Abstract
25-hydroxyvitamin D3-3-sulfate (25-OHD3-S) and 25-hydroxyvitamin D3-3-glucuronide (25-OHD3-G) are major conjugative metabolites of vitamin D3 found in the systemic circulation and potentially important reservoirs for 25-hydroxyvitamin D3. Simultaneous and accurate quantification of these metabolites could advance assessment of the impact of vitamin D3 on health and disease. In this study, a highly sensitive and accurate liquid chromatography-tandem mass spectrometry (LC-MS/MS) method was developed and validated for simultaneous quantification of 25-OHD3-S and 25-OHD3-G in human serum or plasma. Following protein precipitation, the analytes of interest were partially purified by solid-phase extraction and subjected to derivatization with 4-(4’-dimethylaminophenyl)-1,2,4-triazoline-3,5-dione (DAPTAD). Quantification of the analytes was based on multiple reaction monitoring (MRM) operated in the positive ion mode, and deuterated internal standards were used for each conjugative metabolite. Applying this method to the analysis of 25-OHD3-S and 25-OHD3-G concentrations in human serum or plasma samples achieved satisfactory reproducibility, accuracy and sensitivity. We subsequently used this method to simultaneously determine serum concentrations of the two metabolites in archived samples from a rifampin treatment study. Drug treatment had no effect on metabolite concentrations, but significantly increased the 25-OHD3-S/25-OHD3 concentration ratio (p = 0.01). The availability of this new method should improve sample throughput and our ability to quantify and monitor circulating 25-OHD3-S and 25-OHD3-G concentrations.
Keywords: Vitamin D3, 25-hydroxyvitamin D3-3-sulfate, 25-hydroxyvitamin D3-3-glucuronide, LC-MS/MS, derivatization
1. Introduction
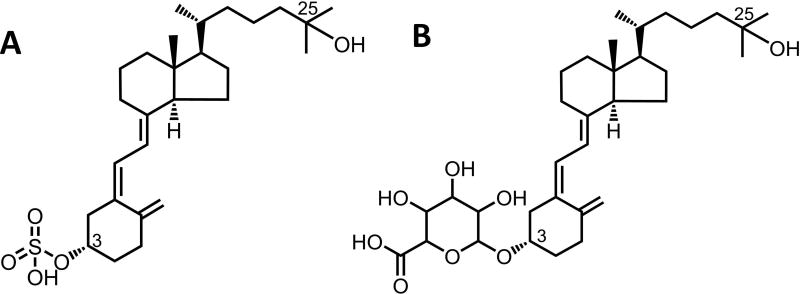
Vitamin D3 is a prohormone critical for the regulation of calcium and phosphate homeostasis, interacting with many biologically, physiologically important receptors and enzymes [1–3]. Vitamin D3 exerts most of its biological and physiological functions through an active metabolite, 1α,25-dihydroxyvitamin D3, that binds to the nuclear transcription factor vitamin D receptor (VDR), which in turn activates the transcription of target genes [4–6]. 1α,25-dihydroxyvitamin D3 is generated following two metabolic steps: 25-hydroxlyation of vitamin D3 primarily by CYP2R1 in the liver and 1α-hydroxylation of 25-OHD3 by CYP27B1 in the kidney and certain extra-renal tissues [7–10]. In addition to 1α–hydroxylation, 25-OHD3 also undergoes a series of hydroxylation reactions that are catabolic (e.g., 23S, 24R and 4β) as well as extensive sulfonation and glucuronidation in the liver, which generates two conjugative circulating metabolites 25-OHD3-3-sulfate (25-OHD3-S, Fig. 1A) and 25-OHD3-3-glucuronide (25-OHD3-G, Fig. 1B) [11, 12]. The detection of 25-OHD3-G in biological fluids from rats was reported by Shimada et al [13] and some studies also found that the glucuronides of vitamin D3 metabolites are excreted in the urine in humans [14, 15]. In addition, both 25-OHD3-S [16, 17] and 25-OHD3-G [12] are found in human plasma and bile, suggesting that conjugates formed in hepatocytes are transported out of the cells by as yet unknown mechanisms [12, 18]. Indeed, the relatively high concentration of 25-OHD3-S in plasma [11, 16, 19] suggests that it is a major metabolite of 25-OHD3. Although the biological roles of 25-OHD3-S and 25-OHD3-G are still not fully understood, the two conjugative metabolites could serve as reservoirs for 25-OHD3 through metabolic deconjugation [12, 20]. Importantly, the conjugates excreted into the bile may act directly on the intestinal epithelia after deconjugation and bioactivation by local CYP27B1 [10, 21]. Therefore, accurate quantification of 25-OHD3-S and 25-OHD3-G in human biological samples may improve our understanding of vitamin D homeostasis and biological activity in the body.
