtained from ATCC and tested negative for mycoplasma in March 2015. Cells were passaged three times prior to in vivo inoculation. All media used was supplemented with plasmocin prophylactic (Invivogen). Cells were not re-authenticated during the course of these experiments. Tumors were established on the right flank of C57BL/6 male mice for 7 days. One day prior to ACT mice were irradiated (5Gy). Ex vivo–expanded TRP-1 TCR-transgenic T cells (2 × 106) treated for 7 days with vehicle or 2-APB were injected into the tail vein and tumor growth was assessed every other day until the endpoint tumor size (≥ 400mm2) was reached. For tumor-infiltrating lymphocyte (TIL) analysis, a tumor dissociation kit (Miltenyi) was used.
RESULTS
The ER stress chaperone gp96 is upregulated in response to TCR activation
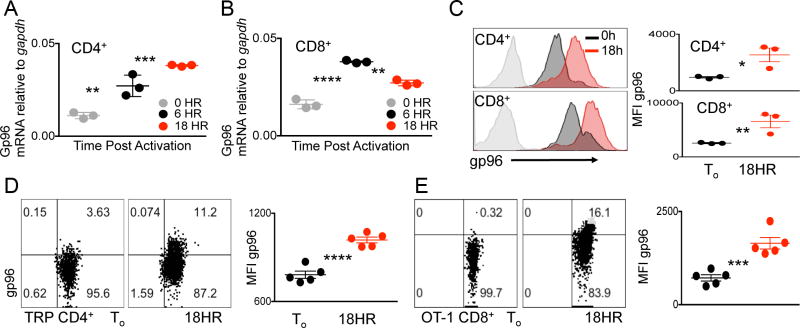
Due to the fact that ER stress chaperone gp96 folds proteins, such as the integrins, which are involved in various T-cell functions, we asked whether the chaperone itself was modulated in response to T-cell activation (12) (13) (17). We purified CD4+ or CD8+ T cells from wild-type (WT) mouse spleens and assessed gp96 gene expression after 6 and 18 h of activation. Both CD4+ and CD8+ T cells upregulated gp96 gene expression 6 and 18 h post activation, indicating acute ER stress response (Fig. 1A and B). To determine whether TCR activation induces the generalized ER stress response-associated with increased gene expression of chaperone proteins, we measured grp78 expression in the aforementioned conditions in both CD4+ and CD8+ T cells. In line with a general and acute ER stress response, grp78 gene expression was also increased at 6 h and 18 h post T-cell activation (Supplementary Fig. S1). To confirm the gene product of gp96 we measured gp96 protein expression via intracellular staining and flow cytometry at 18 h post T-cell activation in both CD4+ and CD8+ T cells. At this time point gp96, as measured by mean fluorescent intensity (MFI), was significantly upregulated in both CD4+ and CD8+ T-cell subsets well above unstimulated naïve controls (Fig. 1C).
Figure 1. ER stress chaperone gp96 is upregulated in response to TCR stimulation.
(A) Quantitative RT-PCR analysis of gp96 expression in naive CD4+ or (B) CD8+ T cells isolated from WT spleens stimulated with CD3/28 for 0, 6, or 18 hours. (C) Representative flow cytometry histogram and quantification of intracellular staining for gp96 in CD4+ (top panel) or CD8+ (bottom panel) T cells 0 or 18 h post CD3/28 stimulation. Isotype controls shaded in gray. Representative flow cytometry plots and quantification of intracellular gp96 levels in (D) CD4+ T cells from TRP TCR transgenic mice or (E) CD8+ T cells from OT-1 TCR transgenic mice at To or 18 h after peptide activation. Data points represent separate mice. Data are mean ± SEM. Numbers in (D) and (E) represent percentage of cell in each quadrant. *P <0.05, **P <0.01, ***P < 0.001, ****P <0.0001, 2-tailed Student t test. Three independent repeats were performed for each experiment.
Due to the upregulation of gp96 gene and protein in both CD4+ and CD8+ T cells upon CD3/28 activation (Fig. 1A–C), we asked whether this response was specific to TCR activation induced by cognate MHC class I–peptide complexes. We assessed the CD4+ T cell response using T cells from TCR transgenic mice with a TCR specific for tyrosinase-related protein-1 (TRP-1). CD4+ T cells were activated for 18 h in the presence of cognate peptide, and the induction of intracellular gp96 was measured. TCR transgenic CD4+ T cells had significantly increased gp96 protein expression in response to cognate peptide, compared to naïve CD4+ T-cell controls (Fig. 1D) (18). We next measured induction of gp96 in OT-1 TCR transgenic CD8+ T cells specific for chicken ovalbumin peptide, (19). Similar to CD4+ T cells, activation of CD8+ T cells with cognate antigen induced gp96 protein expression (Fig. 1E). Given the critical role of the acute ER stress response in cellular activation and differentiation, our results agree with published data (20). However, our data demonstrate that TCR ligation is a form of acute stress that can induce ER stress chaperones in both primary CD4+ and CD8+ T cells.
