Abstract
Background/Objectives
Endothelial dysfunction predicts mortality but it is unknown whether childhood obesity predicts adult endothelial dysfunction. The aim of this study was to determine whether anthropometric indices of body fat in childhood, adolescence and early midlife are associated with endothelial dysfunction in early midlife.
Subjects/Methods
Participants belonged to a representative birth cohort of 1037 individuals born in Dunedin, New Zealand in 1972 and 1973 and followed to age 38 years, with 95% retention (the Dunedin Multidisciplinary Health and Development Study). We assessed anthropometric indices of obesity at ages 3, 5, 7, 9, 11, 13, 15, 18, 21, 26, 32 and 38 years. We tested associations between endothelial function assessed by peripheral arterial tonometry at age 38 and; age-38 cardiovascular risk factors; age-3 body mass index (BMI); and four BMI trajectory groups from childhood to early midlife.
Results
Early midlife endothelial dysfunction was associated with BMI, large waist circumference, low high-density lipoprotein cholesterol, low cardiorespiratory fitness, and increased high-sensitivity C-reactive protein. After adjustment for sex and childhood socioeconomic status, 3-year-olds with BMI one standard deviation above the mean had Framingham-reactive hyperemia index ratios that were 0.10 below those with normal BMI (β = −0.10, 95% CI −0.17 to −0.03, p=0.007) at age 38. Cohort members in the ‘overweight’, ‘obese’, and ‘morbidly obese’ trajectories had Framingham-reactive hyperemia index ratios that were 0.08 (β = −0.08, 95% CI −0.14 to −0.03, p=0.003), 0.13 (β = −0.13, 95% CI −0.21 to −0.06, p<0.001), and 0.17 (β = −0.17, 95% CI −0.33 to −0.01, p=0.033), respectively, below age-peers in the ‘normal’ trajectory.
Conclusions
Childhood BMI and the trajectories of BMI from childhood to early midlife predict endothelial dysfunction evaluated by peripheral arterial tonometry in early midlife.
Introduction
The prevalence of obesity and overweight is increasing worldwide in children as well as adults.1 Childhood obesity is a potent risk factor for mortality with rates of death from endogenous causes among children in the highest quartile of body mass index (BMI) more than double those among children in the lowest BMI quartile.2 Obesity has been shown to be associated with coronary endothelial dysfunction,3 impaired brachial flow-mediated dilation in adults,4 and impaired reactive hyperaemia-peripheral arterial tonometry (PAT) in adolescents.5 Flow-mediated dilation measures macrovascular endothelial function and PAT measures digital microvascular function; both measures correlate with coronary artery endothelial function but likely measure different aspects of vascular biology.6 Endothelial dysfunction is associated with cardiovascular risk factors,7 an increased risk of cardiovascular events and all cause mortality8, 9 implicating it as a possible contributor to the increased mortality observed with obesity.10
Severe obesity in childhood has been shown to be associated with impaired flow-mediated dilation, a measure of endothelial function.11, 12 It is unknown whether childhood obesity is associated with continued endothelial dysfunction into adulthood. Determining if there is an association may provide further impetus for interventions to prevent overweight and obesity in childhood with the goal of improving long term cardiovascular health.
The aim of this study was to determine if anthropometric indices of body fat in childhood, adolescence and early adulthood were associated with endothelial dysfunction assessed by PAT in early midlife.
Subjects and Methods
Participants
Participants were members of the Dunedin Multidisciplinary Health and Development (‘Dunedin’) Study, a longitudinal investigation of the health and behavior of a complete birth cohort of 1037 consecutive births (52% male) between April 1 1972 and March 31 1973 in Dunedin, New Zealand.13 To be eligible for inclusion participants had to be living in the greater Dunedin Metropolitan area three years after their birth at Queen Mary Maternity Hospital – the only maternity hospital in Dunedin at the time. The 9% who declined or were unable to participate were no different from the 91% who agreed to take part in terms of maternal prenatal complications, birthweight, neonatal complications, or family socioeconomic status.14 Cohort families represented the full range of socioeconomic status in New Zealand in the early 1970’s, as compared to the New Zealand census. Cohort members are primarily white; 7.5% self-identify as being Maori which matches the ethnic distribution of the South Island. Research participation has not improved Study members’ mental or physical health as compared to same-aged participants in the New Zealand National Health and Nutrition Survey.15 Day-long assessments have been conducted at ages 3, 5 (n=991, 96%), 7 (n=954, 92%), 9 (n=955, 92%), 11 (n=925, 90%), 13 (n=850, 82%), 15 (n=976, 95%), 18 (n=933, 97%), 21 (n=922, 97%), 26 (n=980, 96%), 32 (n=972, 96%) and most recently at age 38 years (in 2010–2012) when 95% (n=961) of the living Study members took part. The Otago Ethics Committee approved each phase of the study. Study members gave informed consent before participating.
