Abstract
Objectives
Patients with Parkinson’s disease (PD) and essential tremor (ET) have a higher risk of cognitive impairment than age-matched controls. Only a few small studies (11–18 subjects per group) have directly compared the cognitive profile of these conditions. Our aim was to compare the cognitive profile of patients with these two conditions to each other and to healthy individuals in a population-based study of non-demented participants.
Materials & Methods
This investigation was part of the NEDICES study, a survey of the elderly in which 2,438 dementia-free participants underwent a short neuropsychological battery. We used non-parametric techniques to evaluate whether there are differences and/or a gradient of impairment across the groups (PD, ET and controls). Also, we performed a head-to-head comparison of ET and PD, adjusting for age and education.
Results
Patients with PD (N=46) and ET (N=180) had poorer cognition than controls (N=2,212). An impaired gradient of performance was evident. PD scored lower than ET, and then each of these lower than controls, in memory (P < 0.05) and verbal fluency (P < 0.001) tasks. When we compared PD and ET, the former had lower scores in verbal fluency (P < 0.05), whereas the later had a poorer cognitive processing speed (P < 0.05).
Conclusions
This large population-based study demonstrates that both conditions influence cognitive performance, that a continuum exists from normal controls to ET to PD (most severe), and that although deficits are in many of the same cognitive domains, the affected cognitive domains do not overlap completely.
Keywords: Epidemiology, Movement Disorders, Neurobehavioral Manifestations, Neuropsychology, Neuropsychological profile
1 INTRODUCTION
Patients with Parkinson’s disease (PD) and essential tremor (ET) have a higher risk of cognitive impairment than healthy individuals who are matched by age and education 1,2. Their pattern of dysfunction has traditionally been labeled as that of a fronto-subcortical type 3. Deficits in areas such as attention 4,5, verbal fluency 2,5 and memory 6 may be noted even at early stages of both conditions 2,7. In PD, this impairment has been linked with degeneration of cortical association areas 8. In ET, the neuropathological correlates of cognitive impairment have not been completely elucidated 9.
While considerable efforts are being made to fully characterize and better understand the non-motor features of ET and PD, the two most common tremor disorders 10–12, only three studies have directly compared the cognitive profile of these two disorders, and each of these had small sample sizes (11–18 patients in each group) 6,13,14. Furthermore, two of the studies [6,13] enrolled surgical patients, a highly selected group whose cognitive deficits are likely to differ from (i.e., be less marked than) those of the average patient. All three studies assessed patients who self-selected for treatment (i.e., patients attending clinics) rather than those sampled directly from the population. Clinic patients often differ phenotypically from those in the population. Finally, patients with both ET and PD may have Alzheimer’s disease (AD) and other forms of dementia, and none of the studies explicitly removed these from the sample in order to obtain a less-confounded and cleaner picture of cognition. Hence, there is a need for more comprehensive study.
In this population-based study of non-demented participants, we performed a head-to-head comparison of the cognitive profile of 180 individuals with ET and 46 with PD, comparing them to 2,212 healthy aged controls. Our four a priori hypotheses were that (1) both conditions affect cognitive performance relative to that of controls, even in the absence of a clinical diagnosis of dementia, (2) a continuum exists in this performance from normal controls to ET to PD (most severe), (3) the deficits in ET and PD are in many of the same cognitive domains; however, (4) the affected cognitive domains do not overlap completely, and there are demonstrable differences between ET and PD.
2 MATERIAL AND METHODS
2.1 Study population
This investigation was part of the Neurologic Disorders in Central Spain (NEDICES), a population-based survey of the prevalence, incidence, and determinants of major elderly-associated conditions. Detailed accounts of the background, study population, and methods of the survey have been reported 15–17.
2.2 Standard protocol approvals, registrations, and patient consents
All procedures were approved by the ethical standards committees on human experimentation at “12 de Octubre” Hospital (Madrid). Written (signed) informed consent was obtained from all enrollees.
2.3 Study evaluation
Detailed accounts of the study assessments have been published 16,18,19. Face-to-face evaluations were performed at baseline (1994 – 1995) and at one follow-up (1997–1998). The face-to-face interview included data collection on demographics, current medications (including medications with central nervous system [CNS] effects), medical conditions (e.g., diabetes mellitus, hypertension, and heart disease), lifestyle habits, and the presence of depressive symptoms (the question, “do you suffer from depression?”). As described 18, a neurological examination was performed, comprising a general neurological examination, a tremor examination, and the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS) 20 and the Hoehn-Yahr scale 21.
2.4 Diagnostic criteria for Parkinson’s disease and essential tremor
Diagnostic criteria for ET 18, PD 19 and dementia 1 have been described elsewhere. For the diagnosis of Parkinson’s disease, the study questionnaire had 3 questions to screen for parkinsonism (i.e. previous diagnosis of PD, presence of tremor, and presence of bradykinesia). Persons who screened positive (i.e. they responded positively to ≥ 1 of these 3 questions) for PD underwent a general neurological examination and the motor portion of the Unified Parkinson’s Disease Rating Scale (UPDRS) 20. Parkinsonism was diagnosed when at least 2 cardinal signs (resting tremor, rigidity, bradykinesia, and impaired gait/postural reflexes) were present. PD was diagnosed in patients without secondary causes of parkinsonism or atypical features 19. A Hoehn and Yahr stage was assigned to each case 21.