T cell-specific gp96 mutants have altered CD4+ T-cell subsets
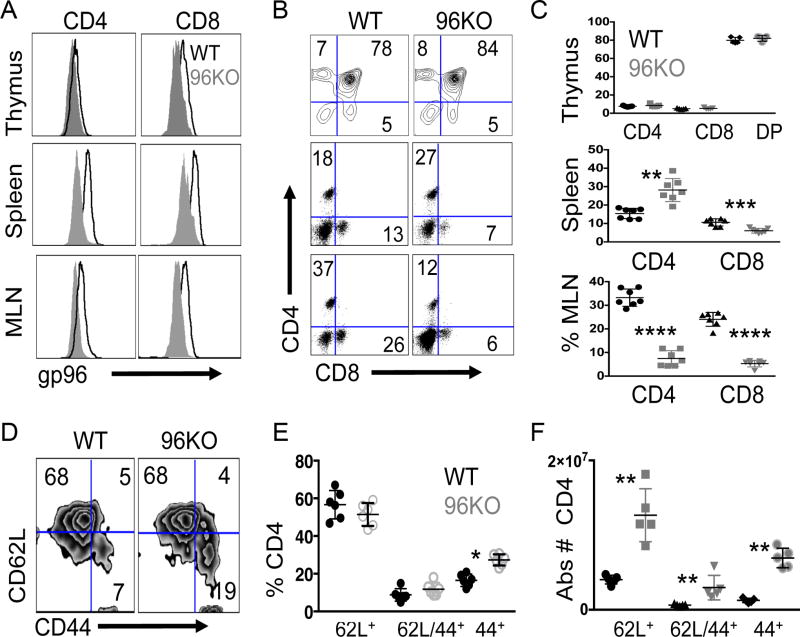
We have previously demonstrated that the function of regulatory T cells is greatly impaired by deletion of gp96, due to loss of GARP, which is a client protein of gp96 (17). We now investigated whether gp96 deficiency in the effector cell subset may impact T-cell activation and phenotype. We generated CD4+ T cell–specific gene deletion mutants for gp96 via CD4cre × Hsp90b1flox/flox (96KO) genetic cross and confirmed gene deletion and loss of protein expression in naïve CD4+ bead–isolated fractions from both spleen and thymus (Supplementary Fig. S2A and B). Given reduced gp96 protein expression in CD4 single-positive (SP) thymocytes, we measured the expression of thymic differentiation markers CD25, CD44, and CD127 among WT or 96KO mice and found no differences (data not shown). To assess alterations in SP and double-positive (DP) thymocyte subsets we measured CD4+/CD8+ T cells among thymocytes and found no differences in the size of the DP populations between WT or 96KO groups (Fig. 2A–C). Given that we aimed to assess the role of gp96 in T-cell activation, we examined TCR Vβ chain usage via flow cytometry in WT and 96KO mice. In both spleen and thymus, 14 commonly expressed Vβ chains were expressed to a similar extent in both WT and 96KO mice (Supplementary Fig. S2C).
Figure 2. Gp96 T cell specific gene deletion induces CD4+ subset changes.
(A) Representative histograms of gp96 expression in CD4+ or CD8+ single-positive thymocytes, splenocytes, or mesenteric lymph node (MLN) T cells preparations from CD4creHsp90b1flox/WT (WT) or CD4creHsp90b1flox/flox (96KO) mice. (B) Representative FACS plots for CD4+/8+ populations from WT or 96KO mice from thymus, spleen, or MLNs and (C) quantification of populations. (D) Representative FACS plots gated from CD4+/CD25− populations from WT or 96KO splenocytes and (E) percentage or (F) absolute number quantification of splenic subpopulations. Data are mean ± SD of 5–7 mice per group. *P < 0.05, **P < 0.01,****P < 0.000,1 2-tailed Student t test.
CD4cre, expressed in the double-positive stage of thymoctye maturation, induced loxp-mediated gp96 gene deletion in >90% of CD4+ and CD8+ T cells. We undertook phenotypic analysis of CD4+ and CD8+ thymocytes, splenocytes, and lymph node-associated populations between WT and 96KO mice (21). In agreement with our protein and gene deletion data (Supplementary Fig. S2A and B) we found that splenic populations were significantly reduced in gp96 expression in both CD4+ and CD8+ T-cell subsets (Fig. 2A). We noticed that splenic populations of CD4+ T cells significantly increased in 96KO mice compared to WT controls. CD8+ T-cell percentages in 96KO mice appeared slightly reduced (Fig. 2B and C, Supplementary Table S1). Our lab has shown that gp96 chaperones multiple integrins, many of which are essential for T-cell homing to lymph nodes. Thus, a direct functional consequence of T cell–specific gene deletion of gp96, was reduced percentages of CD4+ and CD8+ T cells that had trafficked to mesenteric lymph nodes in 96KO mice compared to WT controls (Fig. 2B and C, Supplementary Table S1) (13).
Analysis of absolute T-cell numbers in both CD4+ and CD8+ T-cell subsets showed that absolute CD8+ T-cell numbers between WT and 96KO mice were not significantly different. However, the absolute numbers of CD4+ T-cell populations were affected at absolute levels between WT and 96KO mice (Supplementary Fig. S3A, Supplementary Table S1). Assessment of subpopulations of CD4+ T cells in spleens showed an increase in percent CD44+/CD4+ T cells indicative of activated and differentiated T cells in 96KO mice compared to WT controls (Fig. 2D and E). Detailed analysis of subpopulations via absolute quantification unveiled that all subpopulations within 96KO splenocytes were significantly elevated (Fig. 2F).
Loss of gp96 results in CD4+ T-cell activation defects
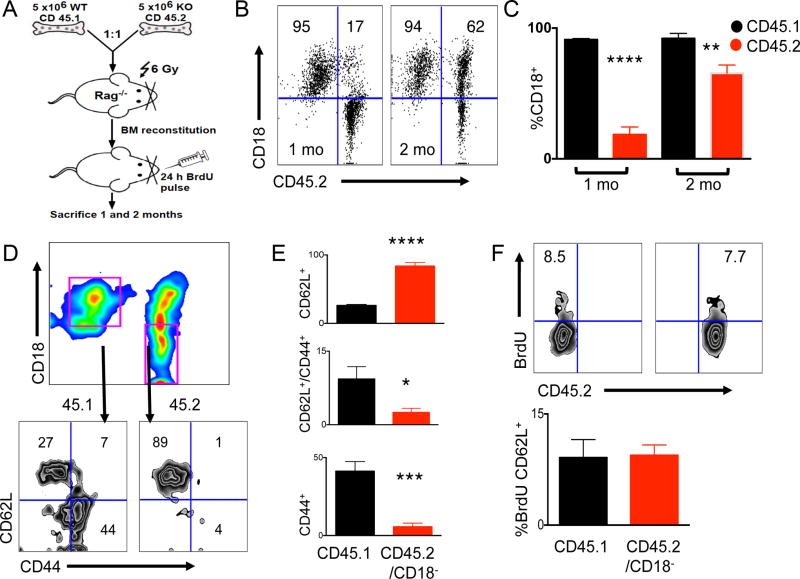
In order to rectify the finding that although 96KO mice possessed greater numbers of both naive and less activated CD4+ T cell, they also had highly activated CD44+ T cells, we used the gp96-folded molecule CD18 as a marker of gp96 deletion in vivo among CD4+ T-cell subsets. Our data show that the highly activated CD44+ T-cell subset in 96KO mice was an outgrowth of a remnant WT population (Supplementary Fig. 3B). To further substantiate this finding and investigate the role of gp96 in CD4+ T-cell activation, we CFSE-labeled CD4+ T cells isolated from splenocytes of WT or 96KO mice (depleted of CD4+/CD25+ cells), and transferred them into sublethally irradiated RAG1−/− mice. Aftter 14 days we assessed splenic populations of recipient RAG1−/− mice for CFSE/CD62L+ populations. Transfers from 96KO mice contained CFSE+/CD62L+ populations, whereas transfers from WT mice showed complete activation, as shown by robust cell division and loss of CFSE+ transferred CD4+ T cells (Supplementary Fig. S4). due to loss of Treg function (17). To address the role of a cell intrinsic activation defect in 96KO CD4+ T cells not due to loss of Treg function, we undertook mixed bone marrow chimera analysis. As Fig. 3A indicates, T cell-depleted bone marrow
Figure 3. WT CD4+ T cells outcompete 96KO CD4+ T cells, indicative of a cell intrinsic defect.