Childhood socioeconomic status (SES) was measured on a six-point scale assessing parents’ self-reported occupational status.16 The scale places each occupation into one of six categories (1=professional, 6=unskilled laborer) based upon the educational level and income associated with that occupation in data from the New Zealand census. The variable used in our analyses, childhood SES, was the average of the highest SES level of either parent, assessed repeatedly from the study members’ birth through age 15 years. This variable was used to capture early life SES experiences.
BMI was calculated in kilograms per square meter (weight (kg)/height (m)2).17 At each assessment age from age 3 to age 38, height was recorded to the nearest millimeter using a portable stadiometer (Harpenden; Holtain, Ltd). Weight was measured to the nearest 0.1 kg using calibrated scales. Participants were weighed in light clothing.
Cardiovascular risk indicators at age 38
Waist girth was measured by averaging two measurements taken using a steel tape calibrated in centimeters with millimeter gradations. Waist girth was taken as the perimeter at the level of the noticeable waist.
Blood pressure was measured in a quiet room, using a cuff of appropriate size, with the study member in a seated position, by medically trained assessors. A Hawksley random-zero sphygmomanometer (Hawksley and Sons Ltd., Sussex, United Kingdom) with a constant deflation valve was used. Systolic blood pressure was assessed as the first Korotkoff sound and based on the mean of either two or three measures taken at five minute intervals according to a standard protocol.
Cardiorespiratory fitness was assessed by having study participants complete a submaximal exercise test on a friction-braked cycle ergometer (Monark, Sweden). Depending on the extent that heart rate increased during a 2-min 50 Watt warm-up, the workload was adjusted to elicit a steady heart-rate in the range 130–170bpm. After a further 6-min constant power output stage the maximal heart rate was recorded and used to calculate predicted maximum oxygen consumption (VO2 max: l/min) according to standard protocols.18
Biomarkers were obtained at age 38 and venepuncture was conducted at the same time each day (4:15–4:45 pm). Ninety five percent of the sample consented to phlebotomy. Pregnant women at this age were excluded from the analyses.
Nonfasting total cholesterol (mmol/L) and high density lipoprotein (HDL) cholesterol level (mmol/L) were measured using a colorimetric assay. Total cholesterol was determined enzymatically by cholesterol oxidase and cholesterol esterase. The between day coefficient of variation ranged from 2.3 to 3.5 percent. HDL cholesterol level was also determined enzymatically by cholesterol oxidase and cholesterol esterase coupled with polyethylene glycol (PEG) to the amino groups.
High-sensitivity C-reactive protein (hsCRP) was measured using a high-sensitivity particle enhanced immunoturbidimetric assay (coefficient of variation 2.8%, Roche Diagnostics, Germany).
Glycated hemoglobin levels (HbA1c) were measured in the serum and glycated hemoglobin concentration (expressed as a percentage of total hemoglobin) was measured by ion exchange high performance liquid chromatography (Variant II: BioRad, Hercultes, Calif.) (between day coefficient of variation, 1.6–2.1%), a method certified by the US National Glycohemoglobin Standardization Program (http://www.missourie.edu/~diabetes/ngsp.html).
Endothelial function assessment
Study members’ endothelial function was assessed in the morning in the non-fasting state after lying supine for 10 minutes in a temperature controlled room at 20°C. A PAT device was placed on the index finger of each hand (Endo-PAT2000, Itamar Medical Ltd, Caesarea, Israel). A blood pressure cuff was placed on the right upper arm and baseline measurements obtained over two minutes. The blood pressure cuff was then inflated to 200 mm Hg or 60 mm Hg above systolic blood pressure, whichever was higher and occlusion of flow was confirmed. After 5 minutes the cuff was deflated and recording continued for five minutes. Pulse amplitude was recorded from both fingers and analyzed by a computerized, automated algorithm (Itamar Medical) that provided the average pulse amplitude for each 30-second interval after forearm cuff deflation up to 5 minutes. PAT measured the digital pulse volume after reactive hyperemia induced by brachial artery occlusion and the ratio of hyperaemic to baseline pulse amplitudes was calculated as the Framingham-reactive hyperemia index (F-RHI). F-RHI was defined as F-RHI = ln(RHo/RHc), where RHo and RHc are the mean pulse amplitudes of the period between 90 and 120 seconds after cuff deflation divided by mean baseline amplitudes in the occluded arm and control arm, respectively.19 Lower values of the F-RHI are indicative of impaired endothelial function.