The evaluation of tremor and diagnosis of ET involved a screening question in the questionnaire (“have you ever had tremor of the head, hands, or legs that has lasted longer than several days?”) with 68.6% sensitivity22. The same examination as in PD was performed. It also included an assessment of postural and kinetic tremors (sustained bilateral arm extension, bilateral finger–nose–finger maneuver, drawing Archimedes spirals) and UPDRS 20. Participants were diagnosed with ET if they had an action tremor of the head, limbs, or voice without any other recognizable cause. Second, the tremor had to be of gradual onset (i.e., slow and progressive) and 1) present for at least 1 year or 2) accompanied by a family history of the same disorder (at least one reportedly affected first-degree relative). In addition, on an Archimedes spiral, tremor severity had to be moderate or greater (rating >2 according to the Washington Heights-Inwood Genetic Study of ET Rating Scale) 23. Participants with tremor related to alcohol withdrawal, hyperthyroidism, anxiety, PD, anti-dopaminergic drug intake, lithium therapy, or other known causes of tremor were not considered to have ET. If ET was diagnosed, the age of onset of tremor was elicited 22.
2.5 Neuropsychological tests
During the follow-up evaluation (1997–1998), all participants underwent a short neuropsychological evaluation 24,25. The tests used were:
Global cognitive performance: This was assessed with an expanded, 37-item version of the Mini Mental-State Examination (37-MMSE) that ranged from 0 to 37 26,27.
Psychomotor or cognitive-processing speed: This was assessed with the Trail Making test part A 28. Here, we report the number of errors while performing the task.
Verbal fluency: For semantic fluency, participants were asked to name as many different animals and fruits as they could within 60 s 29.
Memory: This was evaluated with the following tests: a) Six-objects test 24,30(Naming Test): This was the ability to recall six objects 5 min later [range 0 (greater cognitive impairment) to 6]). b) Story recall: The “story recall” task was derived from the Wechsler Memory Scale-Rev. and measured memory (delayed logical memory) for aurally presented contextual material. The total number of words recalled was summed (range 0 [greater cognitive impairment] to 6) 31.
Premorbid intelligence: This was evaluated with the “word accentuation” test. This test assesses the accentuation of 30 infrequently used Spanish words written without the accent marks (range 0 [greater impairment]-30) 32.
2.6 Final selection of participants
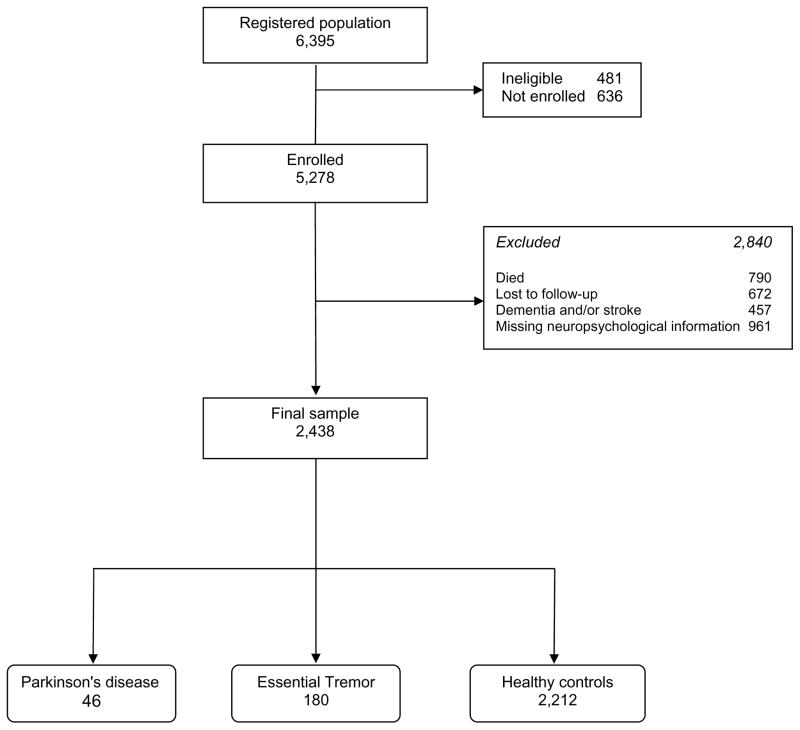
Of the 5,278 participants who were enrolled at baseline, 672 were lost to follow-up (112 declined and 560 were unreachable), and 790 died before they were contacted the second time. Of the remaining 3,816, we excluded 921 with missing neuropsychological information and 457 subjects with stroke or dementia. The final sample (N = 2,438) included patients with PD (N = 46) and ET (N = 180), as well as the remaining healthy controls (N = 2,212) (Figure 1). This sample was similar to the initial 3,816 individuals in terms of sex (1,403 [57.4%] vs. 2,231 [58.5%] women, chi-square = 0.47, p = 0.489), but on average, was 0.9 years younger (75.6 ± 5.7 vs. 76.5 ± 6.4 years, p < 0.001) and included a lower percentage of illiterate subjects (238 [10.0%] vs. 489 [12.9%], chi-square = 11.50, p < 0.001).
Figure 1.
Flow-chart of the Study
2.7 Statistical analyses
Statistical analyses where performed using R 33. Significance was accepted at the 5% level (two-sided). Neuropsychological tests scores were not normally distributed (Kolmogorov-Smirnov tests for all items, p < 0.001). Therefore, although mean and median values were reported, differences across groups and cognitive domains were assessed with a Kruskal-Wallis test correcting for post-hoc multiple comparisons. The chi-square test was used to analyze categorical variables. We used the Jonckheere-Terpstra test, a non-parametric method, to detect a trend (gradient) of performance in the neuropsychological results. In addition, we computed adjusted T-scores for each test 34. To do this, healthy participants were divided in twelve groups by age in tertiles (≤ 68 years, from 69 to 74 years, and ≥ 75 years), and educational category (illiterate, can write and read, primary studies, secondary studies or higher). Then, with the mean and standard deviation of their respective healthy control group the T-score was calculated for each PD and ET patient. With these adjusted T-scores, we compared the performance of the ET and PD groups in the different cognitive domains avoiding for the confounding effects of age and education. The cutoff for normality was defined as one (T-score < 40) or two standard (T-score < 30) deviations from the reference values 34.