(A) Experimental design of 1:1 WT (CD45.1) or 96KO (CD45.2) T-cell depleted mixed bone marrow chimeras (BMC) transferred to irradiated (10Gy) Rag1−/− mice and harvested 1 and 2 months post transfer. (B) Representative flow cytometry plots of splenocytes from 1 and 2 months post bone marrow reconstitution, gated on CD4+ T cells and probed for CD45.2+/CD18− as a measure of gp96 deletion. (C) Quantification of CD4+ T cells probed for CD45.1/CD45.2/CD18 1 and 2 months post BMC reconstitution. (D) Representative flow cytometry plots of splenocytes from 2 months post BMC reconstitution, gated on CD4+ T cells and further gated from (pink gates) CD45.1/CD18+ (WT) or CD45.2/CD18− (96KO) populations assessed for CD62L/CD44 expression and (E) quantification of subset populations. (F) Representative flow cytometry plots of BrdU uptake gated from CD4+ splenic T cells from 24 hour pulsed 1 month BMC mice and (G) quantification of BrdU uptake in CD4/CD62L+ T cells gated from WT (CD45.1/CD18+) or 96KO (CD45.2/CD18−) populations. Data are mean ± SD of 4 mice per group. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, 2-tailed Student t test. Two independent repeats were performed for these experiments.
Ca2+ flow and mitochondrial membrane potential in CD4+ 96KO T cells
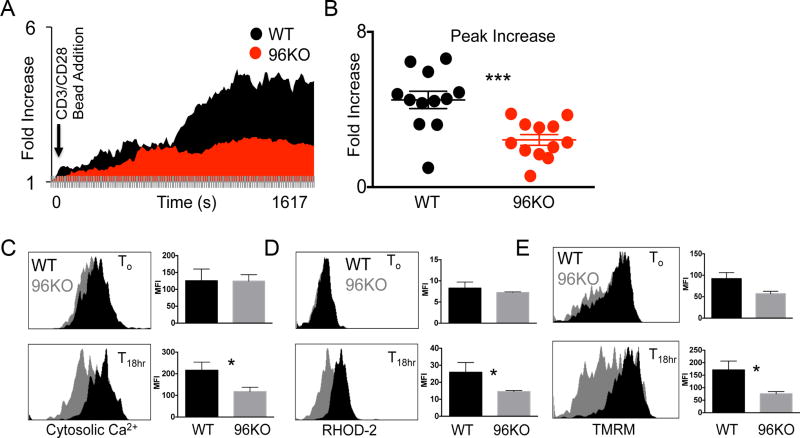
ER stress response is tightly tied to fluctuations in cell Ca2+ concentrations. ER-associated and chaperone proteins have secondary capacities as Ca2+ buffering proteins (15,23). For example, T cell–specific deletion of calreticulin greatly impairs cell Ca2+ oscillations resultant in a pool of less activated T cells and autoimmune conditions in vivo (24). We undertook ex vivo Ca2+ imaging of single cells from WT or 96KO naïve CD4+ T-cell preparations. Bead-isolated naïve WT or 96KO CD4+ T cells were labeled with cytosolic Ca2+ imaging dye Fluo-4. This dye fluoresces upon cytosolic free Ca2+ elevation. We pre-labeled CD4+ T cells with Fluo-4 and live-imaged fluorescent signal upon CD3/28 bead addition. Fluorescence in WT CD4+ T cells increased over the first 30 minutes of TCR engagement, whereas 96KO CD4+ T cells did not mobilize cytosolic free Ca2+ similar to WT patterns (Fig. 4A). Direct quantification of mobilization was obtained by measurement of peak Ca2+ intensity with the first 30 minutes of TCR ligation between WT and 96KO groups (Fig. 4B). 96KO CD4+ T cells did not mobilize cytosolic free Ca2+ to the extent that WT CD4+ T cells did, lending potential mechanistic support to explain the activation defect in 96KO CD4+ T cells. However, more work is needed to attribute this effect directly to the Ca2+ buffering capacity, and not the chaperone function, of gp96.
Figure 4. Gp96 is necessary for naïve CD4+ T-cell mobilization of intracellular Ca2+.
(A) Real time single cell imaging in WT or 96KO CD4+/CD62L+ T cells. Naïve T cells were isolated and labeled with Fluo-2 (FITC) and CD4 (APC) and adhered to glass slide incubating chambers. At time 0 CD3/28 beads were added to media. Data are plotted as average fold increase in Fluo-4 for 12–16 cells per group over 11 second intervals for ~30 minutes. Analysis was performed with Image J software. (B) Peak fluorescent increase in Fluo-4 was recorded. Data are mean ± SEM of 12 cells per group. ***P < 0.0001, 2-tailed Student t test. Similar data were obtained for 4 mice per group in 4 separate experiments. Representative histograms and quantification from 0 or 18 hour CD3/28 activated splenocytes from WT or 96KO mice gated on CD4+/CD18+ (WT) or CD4+/CD18− (96KO) cells and probed for (C) cytosolic Ca2+ with Fluo-4 (D) mitochondrial Ca2+ with Rhod-2 (D) or mitochondrial membrane potential with TMRM. Data are means from 3 mice, three independent experimental repeats were performed. *P < 0.05, 2-tailed Student t test.