Statistical methods
Associations between endothelial function and age-38 cardiovascular risk factors were assessed using ordinary least squares regression analysis, with F-RHI score as the continuous outcome, and the age-38 cardiovascular risk factors as categorical predictors. The associations were controlled for sex. Standard cut-offs were used for all measures except cardio-respiratory fitness, for which sex-specific quartiles were used.20 Specifically, for BMI, “overweight” was defined as ≥25kg/m2 & <30kg/m2, “obese” was defined as ≥30kg/m2; for waist circumference, “large” was defined as >102cm for men and >88cm for women; for systolic blood pressure, “hypertensive” was defined as ≥140mmHg; for HDL cholesterol, “low HDL” was defined as <1.04mmol/L for men and <1.30mmol/L for women; for total cholesterol, “high” was defined as >6.22mmol/L; for glycated haemoglobin, “high” was defined as >5.7%; and for hsCRP, “high” was defined as > 3mg/L.21
Group-based trajectory modelling was used to identify distinctive groups of individual BMI trajectories in the population from ages 3 to 38 years. All sample members with at least 6 of the 12 BMI assessments were included in the trajectory modelling (n=982). We used Proc Traj, a Statistical Analysis Software macro to estimate the model parameters22 using a censored normal model appropriate for continuous normally distributed data.23 A number of criteria23, 24 were used to determine the number of BMI trajectory groups and the trajectory shapes (e.g. cubic in age) including (i) an a priori knowledge of BMI change over time, (ii) tendency toward a parsimonious model, (iii) the Bayesian Information Criterion as a model fit statistic with larger values indicating better fit and, (iv) each group having an average posterior probability of group membership exceeding 0.7. Despite the ability of trajectory modelling to handle missing data, we took a conservative approach by including participants’ data if they had body mass index information at six or more ages (n=982). To determine the best model, 14 models were fitted.23
Associations between age 38 endothelial function and (i) body mass index in childhood (age 3); and (ii) body mass index life-course trajectories (across ages 3–38) were also assessed using ordinary least squares regression analysis, with F-RHI score as the continuous outcome, and each of the measures as categorical predictors. Associations were controlled for (model i) sex and, (model ii) sex and family SES in childhood (averaged from birth to age 15). Analyses were conducted using SPSS version 22 (IBM Corporation, 2013).
Results
Sex comparisons
Endothelial function as assessed by F-RHI was better in women compared to men at the age of 38 years (0.71 ± 0.38 vs 0.50 ± 0.35, p < 0.001, Table 1). Women had lower waist circumference, systolic blood pressure, cardiorespiratory fitness, total cholesterol, glycated haemoglobin, and higher HDL cholesterol and hsCRP at 38 years of age (all p < 0.001). BMI was similar in men and women at age 38 years but lower in girls at 5 years of age (p = 0.013).
Table 1.
Descriptive statistics for cardiovascular outcomes at age 38 and childhood covariates.
| Full sample | Female | Male | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | N | Min | Max | Mean | SD | p | |
| Age 38 measures | ||||||||||||||||
| Framingham Reactive Hyperemia Index | 918 | −0.15 | 1.92 | 0.61 | 0.38 | 458 | −0.14 | 1.92 | 0.71 | 0.38 | 460 | −0.15 | 1.71 | 0.50 | 0.35 | <0.001 |
| Body Mass Index, kg/m2 | 934 | 16.0 | 54.4 | 27.2 | 5.3 | 460 | 16.0 | 54.4 | 26.9 | 5.9 | 474 | 18.9 | 49.5 | 27.5 | 4.6 | 0.127 |
| Waist circumference (cm) | 935 | 62.1 | 136.0 | 86.4 | 12.6 | 461 | 62.1 | 136.0 | 81.5 | 12.3 | 474 | 67.3 | 135.5 | 91.2 | 11.1 | <0.001 |
| Systolic Blood Pressure, mm Hg | 934 | 86.7 | 180.7 | 120.3 | 12.1 | 460 | 86.7 | 161.3 | 116.4 | 11.2 | 474 | 96.7 | 180.7 | 124.0 | 11.8 | <0.001 |
| HDL cholesterol, mmol/L | 911 | 0.40 | 3.54 | 1.45 | 0.43 | 455 | 0.59 | 3.54 | 1.61 | 0.43 | 456 | 0.40 | 3.13 | 1.29 | 0.35 | <0.001 |
| Total Cholesterol, mmol/L | 911 | 2.40 | 11.30 | 5.21 | 1.02 | 455 | 2.40 | 11.30 | 5.00 | 0.97 | 456 | 2.70 | 8.90 | 5.42 | 1.03 | <0.001 |
| Cardiorespiratory fitness, VO2max ml/kg/min | 900 | 11.6 | 53.4 | 29.4 | 7.9 | 437 | 11.5 | 46.9 | 23.7 | 4.9 | 463 | 18.1 | 53.4 | 34.7 | 6.2 | <0.001 |
| Glycated Haemoglobin, % | 901 | 4.16 | 13.50 | 5.41 | 0.54 | 451 | 4.16 | 9.47 | 5.34 | 0.42 | 450 | 4.26 | 13.50 | 5.48 | 0.63 | <0.001 |
| High sensitivity C-reactive protein log mg/L | 907 | 0.10 | 3.69 | 0.96 | 0.65 | 453 | 0.10 | 3.53 | 1.05 | 0.72 | 454 | 0.10 | 3.69 | 0.87 | 0.55 | <0.001 |
| Childhood measures | ||||||||||||||||
| Body Mass Index, age 5, kg/m2 | 893 | 12.0 | 24.3 | 15.9 | 1.2 | 436 | 12.0 | 21.1 | 15.8 | 1.2 | 457 | 12.8 | 24.4 | 16.0 | 1.2 | 0.013 |
| Socioeconomic Status, average across ages 1 thru 15, 1 = low, 6 = high | 1031 | 1 | 6 | 3.75 | 1.14 | 499 | 1 | 6 | 3.76 | 1.14 | 532 | 1 | 6 | 3.75 | 1.14 | 0.879 |
Abbreviation: SD, standard deviation. P-values for sex differences are shown; significant associations (p<0.05) are in bold.