In order to further adjust for confounding, a series of non-parametric stratified analyses was performed. The purpose of these tests was to understand the magnitude and directionality of the effect of these confounders on the cognitive tests results rather than their significance (i.e., if PD or ET scored lower than the controls’ across the different strata). The strata were defined by age in tertiles (≤ 68 years; from 69 to 74 years and ≥ 75 years), gender (male vs. female), education (illiterate, can write and read, primary studies, secondary studies or higher), presence of depressive symptoms (no vs. yes), and medications with CNS effects (no vs. yes). In addition, we double-checked the results of the stratified results in the younger and more educated subjects. We performed a final subanalysis to define the influence of the age of onset (≥ 65 years) on the cognitive performance of the ET patients. The purpose was to evaluate whether there was a different cognitive phenotype of those with an older onset 35.
3 RESULTS
3.1 Demographic features
The final sample included 46 PD patients, 180 ET patients and 2,212 healthy controls (Table 1). The groups differed in terms of age, sex, educational level, intake of medications with CNS effects, and depressive symptoms. The patients had mean disease duration of 5.3 [4.0] ± 5.1 years (PD) and 10.4 [6.0] ± 12.2 years (ET). In the PD group, 29 [63.0%] had unilateral disease (stage I) or bilateral disease without axial symptoms (stage II), based on the Hoehn-Yahr scale.
Table 1.
Demographic and baseline features of the participants (N=2,438)
| Parkinson’s disease [PD] (N= 46) | Essential Tremor [ET] (N=180) | Healthy Controls [CNT] (N=2,212) | Significance test* Post-hoc comparison |
|
|---|---|---|---|---|
|
| ||||
| Age (years) | 78.1 [77.5] ± 5.5 | 76·2 [75.5] ± 5.7 | 75.4 [74.5] ± 5.7 |
P < 0.001 PD > CNT |
|
| ||||
| Sex (female) | 17 (37.0) | 107 (59.4) | 1279 (57.8) |
P = 0.016 PD < CNT; PD < ET |
|
| ||||
| Education | ||||
| Illiterate | 9 (19.6) | 31 (17.2) | 198 (9.0) |
P = 0.006 (Illiterate percentage) PD > CNT; ET > CNT |
| Can write and read | 18 (39.1) | 73 (40.6) | 942 (42.6) | |
| Primary studies | 17 (37.0) | 52 (28.9) | 743 (33.6) | |
| Secondary or higher | 2 (4.3) | 24 (13.3) | 328 (14.8) | |
|
| ||||
| Medications with CNS effects (yes) 1 | 18 (39.1) | 67 (37.2) | 431 (19.5) |
P < 0.001 PD > CNT; ET > CNT |
|
| ||||
| Answered yes to the question “do you suffer from depression?” | 16 (34.8) | 75 (41.7) | 476 (21.5) |
P < 0.001 ET > CNT |
Quantitative data as mean [median] ± standard deviation for continuous variables or frequencies (percentages) for categorical variables; significant results are in bold;
Kruskal-Wallis and Chi-square tests adjusted for post-hoc pairwise comparisons
Medications potentially influencing cognitive performance (anxiolytics, stimulants, antipsychotics, antidepressants, antihistamines, antihypertensive agents, or antiepileptic drugs).
3.2 Cognitive performance - ET and PD vs. controls (Hypothesis 1)
ET and PD patients performed significantly more poorly than controls in several tests and in global cognition (Table 2; Supplementary Figure). When the PD and ET patients were compared with the controls, the mean raw difference for the global cognition test (i.e., the MMSE-37) was −2.3 points and −1.6 points, respectively. The six object and the story delayed recall tests scores were −0.7 and −0.8-points significantly worse in the PD patients than the controls. In the verbal fluency tasks, there was a greater than one-point difference for both the animal and fruit naming tests between the PD group and the controls. Finally, the ET patients erred one point more than the controls in the cognitive processing speed task, the Trail Making Test part A, although the significance was lost in the post-hoc comparison. In analyses that stratified by age in tertiles, gender, prior education, presence of depressive symptoms and CNS drug intake), the group differences observed in the primary analyses persisted (data not shown).
Table 2.