Figure 1. Chemical structures of 25-OHD3-3-sulfate (A) and 25-OHD3-3-glucuronide (B).
Methods using high-performance liquid chromatography coupled with ultraviolet detection (HPLC-UV) or mass spectrometry (LC-MS/MS) have been reported for measurement of 25-OHD3-S and 25-OHD3-G in human serum or plasma[12, 16, 17, 19]; however, thus far reliable quantification of 25-OHD3-G is limited by analytical sensitivity. The objective of this study was to develop and validate a LC-MS/MS method for simultaneous, sensitive and accurate determination of 25-OHD3-S and 25-OHD3-G in human serum or plasma to enhance experimental and clinical monitoring of the two conjugative metabolites.
2. Materials and Methods
2.1. Materials
25-OHD3-S, 25-OHD3-G and d6-25-OHD3 were purchased from Toronto Research Chemicals (Toronto, Canada). The purity of d6-25-OHD3, used to prepare the deuterated internal standards, was 96.6%. Iodobenzene I,I-diacetate, 3’-phosphoadenosine-5’-phosphosulfate (PAPS), uridine 5’-diphosphoglucuronic acid ammonium salt (UDPGA), alamethacin and 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD) was purchased from Sigma-Aldrich (St. Louis, MO, USA).
4-(4’-dimethylaminophenyl)-1,2,4-triazolidine-3,5-dione (precursor of DAPTAD) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). HPLC-grade acetonitrile, methanol, ethyl acetate, methyl tert-butyl ether, formic acid, acetic acid, and ammonium hydroxide were obtained from Fisher Chemicals (Waltham, MA, USA). Vitamin D3-free human serum was purchased from Golden West Biologicals (Temecula, CA, USA). Human plasma was obtained from the Puget Sound Blood Bank (Seattle, WA). Pooled human liver cytosol and microsomes were prepared in-house using liver tissue provided by the University of Washington School of Pharmacy Human Tissue Bank.
2.2. Preparation of DAPTAD solution
DAPTAD solution was prepared as previously described [22]. Briefly, 6 mg of iodobenzene I,I-diacetate (18.5 µmol) was mixed with 4 mg of the precursor of DAPTAD (18.2 µmol) in 4 mL of ethyl acetate and the mixture was stirred at room temperature for 3 hours until the color of the mixture changed to red. The resulting DAPTAD solution was aliquoted and stored at −80°C until used for derivatization. The solution was stable at −80°C for at least two months.
2.3. Preparation of deuterated internal standards
Deuterated 25-OHD3-S and 25-OHD3-G, used as internal standards, were generated biologically. For preparation of deuterated 25-OHD3-S, incubations were performed in 50 mM Tris/HCl buffer (pH 7.5) containing 25 µM d6-25-OHD3, 0.1 M PAPS and 4 mg/mL pooled human liver cytosolic fractions for 5 hours. Incubation mixtures containing d6-25-OHD3-S were used directly after protein precipitation with acetonitrile at a mixture/acetonitrile volume ratio of 2:1. For preparation of deuterated 25-OHD3-G, incubations were performed in 50 mM Tris/HCl buffer (pH 7.5) containing 25 µM d6-25-OHD3, 50 µg/mL alamethicin, 5 mM MgCl2, 5 mM UDPGA and 1 mg/mL pooled human liver microsomes for 3 hours. To isolate d6-25-OHD3-G from multiple isomeric glucuronide metabolites and reduce the excess d6-25-OHD3, the incubation mixtures were subjected to solid-phase extraction (SPE) and HPLC fraction collection. Following incubation, proteins were precipitated with an equal volume of acetonitrile and the supernatant was acidified with acetic acid to pH 3. d6-25-OHD3-G was partially purified by solid-phase extraction using Oasis WAX (3 cc, 30 mg, 60 µ m) anion exchange cartridges (Waters, Milford, MA) according to the manufacturer’s instructions. The eluate from SPE was evaporated and the residue was reconstituted in methanol:water (1:1, v/v) and subjected to metabolite isolation by HPLC-UV following a previously reported method [12].