Endothelial function and cardiovascular risk factors at age 38yrs
After controlling for sex, the following age-38 risk factors were associated with significantly lower F-RHI. Compared to those without the risk factor (Table 2): (i) those overweight had F-RHI ratios 0.09 lower (p=0.002), while those obese had F-RHI ratios 0.19 lower (p<0.001); (ii) those with large waist circumference had F-RHI ratios 0.13 lower (p<0.001); (iii) those with low HDL cholesterol had F-RHI ratios 0.11 lower (p<0.001); and (iv) those with high hsCRP also had F-RHI ratios 0.11 lower (p<0.001). Additionally, cardio-respiratory fitness had a dose-response association with F-RHI, with worsening levels of fitness associated with lower F-RHI ratios. Compared to those in the most fit quartile, those in the second-least fit quartile had F-RHI ratios that were 0.11 lower (p=0.001) and those in the least fit quartile had F-RHI ratios that were 0.20 lower (p<0.001).
Table 2.
Associations between cardiovascular measures and endothelial function at age 38.
| FRHI ratio, Mean (se) | β (95% CI) | p | |
|---|---|---|---|
| Body mass index (n=908) | |||
| Normal (n=353, 38.9%) | 0.68 (0.02) | – | |
| Overweight (n=339, 37.3%) | 0.60 (0.02) | −0.09 (−0.14 – −0.03) | 0.002 |
| Obese (n=216, 23.8%) | 0.49 (0.02) | −0.19 (−0.25 – −0.13) | <0.001 |
| Waist circumference (n=908) | |||
| Normal (n=732, 80.6%) | 0.63 (0.01) | – | |
| Large (men: >102cm; women: > 88cm; n=176, 19.4%) | 0.50 (0.03) | −0.13 (−0.19 – −0.07) | <0.001 |
| Systolic blood pressure (n=908) | |||
| Normal (n=853, 93.9%) | 0.60 (0.01) | – | |
| Hypertensive (n=55, 6.1%) | 0.66 (0.05) | 0.05 (−0.05 – 0.15) | 0.288 |
| HDL cholesterol (n=894) | |||
| Normal (n=663, 74.2%) | 0.64 (0.01) | – | |
| Low (men: <1.04mmol/L; women: <1.30mmol/L; n=231, 25.8%) | 0.52 (0.02) | −0.11 (−0.17 – −0.06) | <0.001 |
| Total cholesterol (n=894) | |||
| Normal (n=774, 86.6%) | 0.61 (0.01) | – | |
| High (>6.22mmol/L; n=120, 13.4%) | 0.58 (0.03) | −0.03 (−0.10 – 0.04) | 0.388 |
| Cardiorespiratory fitness (n=889) | |||
| Quartile 1: Fit (n=225, 25.3%) | 0.68 (0.02) | – | |
| Quartile 2 (n=222, 25.0%) | 0.65 (0.02) | −0.03 (−0.10 – 0.04) | 0.356 |
| Quartile 3 (n=223, 25.1%) | 0.57 (0.02) | −0.11 (−0.18 – −0.05) | 0.001 |
| Quartile 4: Unfit (n=219, 24.6%) | 0.50 (0.02) | −0.18 (−0.25 – −0.12) | <0.001 |
| Glycated haemoglobin (n=884) | |||
| Normal (n=724, 81.9%) | 0.62 (0.01) | – | |
| High (>5.7%; n=160, 18.1%) | 0.56 (0.03) | −0.06 (−0.12 – 0.00) | 0.057 |
| High sensitivity C-reactive protein (n=894) | |||
| Normal (n= 708, 79.2%) | 0.63 (0.01) | – | |
| High (>3mg/L; n= 186, 20.8%) | 0.52 (0.03) | −0.11 (−0.17 – −0.05) | <0.001 |
Abbreviations: CI, Confidence Interval; FHRI, Framingham Reactive Hyperemia Index; HDL, High Density Lipoprotein; se, standard error. Panels show sex-adjusted FRHI scores, and sex-adjusted beta coefficients (95% CI) for each cardiovascular measure. Significant associations (p<0.05) are in bold.