Neuropsychological performance of the NEDICES participants grouped by diagnostic category (N=2,438)
| Parkinson’s disease [PD] (N= 46) | Essential Tremor [ET] (N=180) | Healthy Controls [CNT] (N=2,212) | Significance test* Post-hoc comparison |
Trend analysis Jonckheere-Terpstra test |
|
|---|---|---|---|---|---|
| Global cognition 37-Mini Mental State Examination |
27.8 [28.5] ± 6.0 | 28.5 [29.0] ± 5.2 | 30.1 [31.0] ± 4.8 |
P < 0.001 PD < CNT; ET < CNT |
P < 0.001 |
| Memory Six Object test delayed recall |
3.4 [4.0] ± 1.7 | 3.9 [4.0] ± 1.7 | 4.1 [4.0] ± 1.5 |
P = 0.005 PD < CNT |
P = 0.011 |
| Memory Story delayed recall |
2.9 [3.0] ± 2.2 | 3.5 [4.0] ± 2.1 | 3.7 [4.0] ± 2.0 |
P = 0.007 PD < CNT |
P = 0.007 |
| Verbal fluency Number animals in 1’ |
12.0 [11.0] ± 5.6 | 13.5 [13.0] ± 4.7 | 13.4 [13.0] ± 4.4 |
P = 0.020 PD < CNT; PD < ET |
P = 0.200 |
| Verbal fluency Number fruits in 1’ |
9.3 [8.5] ± 3.2 | 9.9 [10.0] ± 3.1 | 10.5 [10.0] ± 3.0 |
P = 0.001 PD < CNT |
P = 0.001 |
| Cognitive Speed Processing** Trail Making Test errors |
1.7 [0.0] ± 4.1 | 2.8 [0.0] ± 5.7 | 1.8 [0.0] ± 4.4 |
P = 0.047 Post-hoc comparison N.S |
P = 0.071 |
| Premorbid Intelligence** Word Accentuation Test |
13.8 [15.0] ± 8.2 | 15.6 [16.0] ± 9.1 | 16.4 [16.0] ± 8.7 | P = 0.226 | P = 0.160 |
Data as mean [Median] ± standard deviation for continuous variables or frequencies (percentages) for categorical variables; significant results are in bold; N.S=non-significant
Kruskal-Wallis test adjusted for post-hoc pairwise comparisons
N=2,171 for Trail Making Test part A time (PD=34; ET= 148; Controls=1,989), N=2,161 for Trail Making Test part A errors (PD=35; ET= 144; Controls=1,982), and N=1,823 for the Word Accentuation Test (PD=33; ET= 133; Controls=1,657)
3.3 Cognitive performance - trend analyses (Hypothesis 2)
Based on the Jonckheere-Terpstra test (Table 2), a significant trend was detected: the PD patients scored less well than the ET patients, and these scored less well than the controls. This trend was evident for global cognition (P < 0.001), both memory tasks (P < 0.05) and the fruit-naming test (P < 0.001). The only test in which ET patients performed more poorly than PD patients and controls was cognitive speed processing, in which ET patients showed a non-significant trend of increased number of errors (P = 0.071). Again, these relationships continued in stratified analyses that considered the same variables as in hypothesis 1 (data not shown).
3.4 Cognitive profile - ET vs. PD (Hypotheses 3 and 4)
The head-to-head comparison of the ET and PD patients is detailed in Table 3. The calculated T-scores were consistently lower in the PD patients, except for the cognitive processing speed task, but they only reached significance in the lower number of animals that the PD individuals named (P = 0.05). With respect to the percentage of patients with abnormal results, defined by a T-score below forty, in all tests there were subjects who could be considered impaired. In the animal-naming test, 12 PD patients (26.1%) scored abnormally in comparison with 25 ET patients (13.9%) (P = 0.07). When all of the cognitive tests were considered, there were 26 PD (56.5%) and 82 ET (45.6%) patients with at least one abnormal domain. By domain, there were 19 PD (41.3%) and 59 ET (32.8%) patients with a poorer performance in memory tasks, 16 PD (34.8%) and 41 ET (30.0%) with abnormal language functioning and 2 PD (5.7%) vs. 18 ET (12.5%) patients showing more errors in the cognitive speed-processing task. None of these differences reached significance.
Table 3.
Comparison of the different cognitive domains (“T-scores”) in the Parkinson’s disease (N = 46) and essential tremor (N = 180) participants
| Parkinson’s disease (PD) | Essential Tremor (ET) | Significance test* | PD vs. ET cases with a T-score < 40 (%) | PD vs. ET cases with a T-score < 30 (%) | |
|---|---|---|---|---|---|
| Global cognition 37-Mini Mental State Examination |
48.1 [50.8] ± 10.5 | 48.4 [50.4] ± 9.93 | P = 0.922 | 15 (32.6) vs. 44 (24.6) | 2 (4.3) vs. 8 (4.5) |
| Memory Six Object test delayed recall |
46.3 [47.5] ± 10.9 | 48.7 [51.4] ± 11.3 | P = 0.115 | 12 (26.1) vs. 36 (20.0) | 5 (10.9) vs. 18 (10.0) |
| Memory Story delayed recall |
47.3 [49.4] ± 10.8 | 49.3 [53.0] ± 10.8 | P = 0.279 | 15 (32.6) vs. 43 (23.9) | 2 (4.3) vs. 13 (7.2) |
| Verbal fluency Number animals in 1’ |
48.6 [46.5] ± 12.8 | 51.4 [51.4] ± 11.0 | P = 0.050 | 12 (26.1) vs. 25 (13.9) | 1 (2.2) vs. 0 (0.0) |
| Verbal fluency Number fruits in 1’ |
47.8 [43.9] ± 10.3 | 49.3 [48.2] ± 10.8 | P = 0.209 | 8 (17.4) vs. 28 (15.6) | 1 (2.2) vs. 3 (1.7) |
| Cognitive Speed Processing1 Trail Making Test errors |
50.9 [54.1] ± 9.4 | 47.8 [53.8] ± 14.3 | P = 0.100 | 2 (5.7) vs. 17 (11.8) | 1 (2.2) vs. 10 (6.9) |
Quantitative data as mean [median] ± standard deviation
Mann-Whitney test
Trail Making Test part A: PD=35; ET= 145; A T-score of 40 or 30 represents, respectively, one or two standard deviations from the normal values of their age and schooling group.