Given that 96KO CD4+ T cells maintained a naïve state in vivo and that these cells showed impaired initial Ca2+ mobilization in response to TCR activation (Fig. 3 & Fig. 4A), we asked whether activation-induced long-term cytosolic Ca2+ concentrations were impaired in these cells. We found that though naïve cytosolic Ca2+ concentrations between WT and 96KO cells did not differ, 18 h post-activation 96KO CD4+ T cells showed significantly diminished cytosolic free Ca2+ (Fig. 4C). Thus, naive CD4+ T cells maintained low cytosolic Ca2+ concentrations, which supported the notion of Ca2+ as a driver of T-cell activation.
Release of ER Ca2+ stores leads to elevated mitochondrial Ca2+ modulated through the IP3R (25). Though not yet described in T cells, mitochondrial activation through cell Ca2+ is a key regulator of cell bioenergetics (26) (27). We asked whether inhibition of mitochondrial Ca2+ signal could be detected in activated 96KO CD4+ T cells. We used Rhodamine-2 (Rhod-2) to assess mitochondrial Ca2+ content 0 and 18 h post T-cell activation. We found that, although no basal differences in mitochondrial Ca2+ could be detected, activated 96KO cells showed impaired mitochondrial Ca2+ content compared to WT T cells (Fig. 4D). These data suggested a link between ER-modulated Ca2+ signals and mitochondrial activation. Ca2+ overload within the mitochondria can lead to induction of apoptotic signaling cascade, whereas impaired mitochondrial Ca2+ uptake can compromise mitochondrial metabolism (28). We used the mitochondrial membrane potential dye tetramethylrhodamine methyl ester (TMRM) to measure mitochondrial activity. We reasoned that initial loss of Ca2+ uptake in 96KO cells would lead to inactive mitochondria as assessed by TMRM and demonstrate that mitochondrial metabolism was compromised in these cells. Indeed, 18 h after activation, WT cells increased TMRM fluorescence compared to 0 hour controls, but 96KO cells were unable to significantly regulate TMRM levels to that of WT controls (Fig. 4E). Together these data present evidence for the role of cell Ca2+ to modulate mitochondrial metabolism in T cells.
Gp96 CD4+ T-cell mutants are impaired in glucose consumption
The role of ER stress chaperones and sensors in T-cell biology and associated metabolomics has not been studied. Upon CD4+ T-cell activation, glucose uptake and entry into glycolysis is modulated by Glut-1 cell surface expression (29). Further, it is known that cytosolic Ca2+ can perpetuate glycolysis as glycolytic product phosphoenolpyruvate (PEP) directly inhibits ER-re-establishment of Ca2+ homeostasis (9). However, the role of initial mobilization of Ca2+ in promotion of glycolysis has not been addressed. We utilized 96KO CD4+ T cells as a tool to study the induction of glycolysis within the first 18 h of T-cell activation in the context of impaired Ca2+ mobilization.
We asked whether there were in vivo differences between WT CD4+ and 96KO CD4+ T cells in ability to consume glucose. We injected fluorescent glucose analog 2-[N-(7-nitrobenz-2-oxa-1,3-diaxol-4-yl)amino]-2-deoxyglucose (2-NBDG) into WT or 96KO mice and measured in vivo glucose uptake in CD4+ populations in peripheral blood (30). 96KO CD4+ T cells were severely impaired in their ability to uptake glucose compared to WT matched controls (Fig. 5A). These results were not unexpected given that cytosolic Ca2+ concentrations may be slightly affected in vivo in the context of homeostatic proliferation in 96KO T cells and highlight the importance of basal glucose uptake impacted by gp96 in vivo for cell survival.
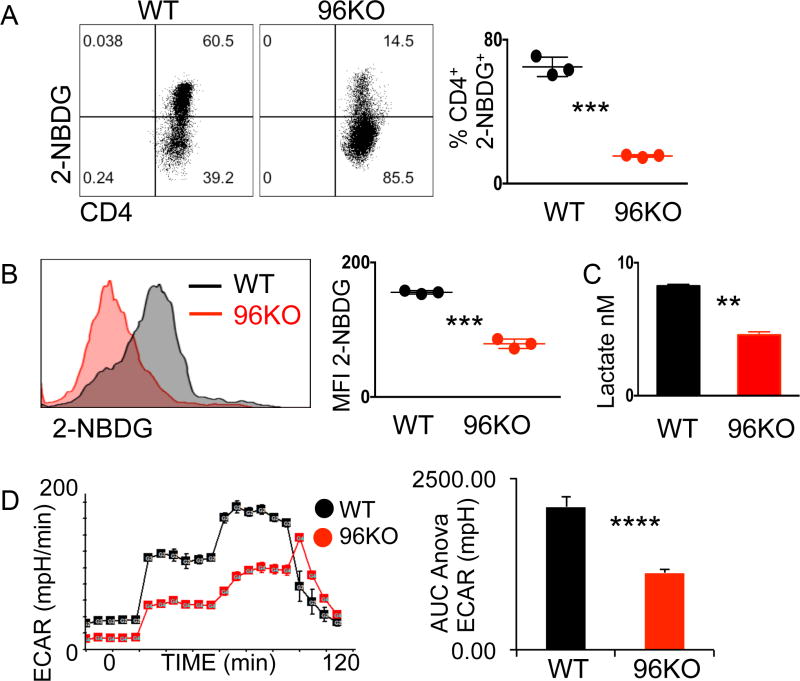
Figure 5. 96KO CD4+ T cells show impaired glucose uptake.
(A) Representative flow cytometry plots or (B) histograms and quantification of 2-NBDG in vivo and in vitro uptake, respectively, by WT or 96KO CD4+ T cells. (C) Lactate secretion to media by WT or 96KO naïve CD4+ T cells cultured for 16 h post CD3/28 activation. Data are mean ± SEM of 3 mice per group, two independent experimental repeats were performed. **P <0.001, ***P <0.0001, 2-tailed Student’s t test. (D) Extracellular acidification (ECAR) rates in response to glucose, oligomycin, and 2-deoxyglucose additions to 16 hour CD3/28 activated WT or 96KO naïve CD4+ T cells. **P <0.001, Area under the curve ANOVA of rates 6–11. Four independent experimental repeats were performed.
In the context of 18 hour TCR activation we saw impaired glucose uptake measured by in vitro 2-NBDG in CD4+ T cells (Fig. 5B). In accordance with diminished processing of glucose, we found that end-stage glycolytic product lactate was significantly reduced in 96KO CD4+ T cells (Fig. 5C). 96KO cells activated for 18 h were also unresponsive to glucose addition, as measured by extracellular acidification rate (ECAR) in comparison to WT control rates (Fig. 5D). Thus, both gp96 and Ca2+ mobilization may be required for CD4+ T-cell activation and subsequent induction of glycolysis.