Trajectory modelling of body mass index
A four-trajectory group model with polynomial shapes (the two lowest BMI groups were quantic in shape, the third and fourth BMI groups were quartic in shape) was identified. Figure 1 shows the plotted predicted trajectory lines for each of the four groups designated as “normal”, “overweight”, “obese” and “morbidly obese”, with 95% confidence intervals based on predicted trajectory means. By age 38 years, the “normal” group (41.6% of the sample population) had a mean BMI in the normal range (20–<25 kg/m2), the “overweight” group (40.4%) had a mean BMI in the overweight range (≥25–<30 kg/m2), the “obese” group (15.4%) in the obese range (≥30–<40 kg/m2) and the “morbidly obese” (2.7%) in the “morbidly obese” range (>40 kg/m2).
Figure 1. Body mass index trajectories ages 3–38 (n = 982).
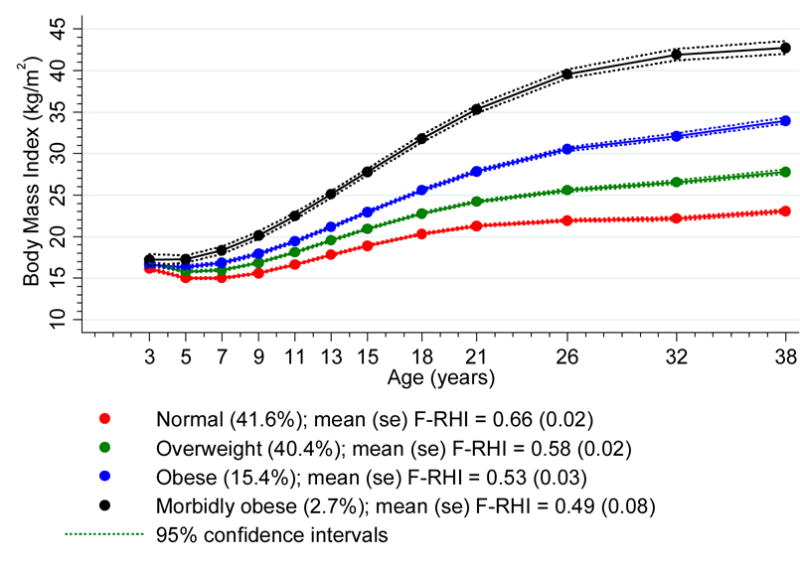
F-RHI, Framingham reactive hyperemia index. se, standard error
Multivariate analyses of life-course influences on endothelial function
BMI in childhood at age 3 predicted endothelial function assessed by F-RHI at age 38 years (upper panel, Table 3). Children whose BMI was greater than one standard deviation above the mean had F-RHI ratios in adulthood that were 0.10 lower than children with normal BMI, after controlling for both sex and childhood SES (β = −0.10, 95% CI −0.17 to −0.03, p=0.007). This effect was found to be partly mediated by age 38 cardiovascular risk factors: the BMI effect reduced to 0.08 lower, after controlling for the risk factors listed in Table 2 (β = −0.08, 95% CI −0.15 to −0.01, p=0.032).
Table 3.
Associations between childhood body mass index at age 3; and body mass index trajectories across ages 3 to 38; and endothelial function at age 38.
| A. Controlled for sex | B. Controlled for sex and childhood socio-economic status | |||||
|---|---|---|---|---|---|---|
| FRHI ratios, Mean (se) | β (95% CI) | p | FRHI ratios, Mean (se) | β (95% CI) | p | |
| BMI, age 3 (n=822) | ||||||
| Normal (n=697, 84.8%) | 0.62 (0.01) | – | 0.62 (0.01) | – | ||
| >1 standard deviation above mean (n=125, 15.2%) | 0.52 (0.03) | −0.10 (−0.17 – −0.03) | 0.005 | 0.52 (0.03) | −0.10 (−0.17 – −0.03) | 0.007 |
| BMI trajectories, age 3–38 (n=905)* | ||||||
| Normal (n=375, 41.4%)* | 0.66 (0.02) | – | 0.66 (0.02) | – | ||
| Overweight (n=374, 41.3%)* | 0.58 (0.02) | −0.08 (−0.14 – −0.04) | 0.002 | 0.58 (0.02) | −0.08 (−0.13 – −0.03) | 0.003 |
| Obese (n=135, 14.9%)* | 0.53 (0.03) | −0.13 (−0.20 – −0.06) | <0.001 | 0.53 (0.03) | −0.13 (−0.21 – −0.06) | <0.001 |
| Morbidly obese (n=21, 2.3%)* | 0.49 (0.08) | −0.17 (−0.33 – −0.01) | 0.032 | 0.49 (0.08) | −0.17 (−0.34 – −0.01) | 0.033 |
Abbreviations: BMI, body mass index; CI, Confidence Interval; FHRI, Framingham Reactive Hyperemia Index; se, standard error. Associations in Panel A are controlled for sex. Associations in Panel B are controlled for sex and childhood socio-economic status. Significant associations (p<0.05) are in bold.