When we excluded the illiterate and older (third tertile i.e. ≥ 75 years) patients (N= 78 ET and 13 PD), the difference in language performance of the two groups was significant (P = 0.02) with 7 PD (53.8%) vs. 15 ET (16.7%) scoring in the abnormal range. The other domains’ differences remained similar and did not reach significance.
3.5 Age of tremor onset and its influence on the cognitive performance of ET
When we stratified ET patients by age of tremor onset, 58 (32.2%) had their onset prior to age 65 years and 122 (67.8%) had an onset ≥ 65 years. The two groups differed in terms of their median current age (71.5 vs. 75.5 years respectively; p < 0.05). Their neuropsychological performance was similar in all tests, with only the one exception of the animal naming task (p < 0.05). When we computed the adjusted T-scores by age and educational attainment, this difference was no longer significant (p = 0.10).
4 DISCUSSION
In this study, we characterized the cognitive performance of a population-based sample of 46 PD patients, 180 ET patients and 2,212 healthy controls. Our goal was to test four a priori hypotheses about the cognitive profile of PD and ET patients relative to that of controls and relative to one another.
Our first a priori hypothesis was that both ET and PD would have poorer cognitive performance relative to that of controls, even in the absence of a clinical diagnosis of dementia. Our results confirm this hypothesis by demonstrating the existence of significant differences in an important percentage of patients with each of these movement disorders when they were compared with healthy individuals. These results support those previously reported by Lombardi et al. in a small sample of 18 ET and 18 PD patients, although that study did not enroll healthy controls; rather, data from enrolled ET and PD patients were compared to published normative data 6.
Our second a priori hypothesis was that a gradient of neuropsychological performance would exist between PD, ET and healthy controls, with the former being the most affected. This continuum was demonstrated by the raw and stratified trend analyses we performed in language and memory domains. Gasparini et al., in a clinic-based study, previously suggested the likely presence of such a continuum of impairment. They studied 15 PD patients, 15 ET patients with familial history of ET, 15 ET patients with a familial history of PD, and 15 controls. Their research highlighted an altered performance in attentional tasks for both ET and PD groups, but with the PD group showing broader deficits 13. A more recent study of surgical patients by Benge et al 14, which validated an executive scale in a sample of deep brain stimulation candidates, included 15 PD patients and 11 ET patients. The PD patients performed more poorly that the ET patients in this scale 14. Most prior studies enrolled and assessed PD or ET patients, but not both. None of these studies were population-based or specifically ruled out AD and other dementia subtypes.
The third and fourth hypotheses suggested an overlap of the affected cognitive domains in ET and PD, yet not a complete overlap. In the study of Lombardi et al., the ET group performed more poorly in verbal fluency tests, whereas the PD patients also had poorer performance in visuospatial, memory and attentional tasks 6. Moreover, Gasparini et al reported an altered performance in some attentional tasks for both ET and PD, but with the PD patients performing less well in some verbal fluency and executive tasks 13. In our cohort, in the trend analyses, the PD participants performed less well than the ET patients, and both groups performed less well than the controls, in tests measuring global cognition, memory and verbal fluency. These results confirm the existence of an overlap of affected domains 36. Nonetheless, when the intensity and the percentage of subjects with an impaired function in a specific domain was compared, the two groups showed differences. In this head-to-head comparison, ET patients consistently scored less well than the PD patients in cognitive-processing speed, although this result was marginally significant in the trend analyses. This fact could be related to greater cerebellar involvement of the ET cases 37,38 as, in our opinion, tremor itself could not justify an increased number of errors 38. Nonetheless, this last point will have to be confirmed in future studies, as it is fairly hypothetical. On the other hand, our results differ from those of Lombardi et al 6 as the PD, and not the ET, had a significantly poorer performance in verbal fluency. This is in agreement with what Gasparini et al. 13 and larger epidemiological studies 39. Overall, the differences noted for some of the tests were modest when compared with previous studies 39. The largest difference was noted for the six objects test in the PD patients and ET patients.
These NEDICES study results highlight the current view that PD and ET patients have non-motor cognitive features even when there are no signs of dementia 7,12. The PD sample mainly included mild cases (63% cases had a Hoehn-Yahr stage ≤ 2) and hence a cortical neuropathological involvement, according to Braak staging, seemed unlikely. Considering the five-year mean disease duration, the chance of misdiagnosing PD with Dementia with Lewy Bodies also seems improbable 40.
In the current sample of ET and PD patients, we observed certain domains to be affected. It is worthwhile noting that there may be some overlap with what may be found in other neurodegenerative conditions. For example, during the preclinical stage of Alzheimer’s disease, selective impairment of memory function may be observed 41. In preclinical stages of frontotemporal dementia, executive and language deficits have been reported 42.
Regarding the clinical consequences of our findings, various reports have evidenced that subtle cognitive deficits, even in the absence of dementia, can impact the quality of life, predict “loss of independence” and impair activities of daily living in these frequent movement disorders 43–46. Nonetheless, the clinical and prognostic implications of these deficits will have to be studied further.
Recently, it has been proposed that late onset of tremor (i.e. ≥ 65 years) could represent a different clinical entity than earlier onset ET 35. With this in mind, we performed a sub-analysis, stratifying ET patients by age of onset. The neuropsychological performance of the two groups was similar in all tests. Based on our data, there does not seem to be a distinct cognitive phenotype in participants with younger tremor onset (i.e. < 65 years) compared with later tremor onset (i.e. ≥ 65 years); however, additional studies are needed before firm conclusions may be reached.