2.4. Preparation of stock solutions and working solutions of analytical standards
25-OHD3-G and 25-OHD3-S standards were prepared as 10 mM primary stock solutions in methanol and stored in amber vials at −80°C until use. Working solutions for calibration standards (CS) and quality controls (QC) were obtained by serial dilution of a combined aliquot of 25-OHD3-G and 25-OHD3-S primary stock solutions in water/methanol (50:50, v/v). All standard solutions were stored at −80°C until use.
2.5. Chromatographic conditions
LC-MS/MS analysis was performed on a Sciex (Framingham, MA) QTRAP 6500 hybrid triple quadrupole/linear ion trap mass spectrometer coupled with a Shimadzu (Kyoto, Japan) liquid chromatography system. Chromatographic separation was achieved on a Hypersil Gold (2.1 × 100 mm, 1.9 µm) column (Thermo Fisher Scientific, Waltham, MA) held at 45°C with a mobile phase at a flow rate of 0.25 mL/min. The mobile phase consisted of 5 mM ammonium acetate (A, pH 4.6) and acetonitrile (B), and linear gradients were applied with B% increasing from 25% to 60% between 1 and 13 min and from 60% to 90% between 13 and 16 min. The autosampler was maintained at 4°C and the injection volume was 20 µL.
2.6. Mass spectrometric conditions
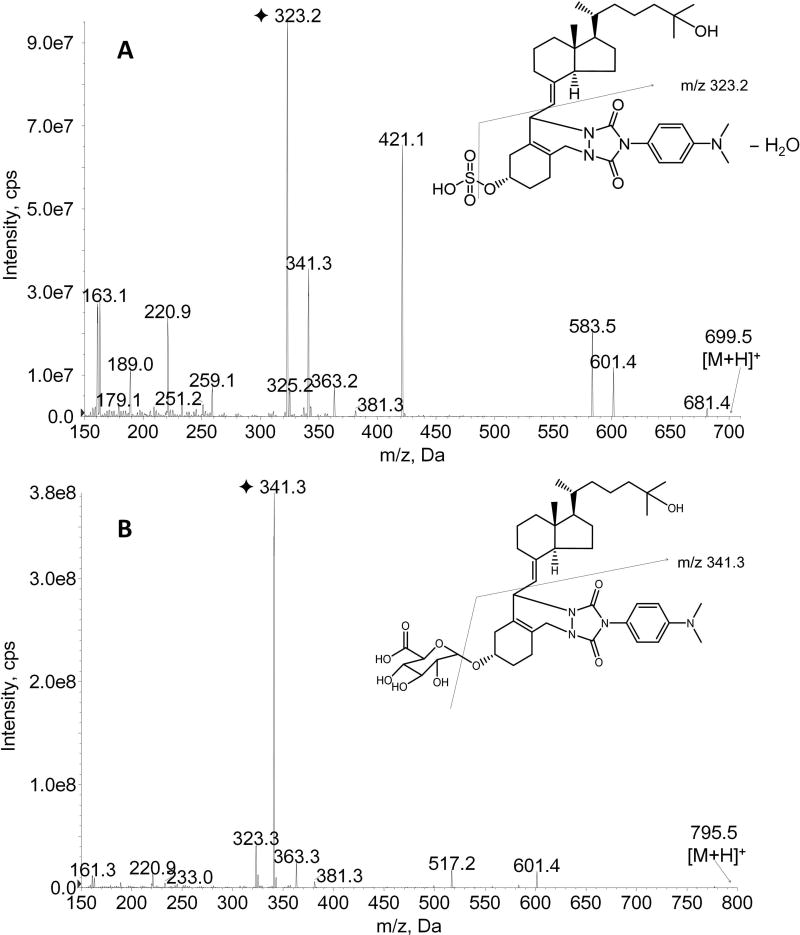
The electrospray ionization (ESI) source of the mass spectrometer was operated in the positive ion mode. Quantitation was achieved by multiple reaction monitoring (MRM) of the transitions at m/z 699.5→323.0 for DAPTAD-25-OHD3-S, m/z 705.5.5→323.0 for DAPTAD-d6-25-OHD3-S, m/z 795.5→341.1 for DAPTAD-25-OHD3-G, and m/z 801.5-341.1 for DAPTAD-d6-25-OHD3-G. Optimal MS parameters for quantitation of the four compounds were as follows: curtain gas, 20 psi; nebulizer gas (GS1) and turbo gas (GS2), 40 psi; ion spray voltage, 5,500 V; source temperature, 400°C; declustering potential, 100 V; collision energy, 45 V (for DAPTAD-25-OHD3-S and DAPTAD-d6-25-OHD3-S) and 42.5 V (for DAPTAD-25-OHD3-G and DAPTAD-d6-25-OHD3-G). Product ion scan spectra (Fig. 2) of DAPTAD-25-OHD3-S and DAPTAD-25-OHD3-G were obtained with the same MS parameters as above, and the scan ranges were m/z 150–700 and 150–800 for the two analytes, respectively.