Numbers in trajectory groups among those with endothelial function data differ slightly from those in the full sample reported in Figure 1.
BMI trajectory groups were associated in a linear fashion with endothelial function as assessed by F-RHI at age 38 years (lower panel, Table 3). After controlling for sex and childhood SES, those in the ‘overweight’, ‘obese’, and ‘morbidly obese’ trajectories had F-RHI ratios that were 0.08 (p=0.003), 0.13 (p<0.001), and 0.17 (p=0.033) lower than those in the ‘normal’ trajectory, respectively. This effect was found to be completely mediated by age 38 cardiovascular risk factors: after controlling for the risk factors listed in Table 2, those in the ‘overweight’, ‘obese’, and ‘morbidly obese’ trajectories had F-RHI ratios that were no different from those in the normal trajectory (differences = 0.01 [p=0.828], 0.07 [p=0.211], and 0.05 [p=0.606], respectively).
Discussion
This study shows that body mass index in childhood is associated with endothelial dysfunction in early midlife. In addition, we observed that body mass index trajectories from childhood to early midlife were associated with endothelial dysfunction in early midlife. This is an important finding as endothelial dysfunction is independently associated with an increased risk of cardiovascular events and all-cause mortality.9 Childhood obesity sustained into adulthood may contribute to an increased risk of cardiovascular events by the mechanism of chronic endothelial dysfunction.
Publications to date have reported a relationship between endothelial function and obesity in childhood. An initial study reported that impaired flow-mediated dilation in severely obese children was correlated with low apolipoprotein A1 and indices of insulin resistance.11 A further study in obese children showed those with impaired endothelial function had higher BMI, body fat content, waist hip ratio, and proportion with hypertension.12 Other investigators have also reported overweight associated with impaired endothelial function in children; BMI being inversely associated with flow mediated dilation.25, 26
Mahmud et al. reported an inverse relationship between and PAT ratio in obese adolescents with insulin resistance.5 More recently Pareyn et al. reported impaired endothelial function assessed by PAT in overweight and obese adolescents. These adolescents had lower median reactive hyperemia - PAT ratios than control subjects.27 However, to our knowledge there have been no reports on the influence of childhood or adolescent measures of obesity on adult endothelial function. We have extended the findings of previous studies by demonstrating that BMI at age 3 years and the trajectories of BMI between the ages of 3–38 years predict adult endothelial dysfunction as assessed by the F-RHI. Endothelial dysfunction is thought to be a precursor to the development and clinical expression of atherosclerosis.7 Endothelial dysfunction in obese children may therefore be a contributor to the early development of vascular disease28 with increased rates of clinical events occurring in adulthood.
Obesity, glucose intolerance, and hypertension in childhood are strongly associated with increased rates of premature death.2 Hamburg et al. utilising PAT to measure endothelial function have previously reported an association between F-RHI and a number of cardiovascular risk factors including male sex, ratio of total to high density lipoprotein cholesterol, diabetes mellitus, smoking and lipid lowering treatment.19 The present study extends these findings demonstrating in addition that elevated levels of hsCRP, an indicator of systemic inflammation and predictor of cardiovascular risk,29 is associated with impaired endothelial function assessed with PAT. Obesity and overweight have been suggested to be associated with a chronic low-grade inflammatory state30 which may contribute to endothelial dysfunction and an increased risk of cardiovascular events.
There is limited information regarding the relationship between endothelial function assessed using PAT and physical fitness in healthy subjects; a small study in older men demonstrated lifelong participation in football training was associated with better physical fitness and higher RHI.31 In the present larger cross-sectional analysis of a birth cohort at age 38 years we found a similar association between higher levels of cardiorespiratory fitness and higher levels of F-RHI. A recently published systematic review confirmed a moderate association between physical fitness and endothelial function determined by endothelium-dependent vasodilator stimuli in healthy subjects.32 Confirmation of our results in other cohorts is required before concluding that PAT derived endothelial function measures are consistently associated with physical fitness.
There are limitations to the present study. The assessment of endothelial function was performed in the non fasting state. This has the potential to cause variability in the PAT measurements between subjects influenced by blood glucose and lipid levels. However all measurements were performed in the morning avoiding diurnal variation. A previous study has shown no change in mean value of RHI when repeated in individuals in the fasting state and following a low calorie meal implying this test may be useful in epidemiological studies.33 We did not perform specific adiposity measures instead analysing BMI and waist circumference. However previous studies have shown strong correlations between body mass index and waist measures with specific indices of adiposity.34, 35 In addition there is good evidence that BMI is associated with all-cause mortality10 suggesting it is a reasonable indicator of adiposity when used in epidemiological studies.