Our study has several limitations. First, we had to exclude a large proportion of subjects because of loss to follow-up, missing neuropsychological data, and other attrition issues. The excluded subjects were slightly older and had a slightly higher percentage of illiterates than those who were included. The potential bias introduced would most likely have been towards a poorer performance among those who were excluded. In addition, the NEDICES study only included participants aged 65 years or older. Therefore, these analyses will have to be replicated in younger subjects. Second, the number of tests we could include was limited because the evaluation was part of a larger epidemiological study. It would have been interesting to characterize additional domains (e.g. visuospatial function). Another important limitation of our study is the lack of additional time-point evaluations, from which the evolution of these cognitive phenotypes could have been addressed. Certainly, future efforts should include follow-up visits to confirm and expand our results. Additionally, our evaluation of depression was limited and we may have under-ascertained depression, resulting in residual confounding. Nevertheless, a validation study showed a high level of agreement between the data generated from the screening question we used and a more detailed in-person psychiatric assessment, suggesting that such residual confounding is likely to have been low 47. Further, we could not completely rule out confounding effects of other covariates. Because of the non-normality of the neuropsychological data, our approach to minimize the effect of these confounders in the tests’ scores was to perform stratified analyses, evaluate T-scores adjusted by age and education and also reproduce some of the analyses in younger subjects with a higher educational category.
We believe that our conclusions are important for the definition and characterization of the cognitive aspects of ET and PD. We have confirmed that PD and ET impair cognitive performance, even in the absence of dementia, and that a continuum of severity exists between the two conditions, with PD showing a greater impact than ET. Also, there is some overlap in the type of impairment that they produce, but with disease-specific features, such as the predominant effect in verbal fluency of PD and in cognitive processing speed of ET. The last aspect should be further investigated to elucidate the mechanisms and structural correlates producing these diverse early cognitive effects. Besides their pathophysiological implications, these data could have added value in the differential diagnosis, prognosis and management of these frequent movement disorders.
Supplementary Material
For each specific test, the diamonds inside the boxplots represent the mean and the horizontal black bar the median.
Acknowledgments
Funding
The Spanish Health Research Agency and the Spanish Office of Science and Technology supported NEDICES. Dr. Alvaro Sanchez-Ferro has received funding from Consejería de Educación, Juventud y Deporte of Comunidad de Madrid and the People Programme (Marie Curie Actions) of the European Union’s Seventh Framework Programme (FP7/2007-2013) under REA grant agreement n° 291820. Dr. Sánchez-Ferro has also been supported by the Madrid-MIT M+Vision Consortium (Comunidad de Madrid).. Dr. Benito-León is supported by the National Institutes of Health, Bethesda, MD, USA (NINDS #R01 NS39422), the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR), and the Spanish Health Research Agency (grant FIS PI12/01602). Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator) and NINDS #R01 NS088257 (principal investigator). Dr. Bermejo-Pareja is supported by NINDS #R01 NS39422 from the National Institutes of Health, Bethesda, MD, USA and from the Commission of the European Union (grant ICT-2011-287739, NeuroTREMOR).
The authors would like to thank Dr. Carmen Gasca for her suggestions to improve this publication. The authors gratefully acknowledge the vital help of the other members of the NEDICES Study Group: additional information about collaborators of the NEDICES Study can be found on the web (http://www.ciberned.es/estudio-nedices).
Footnotes
Authors Contributions
ASF collaborated in the conception, organization and execution of the research project; the statistical analysis design, and the writing of the manuscript first draft and the review and critique of the manuscript. JBL collaborated in the conception, organization and execution of the research project; the statistical analysis design; and the review and critique of the manuscript. EDL collaborated in the conception, organization and execution of the research project; the statistical analysis design; and the review and critique of the manuscript. IC collaborated in the conception, organization and execution of the research project; the interpretation of the results; and the review and critique of the manuscript. JHG collaborated in the conception, of the research project; and the review and critique of the manuscript. VPM collaborated in the interpretation of the results and the review and critique of the manuscript. FBP collaborated in the conception, organization and execution of the research project; the statistical analysis design, and; and the review and critique of the manuscript.
Potential conflicts of Interests
ASF. JBL, EDL, IC, JHG, VPM, and FBP report no disclosures.