Cytosolic Ca2+ defines CD4+ T-cell activation and differentiation
Naïve CD8+ T cells activated in the presence of glycolysis inhibitor 2-deoxyglucose (2-DG) primarily form T memory cells in vitro as marked by preferential dependence on oxidative metabolism (31). Given the emerging role of metabolomics in programming T-cell lineages coupled to our finding that Ca2+ inhibited CD4+ T cells showed severe defects in glucose uptake, we asked whether cytosolic Ca2+ alone could define murine CD4+ T-cell activation and differentiation. We harvested splenocytes from WT mice and used Fluo-4 dye to assess cytosolic free Ca2+ content in CD4+ T cells directly ex vivo. Cytosolic Ca2+ concentrations could definitively differentiate naïve (CD62L+/CD44−), central memory (CD62L+/CD44+), and effector (CD62L−/CD44+) subsets among CD4+ T-cell populations in mice (Fig. 6A–B). In vivo analysis of CD8+ splenocytes from mice showed that cytoplasmic Ca2+ content was only able to differentiate CD44+ effector subsets from naïve and central memory groups (Supplementary Fig. S5A). However, in the context of in vitro cytokine driven differentiation of CD8+ T cells toward effector (IL2) or memory (IL15) lineages cytosolic Ca2+ was able to discriminate subsets (Supplementary Fig. S5B). Thus, cytosolic Ca2+ is a means to discriminate T-cell lineages and suggest that mechanisms of Ca2+ control may be used to modulate lineage outcomes.
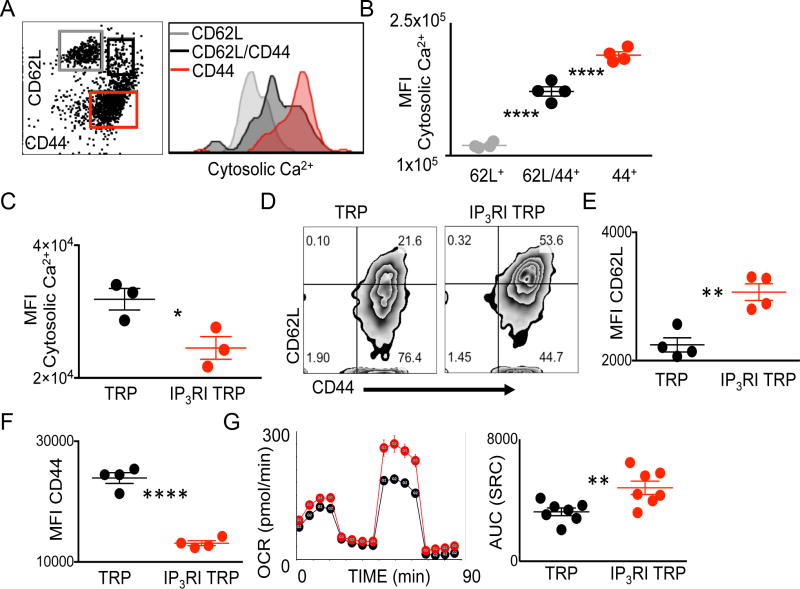
Figure 6. Cell Ca2+ differentiates CD4+ T-cell lineages and modulation of lP3R promotes CD4+ T CD62Lhigh/CD44low phenotype.
(A) Representative flow cytometry plots and (B) quantification of WT CD4+ gated splenocytes differentiated by CD62L/CD44 and probed for cytosolic Ca2+. Data are means from 4 mice, three independent experimental repeats were performed. ****P < 0.0001, 2-tailed Student t test. (C) Quantification of cytosolic Ca2+ content in 18 hour ex vivo peptide expanded TCR transgenic TRP T cells activated in the presence of IP3R inhibitor (IP3RI) 2-APB or vehicle control. (D) Representative flow cytometry plots and quantification of (E) CD62L MFI (F) CD44 MFI of day 7 ex vivo expanded TCR transgenic TRP T cells activated in the presence of IP3RI or vehicle control. (G) Oxygen consumption rates (OCR) and quantification of rates in response to addition of oligomycin, FCCP, and Rotenone/Antimycin A. (C,E-F) Data are mean ± SEM of individual mice and experiments were repeated at least twice, *P < 0.05, **P <0.001,****P < 0.0001, 2-tailed Student t test. (G) Data are mean ± SEM of pooled T cells from 5–7 mice per group, experiment was repeated 3 times, *P < 0.05, Area under the curve ANOVA of rate data, 8–13.
We next asked whether a similar differentiation based on cytosolic free Ca2+ content could be made among human CD4+ populations. We isolated peripheral blood mononuclear cells (PBMC) from donors and utilized CD62L/CD45RO subset analysis to differentiate between naïve (CD62L+/CD45RO−), central memory (CD62L+/CD45RO+), effector memory (CD62L−/CD45RO+), and effector (CD62L−/CD45RO−) subsets. Indeed, we found that in human CD4+ T cells, cytosolic Ca2+ content could distinguish CD4+ lineage populations. Among four PBMC samples assessed there were no significant differences between naïve and central memory cytosolic Ca2+ levels, whereas Ca2+ was able to distinguish both effector memory and effector populations from the naïve group (Supplementary Fig. S6).
Our demonstration that a low cytosolic free Ca2+ signal is a property able to differentiate fully activated effector T-cell populations, coupled to our finding that inhibition of Ca2+ mobilization impairs glucose dependence, led us to ask whether direct inhibition of ER-mitochondrial Ca2+ exchange could modulate CD4+ T-cell phenotype and metabolic dependence. The IP3R is responsible for release of ER Ca2+ stores to both the cytosol and mitochondria, and similar to 96KO CD4+ T-cell phenotype, T cell–specific deletion of IP3R inhibits T-cell activation (25) (32). However, the specific role of IP3R inhibition in the context of CD4+ T-cell activation has not been addressed. We used IP3R inhibitor 2-aminoethoxydiphenyl borate (2-APB) to inhibit IP3R at the time of T-cell activation in CD4+ TCR transgenic T cells. As expected, 18 h after peptide-specific activation of TRP TCR transgenic CD4+ T cells, cytosolic Ca2+ was significantly diminished in the IP3R inhibited T-cell group (Fig. 6C). After 7 days, TCR transgenic T cells activated in the presence of 2-APB maintained a significantly increased CD62Lhigh T-cell pool with less fully activated CD44+ T cells, compared to WT matched controls (Fig. 6D). Measurements for CD62L and CD44 MFI between WT or IP3R-inhibited (IP3RI) populations showed significantly increased CD62L expression and reduced CD44 expression in IP3RI CD4+ T cells compared to WT controls (Fig. 6E–F).