In conclusion the results of this prospective-longitudinal study show that higher BMI in childhood and the trajectories of BMI from childhood to early midlife are associated with impaired endothelial dysfunction in early midlife suggesting an early influence of obesity on long-term cardiovascular risk. Chronic low-grade systemic inflammation associated with obesity may contribute to impaired endothelial dysfunction in adulthood. Further studies to assess the effects of interventions to reduce childhood obesity on endothelial function should be considered.
Acknowledgments
We thank study founder Phil Silva, PhD, the Dunedin Multidisciplinary Health and Development Study of New Zealand members, and the Dunedin Multidisciplinary Health and Development Research Unit research staff.
The Dunedin Multidisciplinary Health and Development Research Unit is supported by the Health Research Council of New Zealand and the New Zealand Ministry of Business, Innovation and Employment (MBIE). This research also received support from the United Kingdom Medical Research Council (Grant G0100527) and from the National Institute of Aging (Grants R01AG032282 and R01AG048895). Reremoana Theodore was supported by a Health Research Council Erihapeti Rehu-Murchie fellowship under grant number 13/579. The funding bodies had no role in the design of the study, collection and analysis of data and the decision to publish.
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.WHO consultation on obesity Obesity: preventing and managing the global epidemic. Geneva: World Health Organization; 2000. [PubMed] [Google Scholar]
- 2.Franks PW, Hanson RL, Knowler WC, Sievers ML, Bennett PH, Looker HC. Childhood obesity, other cardiovascular risk factors, and premature death. N Engl J Med. 2010;362:485–493. doi: 10.1056/NEJMoa0904130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Al Suwaidi J, Higano ST, Holmes DR, Jr, Lennon R, Lerman A. Obesity is independently associated with coronary endothelial dysfunction in patients with normal or mildly diseased coronary arteries. J Am Coll Cardiol. 2001;37:1523–1528. doi: 10.1016/s0735-1097(01)01212-8. [DOI] [PubMed] [Google Scholar]
- 4.Brook RD, Bard RL, Rubenfire M, Ridker PM, Rajagopalan S. Usefulness of visceral obesity (waist/hip ratio) in predicting vascular endothelial function in healthy overweight adults. Am J Cardiol. 2001;88:1264–1269. doi: 10.1016/s0002-9149(01)02088-4. [DOI] [PubMed] [Google Scholar]
- 5.Mahmud FH, Hill DJ, Cuerden MS, Clarson CL. Impaired vascular function in obese adolescents with insulin resistance. J Pediatr. 2009;155:678–682. doi: 10.1016/j.jpeds.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 6.Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, et al. The assessment of endothelial function: from research into clinical practice. Circulation. 2012;126:753–767. doi: 10.1161/CIRCULATIONAHA.112.093245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 8.Al Suwaidi J, Hamasaki S, Higano ST, Nishimura RA, Holmes DR, Jr, Lerman A. Long-term follow-up of patients with mild coronary artery disease and endothelial dysfunction. Circulation. 2000;101:948–954. doi: 10.1161/01.cir.101.9.948. [DOI] [PubMed] [Google Scholar]
- 9.Xu Y, Arora RC, Hiebert BM, Lerner B, Szwajcer A, McDonald K, et al. Non-invasive endothelial function testing and the risk of adverse outcomes: a systematic review and meta-analysis. Eur Heart J Cardiovasc Imaging. 2014;15:736–746. doi: 10.1093/ehjci/jet256. [DOI] [PubMed] [Google Scholar]
- 10.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;309:71–82. doi: 10.1001/jama.2012.113905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tounian P, Aggoun Y, Dubern B, Varille V, Guy-Grand B, Sidi D, et al. Presence of increased stiffness of the common carotid artery and endothelial dysfunction in severely obese children: a prospective study. Lancet. 2001;358:1400–1404. doi: 10.1016/S0140-6736(01)06525-4. [DOI] [PubMed] [Google Scholar]
- 12.Meyer AA, Kundt G, Steiner M, Schuff-Werner P, Kienast W. Impaired flow-mediated vasodilation, carotid artery intima-media thickening, and elevated endothelial plasma markers in obese children: the impact of cardiovascular risk factors. Pediatrics. 2006;117:1560–1567. doi: 10.1542/peds.2005-2140. [DOI] [PubMed] [Google Scholar]
- 13.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: overview of the first 40 years, with an eye to the future. Soc Psychiatry Psychiatr Epidemiol. 2015;50:679–693. doi: 10.1007/s00127-015-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva PA. A thousand Dunedin three year olds A multidisciplinary study of child development; Research report presented to the Medical Research Council of New Zealand; 1976. [Google Scholar]