References
- 1.Bermejo-Pareja F, Louis ED, Benito-León J. Risk of incident dementia in essential tremor: a population-based study. Mov Disord. 2007;22(11):1573–1580. doi: 10.1002/mds.21553. [DOI] [PubMed] [Google Scholar]
- 2.Benito-León J, Louis ED, Posada IJ, et al. Population-based case-control study of cognitive function in early Parkinson’s disease (NEDICES) J Neurol Sci. 2011;310(1–2):176–182. doi: 10.1016/j.jns.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Walterfang M, van de Warrenburg BP. Cognitive impairment in “Other” movement disorders: hidden defects and valuable clues. Mov Disord. 2014;29(5):694–703. doi: 10.1002/mds.25849. [DOI] [PubMed] [Google Scholar]
- 4.Owen AM, James M, Leigh PN, et al. Fronto-striatal cognitive deficits at different stages of Parkinson’s disease. Brain. 1992;115(Pt 6):1727–1751. doi: 10.1093/brain/115.6.1727. [DOI] [PubMed] [Google Scholar]
- 5.Benito-León J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66(1):69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- 6.Lombardi WJ, Woolston DJ, Roberts JW, Gross RE. Cognitive deficits in patients with essential tremor. Neurology. 2001;57(5):785–790. doi: 10.1212/wnl.57.5.785. [DOI] [PubMed] [Google Scholar]
- 7.Benito-León J, Louis ED, Sánchez-Ferro Á, Bermejo-Pareja F. Rate of cognitive decline during the premotor phase of essential tremor: a prospective study. Neurology. 2013;81(1):60–66. doi: 10.1212/WNL.0b013e318297ef2b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Braak H, Rüb U, Jansen Steur ENH, Del Tredici K, de Vos RAI. Cognitive status correlates with neuropathologic stage in Parkinson disease. Neurology. 2005;64(8):1404–1410. doi: 10.1212/01.WNL.0000158422.41380.82. [DOI] [PubMed] [Google Scholar]
- 9.Pan JJ, Lee M, Honig LS, Vonsattel J-PG, Faust PL, Louis ED. Alzheimer’s-related changes in non-demented essential tremor patients vs. controls: links between tau and tremor? Parkinsonism Relat Disord. 2014;20(6):655–658. doi: 10.1016/j.parkreldis.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaudhuri KR, Healy DG, Schapira AHV. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5(3):235–245. doi: 10.1016/S1474-4422(06)70373-8. [DOI] [PubMed] [Google Scholar]
- 11.Benito-León J, Louis ED. Essential tremor: emerging views of a common disorder. Nat Clin Pract Neurol. 2006;2(12):666–78. doi: 10.1038/ncpneuro0347. quiz 2p following 691. [DOI] [PubMed] [Google Scholar]
- 12.Sánchez-Ferro Á, Benito-León J, Louis ED, et al. Rate of cognitive decline in premotor Parkinson’s disease: a prospective study (NEDICES) Mov Disord. 2013;28(2):161–168. doi: 10.1002/mds.25148. [DOI] [PubMed] [Google Scholar]
- 13.Gasparini M, Bonifati V, Fabrizio E, et al. Frontal lobe dysfunction in essential tremor: a preliminary study. J Neurol. 2001;248:399–402. doi: 10.1007/s004150170181. [DOI] [PubMed] [Google Scholar]
- 14.Benge J, Phillips-Sabol J, Phenis R. The Neuropsychological Assessment Battery Categories Test as a Measure of Executive Dysfunction in Patients With Parkinson’s Disease and Essential Tremor: An Exploratory Study. Clin Neuropsychol. 2014;28(October):1008–1018. doi: 10.1080/13854046.2014.950985. [DOI] [PubMed] [Google Scholar]
- 15.Bermejo-Pareja F, Benito-León J, Vega-Q S, et al. The NEDICES cohort of the elderly. Methodology and main neurological findings. Rev Neurol. 2008;46(7):416–423. [PubMed] [Google Scholar]
- 16.Morales JM, Bermejo FP, Benito-León J, et al. Methods and demographic findings of the baseline survey of the NEDICES cohort: a door-to-door survey of neurological disorders in three communities from Central Spain. Public Health. 2004;118(6):426–433. doi: 10.1016/j.puhe.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 17.Vega S, Benito-León J, Bermejo-Pareja F, et al. Several factors influenced attrition in a population-based elderly cohort: neurological disorders in Central Spain Study. J Clin Epidemiol. 2010;63(2):215–222. doi: 10.1016/j.jclinepi.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Benito-León J, Louis ED, Bermejo-Pareja F. Elderly-onset essential tremor is associated with dementia. Neurology. 2006;66:1500–1505. doi: 10.1212/01.wnl.0000216134.88617.de. [DOI] [PubMed] [Google Scholar]
- 19.Benito-León J, Bermejo-Pareja F, Morales-González JM, et al. Incidence of Parkinson disease and parkinsonism in three elderly populations of central Spain. Neurology. 2004;62(5):734–741. doi: 10.1212/01.wnl.0000113727.73153.68. [DOI] [PubMed] [Google Scholar]
- 20.Fahn S. Members of the UPDRS Development Committee. In: Fahn S, Marsden C, Goldstein M, Calne D, editors. Recent Developments in Parkinson’s Disease. Florham Park, NJ: Macmillan Health Care Information; 1987. pp. 153–163. [Google Scholar]
- 21.Hoehn MM, Yahr MD. Parkinsonism: onset, progression and mortality. Neurology. 1967;17(5):427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- 22.Benito-león J, Bermejo-pareja F, Louis ED. Incidence of essential tremor in three elderly populations of central Spain. 2005:1721–1726. doi: 10.1212/01.WNL.0000161852.70374.01. [DOI] [PubMed] [Google Scholar]
- 23.Louis ED, Ottman R, Ford B, et al. The Washington Heights-Inwood Genetic Study of Essential Tremor: methodologic issues in essential-tremor research. Neuroepidemiology. 1997;16(3):124–133. doi: 10.1159/000109681. [DOI] [PubMed] [Google Scholar]
- 24.Peña-Casanova J, Guardia J, Bertran-Serra I, Manero RM, Jarne A. Shortened version of the Barcelona test (I): subtests and normal profiles. Neurologia. 1997;12(3):99–111. [PubMed] [Google Scholar]