Figure 2. Product ion spectra of DAPTAD-25-OHD3-3-sulfate (A) and DAPTAD-25-OHD3-3-glucuronide (B) by ESI-MS/MS in positive ion mode.
The fragmentation was conducted under collision energies of 45 and 42.5 V for DAPTAD-25-OHD3-3-sulfate and DAPTAD-25-OHD3-3-glucuronide respectively. The fragmentation pathways for selected product ions are illustrated by the lines in the figure.
2.7. Quantification and data analysis
Data acquisition and analysis were performed using the Analyst® software (SCIEX, Framingham, MA). Calibration curves were constructed by plotting the peak area ratio of each vitamin D3 metabolite to the respective internal standard against a range of concentrations, and by fitting a curve using linear regression to the data points, with a weighting of 1/x.
2.8. Plasma sample preparation
Human plasma (200 µL) was spiked with d6-25-OHD3-S (~4.0 pmol) and d6-25-OHD3-G (~1.6 pmol) and precipitated with 200 µL acetonitrile. The supernatant was acidified with 1 mL of 0.1 M sodium acetate (pH 3.2) and subjected to SPE using Waters Oasis WAX (1 cc, 30 mg, 60 µm) anion exchange cartridges. SPE cartridges were activated with 1 mL of methanol and pre-conditioned with 2 mL of water containing 2% acetic acid before loading diluted samples. After loading, cartridges were washed with 2 mL of water containing 2% acetic acid and followed by 1 mL of methanol containing 2% formic acid, and then eluted with 1 mL of ammonium hydroxide (28%, w/w) in methanol (3:97, v/v). The eluate from SPE cartridges was dried and the residue was reconstituted in 10 µL of methanol and then mixed with 200 µL of the DAPTAD solution (~4.5 mM). The resulting mixture was vortexed and kept in the dark for 1 hour at room temperature. Derivatization was stopped by evaporating the reaction mixture under N2 flow and the residue was reconstituted in 100 µL of acetonitrile-water (30:70, v/v). A brief clean-up step was performed by centrifuging derivatized samples at 13,362 g for 5 min to remove insoluble particulates (including excess DAPTAD) prior to LC-MS/MS analysis.
2.9. Method validation
2.9.1. Selectivity
Selectivity was evaluated by analyzing MRM chromatograms of the samples under the following three conditions: 1) blank vitamin D3-free human serum; 2) blank serum spiked with the two analytes and their corresponding internal standards; and 3) plasma sample from a healthy individual spiked with internal standards.
2.9.2. Linearity and lower limits of quantification (LLOQ)
Vitamin D3-free human serum was used as the blank matrix for establishing the lower limit of quantification following serial dilutions of the stock 25-OHD3-G and 25-OHD3-S standards. A signal-to-noise ratio of 10 was selected as the LLOQ threshold. Vitamin D3-free human serum was also used to generate calibration curves. Linearity of each calibration curve was determined by spiking seven concentrations of a standard in the blank matrix to obtain a series of concentrations of 0.5, 1, 2, 4, 8, 12 and 20 nM for 25-OHD3-G and of 5, 10, 20, 40, 80, 120 and 200 nM for 25-OHD3-S. Calibration curves were plotted as the peak area ratio of each 25-OHD3 conjugative metabolite to respective internal standard against a series of concentrations of the metabolite.
2.9.3. Accuracy and precision
Six replicates of the QC samples at each of the three concentrations (1, 8, 18 nM and 10, 80, 180 nM for 25-OHD3-G and 25-OHD3-S, respectively) were analyzed in three separate runs conducted on three consecutive days, to determine the intra-day and inter-day precision and accuracy. The precision of determination was calculated as relative standard deviation (RSD %), and the accuracy was represented as the relative error to the nominal concentrations (RE %).