- 15.Poulton R, Hancox R, Milne B, Baxter J, Scott K, Wilson N. The Dunedin Multidisciplinary Health and Development Study: are its findings consistent with the overall New Zealand population? N Z Med J. 2006;119 [PubMed] [Google Scholar]
- 16.Elley WB, Irving JC. Revised socio-economic index for New Zealand. N Z J Educ Stud. 1976;11:25–36. [Google Scholar]
- 17.Belsky DW, Caspi A, Goldman-Mellor S, Meier MH, Ramrakha S, Poulton R, et al. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol. 2013;178:1461–1468. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cullinane EM, Siconolfi S, Carleton RA, Thompson PD. Modification of the Astrand-Rhyming sub-maximal bicycle test for estimating VO2max of inactive men and women. Med Sci Sports Exerc. 1988;20:317–318. [PubMed] [Google Scholar]
- 19.Hamburg NM, Keyes MJ, Larson MG, Vasan RS, Schnabel R, Pryde MM, et al. Cross-sectional relations of digital vascular function to cardiovascular risk factors in the Framingham Heart Study. Circulation. 2008;117:2467–2474. doi: 10.1161/CIRCULATIONAHA.107.748574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Belsky DW, Caspi A, Israel S, Blumenthal JA, Poulton R, Moffitt TE. Cardiorespiratory fitness and cognitive function in midlife: neuroprotection or neuroselection? Ann Neurol. 2015;77:607–617. doi: 10.1002/ana.24356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meier MH, Caspi A, Cerda M, Hancox RJ, Harrington H, Houts R, et al. Associations Between Cannabis Use and Physical Health Problems in Early Midlife: A Longitudinal Comparison of Persistent Cannabis vs Tobacco Users. JAMA Psychiatry. 2016;73:731–740. doi: 10.1001/jamapsychiatry.2016.0637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociol Methods Res. 2001;29:374–393. [Google Scholar]
- 23.Nagin DS. Group-based modeling of development. Cambridge: Harvard University Press; 2005. [Google Scholar]
- 24.Ostbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: results from the National Longitudinal Survey of Youth 1979 cohort (1981–2006) Int J Epidemiol. 2011;40:240–250. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 25.Woo KS, Chook P, Yu CW, Sung RY, Qiao M, Leung SS, et al. Overweight in children is associated with arterial endothelial dysfunction and intima-media thickening. Int J Obes Relat Metab Disord. 2004;28:852–857. doi: 10.1038/sj.ijo.0802539. [DOI] [PubMed] [Google Scholar]
- 26.Zhu W, Huang X, He J, Li M, Neubauer H. Arterial intima-media thickening and endothelial dysfunction in obese Chinese children. Eur J Pediatr. 2005;164:337–344. doi: 10.1007/s00431-005-1642-y. [DOI] [PubMed] [Google Scholar]
- 27.Pareyn A, Allegaert K, Verhamme P, Vinckx J, Casteels K. Impaired endothelial function in adolescents with overweight or obesity measured by peripheral artery tonometry. Pediatr Diabetes. 2015;16:98–103. doi: 10.1111/pedi.12139. [DOI] [PubMed] [Google Scholar]
- 28.Cote AT, Harris KC, Panagiotopoulos C, Sandor GG, Devlin AM. Childhood obesity and cardiovascular dysfunction. J Am Coll Cardiol. 2013;62:1309–1319. doi: 10.1016/j.jacc.2013.07.042. [DOI] [PubMed] [Google Scholar]
- 29.Emerging Risk Factors Collaboration. Kaptoge S, Di Angelantonio E, Pennells L, Wood AM, White IR, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- 31.Schmidt JF, Andersen TR, Andersen LJ, Randers MB, Hornstrup T, Hansen PR, et al. Cardiovascular function is better in veteran football players than age-matched untrained elderly healthy men. Scand J Med Sci Sports. 2015;25:61–69. doi: 10.1111/sms.12153. [DOI] [PubMed] [Google Scholar]
- 32.Montero D. The association of cardiorespiratory fitness with endothelial or smooth muscle vasodilator function. Eur J Prev Cardiol. 2015;22:1200–1211. doi: 10.1177/2047487314553780. [DOI] [PubMed] [Google Scholar]
- 33.Brant LC, Barreto SM, Passos VM, Ribeiro AL. Reproducibility of peripheral arterial tonometry for the assessment of endothelial function in adults. J Hypertens. 2013;31:1984–1990. doi: 10.1097/HJH.0b013e328362d913. [DOI] [PubMed] [Google Scholar]
- 34.Bouchard C. BMI, fat mass, abdominal adiposity and visceral fat: where is the ‘beef’? Int J Obes. 2007;31:1552–1553. doi: 10.1038/sj.ijo.0803653. [DOI] [PubMed] [Google Scholar]
- 35.Camhi SM, Bray GA, Bouchard C, Greenway FL, Johnson WD, Newton RL, et al. The relationship of waist circumference and BMI to visceral, subcutaneous, and total body fat: sex and race differences. Obesity. 2011;19:402–408. doi: 10.1038/oby.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]