A hallmark feature of CD62Lhigh/CD44+ CD8+ T cells is elevated spare respiratory capacity (SRC) demonstrative of continued oxygen consumption in the context of activation (33). Given the striking CD62Lhigh/CD44low phenotype of IP3RI derived CD4+ T cells, we measured oxygen consumption rates (OCR) and associated SRC in these cells 7 days after initial activation. We found IP3RI CD4+ T cells possessed greater OCR and marked SRC compared to WT matched controls (Fig. 6G).
Modulation of cell Ca2+ enhances therapeutic efficacy of tumor-specific T Cells
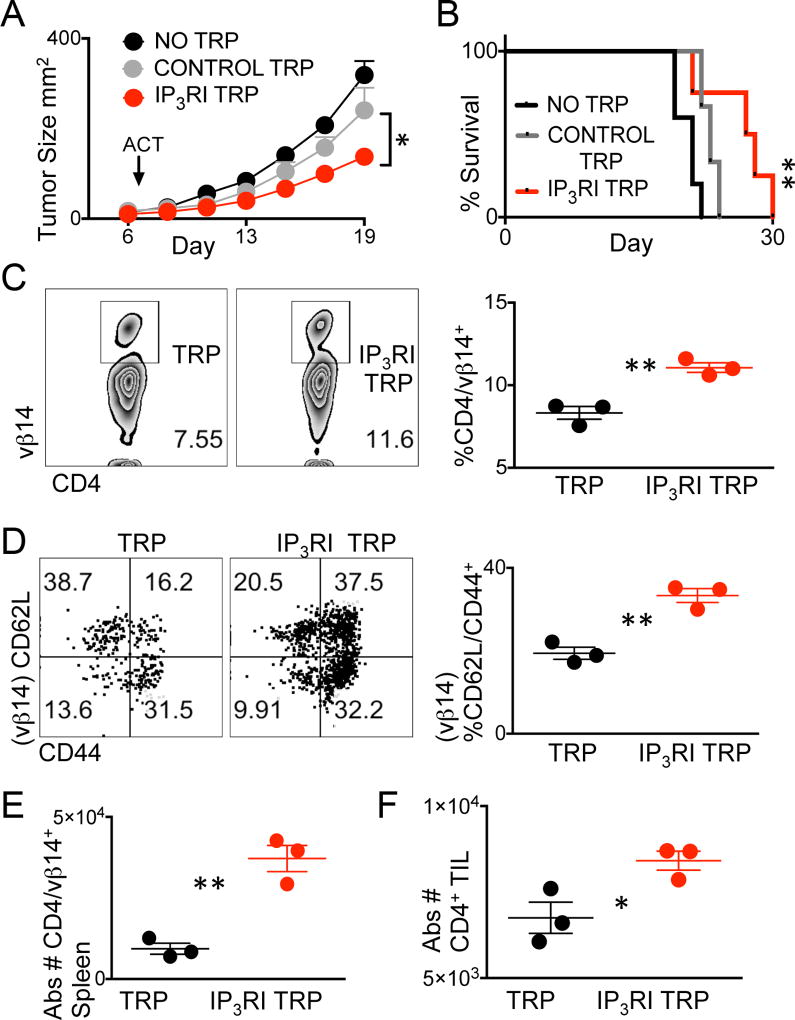
A hallmark feature of T cells shifted toward a CD62Lhigh/CD44+ phenotype through metabolomic modulation is elevated tumor control above glycolytic effector counterparts. Such manipulations are well documented in CD8+ T-cell populations and not yet flourished among CD4+ T-cell counterparts (31) (34). We asked whether IP3RI CD4+ T cells possessed the in vivo property of augmented tumor control. We adoptively transferred CD4+ TCR transgenic T cells activated and expanded in the presence of vehicle or 2-APB into syngeneic B16F10 melanoma-bearing mice. We found that IP3RI 2-APB–treated T cells significantly inhibited tumor growth and extended survival times above vehicle matched control T cells (Fig. 7A–B).
Figure 7. Modulation of cell Ca2+ enhances therapeutic efficacy of tumor-specific T Cells.
Day 7 established B16F10 tumors were treated with no T cells, or 2 × 106 TRP T cells cultured in the presence of IP3R inhibitor 2-APB (IP3R TRP) or vehicle control. (A) Tumor growth and (B) survival were measured. Survival endpoints were considered tumor size ≥ 400mm2 with n = 5 mice per group. Experiment was repeated twice. (A) *P < 0.05, 2-tailed Student t test of Control TRP versus IP3RI TRP and (B) **P < 0.001, Log-rank test. (C) Representative flow cytometry plots and quantification of CD4/Vβ14+ splenocytes or (D) CD62L/CD44 phenotype of CD4+/Vβ14+ splenocytes,5 days post adoptive cell therapy. (E) Absolute numbers of CD4+/Vβ14+ T cells in spleens or of (F) CD4+ T cell in tumors 5 days post ACT. (C–F) Data points represent 3 mice per group, *P < 0.05, **P < 0.01 2-tailed Student t test.
Possible reasons for superior tumor control by CD62Lhigh/CD44+ populations are increased lymph node homing and tumor entry due to expression of CD62L and energy reserves endowed by heightened SRC (35) (33). Given that IP3RI TRP CD4+ T cells showed increased CD62L expression and elevated SRC compared to effector matched controls, we asked whether more Vβ14+ CD4 T cells were found in IP3RI T cell treated mice. Spleens from mice adoptively transferred with IP3RI T cells showed significantly increased Vβ14+ percentages and absolute numbers compared to WT matched controls (Fig. 7C and E). Phenotypic analysis of the Vβ14 populations found in mice treated with control or treated or IP3RI-treated T cells showed that IP3RI populations maintained significantly increased numbers of CD62Lhigh/CD44+ associated populations compared to vehicle treated T-cell group (Fig. 7D). As expected, IP3RI T cell–treated mice had significantly increased total CD4+ T-cell numbers accumulated in tumors as compared to vehicle treated T-cell controls (Fig. 7E). Together, these data show that cytosolic Ca2+ content was a novel property of antitumor immunity that could predict efficacy of adoptively transferred T cells.