2.9.4. Extraction recovery and matrix effect
Extraction recovery of the two analytes was estimated by comparing the peak areas of QC samples and the extracted blank matrix spiked with corresponding standard solutions. Standard solutions and extracted blank matrix were derivatized separately and then mixed together. The peak areas of the resulting mixture were compared against that of derivatized neat standards to obtain the matrix effect. The extraction recovery and matrix effects were determined in triplicate at two QC concentrations (1 and 18 nM for 25-OHD3-G, and 10 and 180 nM for 25-OHD3-S).
2.9.5. Stability
Stability of 25-OHD3-G and 25-OHD3-S in human serum was investigated by analyzing three replicate samples at two QC concentrations (low and high). Blank human serum spiked with a designated amount of 25-OHD3-G or 25-OHD3-S (< 1% vehicle, v/v) were exposed to different test conditions, including leaving samples at room temperature for 2 hours, storing samples at −80°C for 50 days, and three freeze-thaw cycles. Prepared sample stability was also investigated by placing prepared samples in the autosampler at 4°C for 24 hours prior to LC-MS/MS analysis.
2.9.6. Applicability for human plasma analysis
To validate these methods for human plasma, plasma (200 µL) from three donors was spiked with 25-OHD3-S (16 pmol) and 25-OHD3-G (1.6 pmol) in triplicate. After equilibrating for 30 min in the dark, both original and spiked plasma samples were extracted and measured as described above.
2.10. Application to a clinical study
In brief, six healthy volunteers were recruited for a study to assess the effects of a potent cytochrome P450 inducer, rifampin, on vitamin D levels as described previously [23]. The study was approved by the Institutional Review Board at the University of Washington. Serum samples were collected on 4 study visit days. The first two visits (Day 0 and Day 1) occurred before the first dose of rifampin. Beginning on the evening of Day 1, subjects received daily 600 mg doses of rifampin for 7 days. Subjects returned on Day 7 and Day 8 for sampling. Serum samples were stored at −80°C prior to analysis. 25-OHD3 data for this study were reported previously [23].
GraphPad® Software (San Diego, CA), was used for statistical analysis. Results from two study days were averaged for each volunteer pre-rifampin (Day 0 and Day 1) and post-rifampin (Day 7 and Day 8). A paired Student’s t-test was used to compare the data before and after rifampin treatment. A p-value of < 0.05 was considered to be significant.
3. Results and discussion
3.1. Optimization of derivatization
Due to the lack of a sensitive quantification method for 25-OHD3-G, the major consideration during development of the current method was to improve the detection sensitivity for 25-OHD3-G. Unlike underivatized 25-OHD3-S, which generates a product ion with an m/z of 96.6 for MRM detection under negative mode ionization[17], no stable product ion was observed for underivatized 25-OHD3-G under the same conditions. Thus, to obtain a better detection sensitivity, derivatization of the conjugates became a necessary step for LC-MS/MS detection of 25-OHD3-G. We first tested 4-phenyl-1,2,4-triazoline-3,5-dione (PTAD), a Diels-Alder derivatization reagent frequently used for LC-MS/MS analysis of hydroxylated vitamin D metabolites, using a previously described method [24–26]. However, we observed that PTAD derivatization provided poor assay sensitivity and reproducibility, particularly for 25-OHD3-G quantification (data not shown). We next examined DAPTAD, a derivative of PTAD with an extra para-dimethyl amino group in the aromatic ring. As shown in Fig. 2, multiple product ions with good abundance were observed for both DAPTAD-25-OHD3-S (Fig. 2A) and DAPTAD-25-OHD3-G (Fig. 2B) in positive ion mode. The most abundant product ion transitions were selected for the LC-MS/MS acquisition method. Compared to PTAD, DAPTAD derivatization provided about 15-fold enhanced sensitivity, possibly due to the alkali nitrogen atom of the dimethyl amino group, which may facilitate ionization of the analyte in positive ion mode. In addition, we noted that DAPTAD derivatization provided better reproducibility than PTAD.