- 25.Bermejo F, Alom J, Peña-Casanova J, et al. Multicenter register of index cases of dementia. A study by the Spanish Neurological Society’s dementia group. Neurologia. 1994;9(9):401–406. [PubMed] [Google Scholar]
- 26.Amaducci L, Baldereschi M, Amato MP, et al. The World Health Organization cross-national research program on age-associated dementias. Aging (Milano) 1991;3(1):89–96. doi: 10.1007/BF03323983. [DOI] [PubMed] [Google Scholar]
- 27.Baldereschi M, Amato MP, Nencini P, et al. Cross-national interrater agreement on the clinical diagnostic criteria for dementia. Neurology. 1994;44(2):239–239. doi: 10.1212/WNL.44.2.239. [DOI] [PubMed] [Google Scholar]
- 28.Tombaugh T. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol. 2004;19(2):203–214. doi: 10.1016/S0887-6177(03)00039-8. [DOI] [PubMed] [Google Scholar]
- 29.Pena-Casanova J, Quinones-Ubeda S, Gramunt-Fombuena N, et al. Spanish Multicenter Normative Studies (NEURONORMA Project): Norms for Verbal Fluency Tests. Arch Clin Neuropsychol. 2009;24(4):395–411. doi: 10.1093/arclin/acp042. [DOI] [PubMed] [Google Scholar]
- 30.De Yébenes MJG, Otero A, Zunzunegui MV, Rodríguez-Laso A, Sánchez-Sánchez F, Del Ser T. Validation of a short cognitive tool for the screening of dementia in elderly people with low educational level. Int J Geriatr Psychiatry. 2003;18(10):925–936. doi: 10.1002/gps.947. [DOI] [PubMed] [Google Scholar]
- 31.Wechsler D. Wechsler Memory Scale-Revised. San Antonio, TX: The Psychological Corporation; 1987. [Google Scholar]
- 32.Contador I, Bermejo-pareja F, Del Ser T, Benito-león J. Effects of education and word reading on cognitive scores in a community-based sample of Spanish elders with diverse socioeconomic status Effects of education and word reading on cognitive scores in a community-based sample of Spanish elders with diverse. J Clin Exp Neuropsychol. 2015;37(1):92–101. doi: 10.1080/13803395.2014.989819. [DOI] [PubMed] [Google Scholar]
- 33.R Core Team. R: A Language and Environment for Statistical Computing. 2014. [Google Scholar]
- 34.Woods SP, Fields JA, Lyons KE, Pahwa R, Higginson CI. Neuropsychological de ® cits in essential tremor: an expression of cerebello-thalamo-cortical pathophysiology? 2002:143–151. doi: 10.1046/j.1468-1331.2002.00341.x. [DOI] [PubMed] [Google Scholar]
- 35.Deuschl G, Petersen I, Lorenz D, Christensen K. Tremor in the Elderly: Essential and Aging-Related Tremor. Mov Disord. 2015;30(10):1327–1335. doi: 10.1002/mds.26265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benito-León J, Louis ED, Bermejo-Pareja F. Risk of incident Parkinson’s disease and parkinsonism in essential tremor: a population based study. J Neurol Neurosurg Psychiatry. 2009;80(4):423–425. doi: 10.1136/jnnp.2008.147223. [DOI] [PubMed] [Google Scholar]
- 37.Gottwald B, Wilde B, Mihajlovic Z, Mehdorn HM. Evidence for distinct cognitive deficits after focal cerebellar lesions. J Neurol Neurosurg Psychiatry. 2004;75:1524–1531. doi: 10.1136/jnnp.2003.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schwartz M, Badarny S, Gofman S, Hocherman S. Visuomotor performance in patients with essential tremor. Mov Disord. 1999;14(6):988–993. doi: 10.1002/1531-8257(199911)14:6<988::aid-mds1012>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 39.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132(11):2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 40.McKeith IG, Dickson DW, Lowe J, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–1872. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- 41.Dubois B, Feldman HH, Jacova C, et al. Advancing research diagnostic criteria for Alzheimer ‘ s disease: the IWG-2 criteria. 2014;13(June) doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- 42.Stokholm J, Teasdale TW, Johannsen P, et al. Cognitive impairment in the preclinical stage of dementia in FTD-3 CHMP2B mutation carriers: a longitudinal prospective study. J Neurol Neurosurg Psychiatry. 2013;84:170–176. doi: 10.1136/jnnp-2012-303813. [DOI] [PubMed] [Google Scholar]
- 43.Louis ED, Benito-León J, Vega-Quiroga S, Bermejo-Pareja F. Cognitive and motor functional activity in non-demented community-dwelling essential tremor cases. J Neurol Neurosurg Psychiatry. 2010;81(9):997–1001. doi: 10.1136/jnnp.2009.202838. [DOI] [PubMed] [Google Scholar]
- 44.Lawson RA, Yarnall AJ, Duncan GW, et al. Quality of life and mild cognitive impairment in early Parkinson’s disease: does subtype matter? J Parkinsons Dis. 2014;4(3):331–336. doi: 10.3233/JPD-140390. [DOI] [PubMed] [Google Scholar]
- 45.Pirogovsky E, Schiehser DM, Obtera KM, et al. Instrumental activities of daily living are impaired in Parkinson’s disease patients with mild cognitive impairment. Neuropsychology. 2014;28(2):229–237. doi: 10.1037/neu0000045. [DOI] [PubMed] [Google Scholar]
- 46.Bjornestad A, Tysnes O-B, Larsen JP, Alves G. Loss of independence in early Parkinson disease. Neurology. 2016;87(15):1599–1606. doi: 10.1212/WNL.0000000000003213. [DOI] [PubMed] [Google Scholar]
- 47.Louis ED, Benito-León J, Bermejo-Pareja F. Self-reported depression and anti-depressant medication use in essential tremor: cross-sectional and prospective analyses in a population-based study. Eur J Neurol. 2007;14(10):1138–1146. doi: 10.1111/j.1468-1331.2007.01923.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For each specific test, the diamonds inside the boxplots represent the mean and the horizontal black bar the median.