DISCUSSION
This work depicts the initial exploration of a vivid role for the ER in T-cell biology in both control of mitochondrial function and lineage fate. The defects in 96KO CD4+ T cells were not unexpected due to the essential role of this chaperone protein in general immune cell biology previously elucidated by our laboratory (36) (16). Still, the profound impairment presented in 96KO T cells is particularly intriguing given that we show that antigenic stimulation specifically upregulates the general ER stress response. Glucose-regulated proteins were discovered due to their upregulation in the context of glucose deprivation (37). In the face of acute cell stress, ER chaperones are mobilized at both the gene and protein levels to promote protein-folding functions in order to restore cellular homeostasis (38). It stands to reason that, as we have demonstrated, TCR engagement imparts a form of acute cell stress, as measured by upregulation of gp96 and grp78 gene and protein expression. Entry into glycolysis, a time of vigorous new protein synthesis, likely demands increased ER chaperone activity to support de novo protein biosynthesis.
In the context of TCR engagement, the specific role of the ER to promote T-cell metabolism has, to this point, remained largely unknown. We demonstrated that gp96 deficient CD4+ T cells have defective Ca2+ mobilization upon TCR engagement and are unable to undergo activation-induced glycolysis. The T-cell defect in the absence of gp96 is likely multi-factorial and cannot be attributed to dysregulation of Ca2+ mobilization only. However, we specifically addressed the roles of ER Ca2+ exchange in the context of TCR engagement in CD4+ T-cell activation using pharmacological inhibitors of IP3Rs. We found that that inhibition of IP3Rs in T cells results in a critical activation defect, followed by an inability to initiate glycolysis, consistent with the inability to mobilize cytosolic Ca2+ content (32). If results from other cell types may be extrapolated, IP3R-mediated Ca2+ release is a key driver of mitochondrial programming and function. Deletion of IP3Rs from B-lymphocytes show diminished mitochondrial Ca2+ uptake resulting in impaired cellular bioenergetics (39).
Cytosolic Ca2+ content was able to directly discriminate both CD4+ and CD8+ T-cell lineage fates. We thus reasoned that direct graded modulation of ER-mitochondrial Ca2+ exchange might similarly tune T-cell outcomes. Our data show that direct modulation of a second messenger ion could shift CD4+ T-cell fates. This was interesting given that costimulatory signals associated with graded crosslinking of CD28 drive IP3R-associated cytosolic Ca2+ rise only at the strongest levels of engagement (40).
We found in vivo that CD4+ T cells activated in the presence of IP3RI had augmented antitumor responses. Therapeutic efficacy of CD4+ T cells in the context of adoptive cellular therapy generally involves intense cytokine conditioning (18). However, the requirements for generating therapeutically effective CD4+ T cells could be tempered simply by Ca2+ channel modulation. Together, our data suggest a role for Ca2+ in programming CD4+ T-cell fates, and shed light on ER-mitochondrial crosstalk in T cells that may regulate cellular bioenergetics, survival, and function in vivo.
Supplementary Material
Acknowledgments
We thank members of the Zihai Li lab and Drs. Mark Rubinstein and Matthew Scheffel for thoughtful suggestions and Gyda Beeson and Brett Hoover for Seahorse support and guidance.
Financial Support: NIH grants: CA186866, CA188419, AI070603 and AI077283 (to Z.L.), and the Hollings Cancer Center’s Cancer Center K12 support and ACS (to J.E.T.).
Footnotes
Conflicts of Interest Statement: The authors declare no potential conflicts of interest.
Author contributions
J.E.T designed and performed all experiments and prepared manuscript. C.W. and B.R. aided in experimental procedures and discussion of data. Y.Z. and B.L. provided guidance in gp96 knockout mouse genetics and husbandry strategies. C.M.P. and C.C.B. provided guidance for adoptive cellular therapy methods and metabolic assay development, respectively. Z.L. provided mentorship in experimental design, data analysis, and interpretation.
References
- 1.Williams MA, Bevan MJ. Effector and memory CTL differentiation. Annual review of immunology. 2007;25:171–92. doi: 10.1146/annurev.immunol.25.022106.141548. [DOI] [PubMed] [Google Scholar]
- 2.Buck MD, O'Sullivan D, Pearce EL. T cell metabolism drives immunity. The Journal of experimental medicine. 2015;212(9):1345–60. doi: 10.1084/jem.20151159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feske S. Calcium signalling in lymphocyte activation and disease. Nature reviews Immunology. 2007;7(9):690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 4.Le Borgne M, Raju S. Real-Time Analysis of Calcium Signals during the Early Phase of T Cell Activation Using a Genetically Encoded Calcium Biosensor. 2016;196(4):1471–9. doi: 10.4049/jimmunol.1502414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weber KS, Hildner K, Murphy KM, Allen PM. Trpm4 differentially regulates Th1 and Th2 function by altering calcium signaling and NFAT localization. Journal of immunology (Baltimore, Md : 1950) 2010;185(5):2836–46. doi: 10.4049/jimmunol.1000880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feske S, Giltnane J, Dolmetsch R, Staudt LM, Rao A. Gene regulation mediated by calcium signals in T lymphocytes. Nature immunology. 2001;2(4):316–24. doi: 10.1038/86318. [DOI] [PubMed] [Google Scholar]
- 7.Nohara LL, Stanwood SR, Omilusik KD, Jefferies WA. Tweeters, Woofers and Horns: The Complex Orchestration of Calcium Currents in T Lymphocytes. Frontiers in immunology. 2015;6:234. doi: 10.3389/fimmu.2015.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srikanth S, Gwack Y. Orai1-NFAT signalling pathway triggered by T cell receptor stimulation. Molecules and cells. 2013;35(3):182–94. doi: 10.1007/s10059-013-0073-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ho PC, Bihuniak JD, Macintyre AN, Staron M, Liu X, Amezquita R, et al. Phosphoenolpyruvate Is a Metabolic Checkpoint of Anti-tumor T Cell Responses. Cell. 2015;162(6):1217–28. doi: 10.1016/j.cell.2015.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu G, Lee AS. Role of the unfolded protein response, GRP78 and GRP94 in organ homeostasis. Journal of cellular physiology. 2015;230(7):1413–20. doi: 10.1002/jcp.24923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eletto D, Dersh D, Argon Y. GRP94 in ER quality control and stress responses. Seminars in cell & developmental biology. 