3.2. Optimization of sample preparation
The initial step of the plasma sample preparation was protein precipitation with acetonitrile. After protein precipitation, we first tried liquid-liquid extraction (LLE) with ethyl acetate or methyl tert-butyl ether to further clean up the matrix. However, the LLE recovery of 25-OHD3-G was extremely low; therefore, a SPE step was developed and adopted in the method. Given chemical properties of the two conjugative metabolites, which both contain an acidic group, ion exchange columns were chosen over reverse-phase C18 columns. We compared the performance of weak anion-exchange (WAX) columns with mixed-mode anion-exchange (MAX) columns (Waters, Milford, MA). WAX columns showed much higher retention of the analytes of interest than MAX columns and resulted in a higher recovery of both 25-OHD3-S and 25-OHD3-G (data not shown). Therefore, WAX columns were selected for the SPE clean-up step. We further optimized the SPE procedure by testing different washing and elution buffers, based upon the manufacturer’s protocol. We found that the conjugates were retained on the column at pH 3.0–4.0. Consequently, before loading samples, protein precipitation supernatants were acidified using 0.1 M sodium acetate (pH 3.2) to reduce the pH and to simultaneously reduce the percentage of organic solvent. After loading the samples onto the column, the column was subjected to sequential weak (aqueous) and strong (methanol) washes. We also noticed that the addition of acid (2% acetic or formic acid) in the washing buffer was crucial for an optimal recovery of the conjugates. The conjugates were eluted with ammonium hydroxide (28%) in methanol (3:97, v/v), in which the sorbent was uncharged and therefore lost ion-exchange binding capacity.
3.3. Method validation
3.3.1. Linearity and calibration curve
In order to assess linearity of the quantification method, we analyzed vitamin D3-free human serum (200 µL) spiked with d6-25-OHD3-S (~4.0 pmol) and d6-25-OHD3-G (~1.6 pmol) and an increasing amount of the standard 25-OHD3-S (1, 2, 4, 8, 16, 24 or 40 pmol corresponding to plasma concentrations of 5, 10, 20, 40, 80, 120 or 200 nM, respectively) with 25-OHD3-G (0.1, 0.2, 0.4, 0.8, 1.6, 2.4 or 4.0 pmol corresponding to plasma concentrations of 0.5, 1, 2, 4, 8, 12 or 20 nM, respectively) by LC-MS/MS. Regression analysis showed good linearity, with correlation coefficients (r2) of >0.996 for both 25-OHD3-S and 25-OHD3-G. During the three validation runs, the accuracy (RE %) at each concentration levels of calibration curves ranged from 92.6% to 113% for both analytes, and the precision of the determination (RSD %) was 2.5% for 25-OHD3-S and 0.5% for 25-OHD3-G. The concentration range of the calibration curve was based on the estimated range of 25-OHD3-S and 25-OHD3-G concentrations in healthy human subjects.
3.3.2. Selectivity and LLOQ
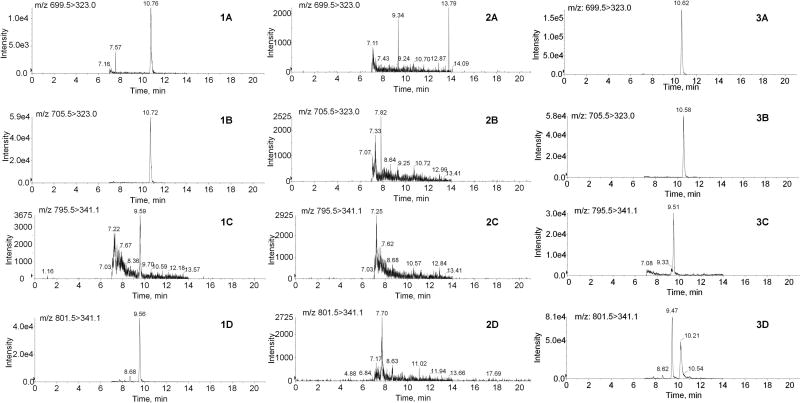
Due to the presence of endogenous vitamin D3 metabolites in human plasma and serum, vitamin D3-free human serum was used as the blank matrix for preparation of calibration curves. To confirm that the blank vitamin D3-free human serum did not have peaks that interfered with detection of the metabolites, vitamin D3-free human serum spiked with both 25-OHD3-S and 25-OHD3-G standards at the lowest standard concentrations and their respective deuterated internal standards were compared with double blank samples without spiking any standards. As shown in Fig. 3, no interference from the blank human serum was observed for either DAPTAD-25-OHD3-S or DAPTAD-25-OHD3-G or their deuterated internal standards. The signal-to-noise ratios at the lowest calibration standards, calculated by dividing the peak responses of the analytes in the samples by the noise level at corresponding retention times in blank serum, were at least 57 and 16 for DAPTAD-25-OHD3-S and DAPTAD-25-OHD3-G, respectively.