2010;21(5):479–85. doi: 10.1016/j.semcdb.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ansa-Addo EA, Thaxton J, Hong F, Wu BX, Zhang Y, Fugle CW, et al. Clients and Oncogenic Roles of Molecular Chaperone gp96/grp94. Current topics in medicinal chemistry. 2016;16(25):2765–78. doi: 10.2174/1568026616666160413141613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Staron M, Yang Y, Liu B, Li J, Shen Y, Zúñiga-Pflücker JC, et al. gp96, an endoplasmic reticulum master chaperone for integrins and Toll-like receptors, selectively regulates early T and B lymphopoiesis. Blood. 2010;115(12):2380–90. doi: 10.1182/blood-2009-07-233031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science (New York, NY) 2011;334(6059):1081–6. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 15.Prins D, Michalak M. Organellar calcium buffers. Cold Spring Harbor perspectives in biology. 2011;3(3) doi: 10.1101/cshperspect.a004069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Liu B, Dai J, Srivastava PK, Zammit DJ, Lefrancois L, et al. Heat shock protein gp96 is a master chaperone for toll-like receptors and is important in the innate function of macrophages. Immunity. 2007;26(2):215–26. doi: 10.1016/j.immuni.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, Wu BX, Metelli A, Thaxton JE, Hong F, Rachidi S, et al. GP96 is a GARP chaperone and controls regulatory T cell functions. The Journal of clinical investigation. 2015;125(2):859–69. doi: 10.1172/jci79014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112(2):362–73. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hogquist KA, Jameson SC, Heath WR, Howard JL, Bevan MJ, Carbone FR. T cell receptor antagonist peptides induce positive selection. Cell. 1994;76(1):17–27. doi: 10.1016/0092-8674(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 20.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell death and differentiation. 2006;13(3):374–84. doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 21.Lee PP, Fitzpatrick DR, Beard C, Jessup HK, Lehar S, Makar KW, et al. A critical role for Dnmt1 and DNA methylation in T cell development, function, and survival. Immunity. 2001;15(5):763–74. doi: 10.1016/s1074-7613(01)00227-8. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Zhu J. Immunologic applications of conditional gene modification technology in the mouse. Current protocols in immunology. 2014;105 doi: 10.1002/0471142735.im1034s105. 10.34.1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Koch G, Smith M, Macer D, Webster P, Mortara R. Endoplasmic reticulum contains a common, abundant calcium-binding glycoprotein, endoplasmin. Journal of cell science. 1986;86:217–32. doi: 10.1242/jcs.86.1.217. [DOI] [PubMed] [Google Scholar]
- 24.Porcellini S, Traggiai E, Schenk U, Ferrera D, Matteoli M, Lanzavecchia A, et al. Regulation of peripheral T cell activation by calreticulin. The Journal of experimental medicine. 2006;203(2):461–71. doi: 10.1084/jem.20051519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annual review of immunology. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Denton RM, Randle PJ, Martin BR. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. The Biochemical journal. 1972;128(1):161–3. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denton RM. Regulation of mitochondrial dehydrogenases by calcium ions. Biochimica et biophysica acta. 2009;1787(11):1309–16. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Duchen MR. Mitochondria and calcium: from cell signalling to cell death. The Journal of physiology. 2000;529(Pt 1):57–68. doi: 10.1111/j.1469-7793.2000.00057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macintyre AN, Gerriets VA, Nichols AG, Michalek RD, Rudolph MC, Deoliveira D, et al. The glucose transporter Glut1 is selectively essential for CD4 T cell activation and effector function. Cell metabolism. 2014;20(1):61–72. doi: 10.1016/j.cmet.2014.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Sullivan D, van der Windt GJ, Huang SC, Curtis JD, Chang CH, Buck MD, et al. Memory CD8(+) T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity. 2014;41(1):75–88. doi: 10.1016/j.immuni.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sukumar M, Liu J, Ji Y, Subramanian M, Crompton JG, Yu Z, et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. The Journal of clinical investigation. 2013;123(10):4479–88. doi: 10.1172/jci69589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jayaraman T, Ondriasova E, Ondrias K, Harnick DJ, Marks AR. The inositol 1,4,5-trisphosphate receptor is essential for T-cell receptor signaling. Proceedings of the National Academy of Sciences of the United States of America. 1995;92(13):6007–11. doi: 10.1073/pnas.92.13.6007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van der Windt GJ, Everts B, Chang CH, Curtis JD, Freitas TC, Amiel E, et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity. 2012;36(1):68–78. doi: 10.1016/j.immuni.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crompton JG, Sukumar M, Roychoudhuri R, Clever D, Gros A, Eil RL, et al. Akt inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer research. 2015;75(2):296–305. doi: 10.1158/0008-5472.can-14-2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nolz JC, Starbeck-Miller GR, Harty JT. Naive, effector and memory CD8 T-cell trafficking: parallels and distinctions. Immunotherapy. 2011;3(10):1223–33. doi: 10.2217/imt.11.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu B, Li Z. Endoplasmic reticulum HSP90b1 (gp96, grp94) optimizes B-cell function via chaperoning integrin and TLR but not immunoglobulin. Blood. 2008;112(4):1223–30. doi: 10.1182/blood-2008-03-143107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kozutsumi Y, Segal M, Normington K, Gething MJ, Sambrook J. The presence of malfolded proteins in the endoplasmic reticulum signals the induction of glucose-regulated proteins. Nature. 1988;332(6163):462–4. doi: 10.1038/332462a0. [DOI] [PubMed] [Google Scholar]
- 38.Lee AS. The glucose-regulated proteins: stress induction and clinical applications. Trends in biochemical sciences. 2001;26(8):504–10. doi: 10.1016/s0968-0004(01)01908-9. [DOI] [PubMed] [Google Scholar]
- 39.Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell. 2010;142(2):270–83. doi: 10.1016/j.cell.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ledbetter JA, Imboden JB, Schieven GL, Grosmaire LS, Rabinovitch PS, Lindsten T, et al. CD28 ligation in T-cell activation: evidence for two signal transduction pathways. Blood. 1990;75(7):1531–9. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.