Figure 3. Representative MRM chromatograms of DAPTAD-derivatized vitamin D3 conjugative metabolites.
Shown are MRM chromatograms of DAPTAD-25-OHD3-3-sulfate (1A), DAPTAD-d6-25-OHD3-3-sulfate (1B), DAPTAD-25-OHD3-3-glucuronide (1C) and DAPTAD-d6-25-OHD3-3-glucuronide (1D) in human serum at the lowest calibration standard levels (5 nM for 25-OHD3-3-sulfate and 0.5 nM for 25-OHD3-3-glucuronide); DAPTAD-25-OHD3-3-sulfate (2A), DAPTAD- d6-25-OHD3-3-sulfate (2B), DAPTAD-25-OHD3-3-glucuronide (2C) and DAPTAD-d6-25-OHD3-3-glucuronide (2D) in the blank vitamin D3-free human serum; DAPTAD-25-OHD3-3-sulfate (3A), DAPTAD-d6-25-OHD3-3-sulfate (3B), DAPTAD-25-OHD3-3-glucuronide (3C) and DAPTAD-d6-25-OHD3-3-glucuronide (3D) in plasma of a healthy individual. The samples were prepared and analyzed with the method described in section 2.
To establish LLOQ levels for 25-OHD3-S and 25-OHD3-G, we tested lower concentrations and found that 1 nM of 25-OHD3-S and 0.3 nM of 25-OHD3-G still yielded signal-to noise ratios above 10. The sensitivity of the current method for quantitation of 25-OHD3-S is comparable to a previous method in which the LLOQ of 2.5 ng/mL (~5 nM) was achieved by using 20 µL of serum [19]. The current method for quantification of 25-OHD3-G gave markedly improved sensitivity and reproducibility compared to what we reported previously, in which the LLOQ of ~1 nM was achieved by using 500 µL of plasma [12].
3.3.3. Precision and accuracy
Table 1 summarizes the precision and accuracy of this method for simultaneous quantification of 25-OHD3-S and 25-OHD3-G. The relative errors of the three QC concentrations tested in three independent determinations were within ±3% and ±12% for 25-OHD3-S and 25-OHD3-G, respectively. The intra-assay (n = 6) variation did not exceed 3% for 25-OHD3-S and 7% for 25-OHD3-G. For both metabolites, the inter-assay (n = 3) variation was within 3%. These results indicate high reproducibility and accuracy of the current method.
Table 1.
Precision and accuracy of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide measurements
| Compound and concentration | Intra-assay (n=6) a | Inter-assay (n=3) b | |||||
|---|---|---|---|---|---|---|---|
| Measuredc | % RE d | % CV e | Measuredc | % RE d | % CV e | ||
| 25-OHD3-3-sulfate | |||||||
| 10 nM | 9.75 ± 0.18 | −2.42 | 1.87 | 9.64 ± 0.15 | −3.63 | 1.53 | |
| 80 nM | 82.2 ± 1.9 | 2.73 | 2.36 | 81.3 ± 2.1 | 1.58 | 2.54 | |
| 180 nM | 176.7 ± 3.2 | −1.85 | 1.81 | 178.9 ± 2.1 | −0.62 | 1.17 | |
| 25-OHD3-3-glucuronide | |||||||
| 1 nM | 0.98 ± 0.06 | −1.83 | 6.26 | 0.99 ± 0.02 | −1.02 | 2.22 | |
| 8 nM | 8.89 ± 0.12 | 11.10 | 1.31 | 8.89 ± 0.07 | 11.15 | 0.83 | |
| 18 nM | 20.0 ± 0.4 | 10.93 | 1.84 | 19.9 ± 0.2 | 10.62 | 1.03 | |
Intra-assay precision was calculated for 6 replicates measured in a single assay.
Inter-assay precision was calculated from 3 independent assays.
Means ± SD of measured concentrations.
Percent relative error (% RE) is defined as the ratio of the deviation to the theoretical value × 100.
Coefficient of variance (% CV) is defined as the ratio of the standard deviation to the mean value × 100.
3.3.4. Recovery and matrix effect
Estimates of the recovery of the 25-OHD3-S and 25-OHD3-G following the optimized extraction procedures are presented in Table 2. The mean recovery rates (n = 6) were 97% and 91% for 25-OHD3-S at 10 and 180 nM, respectively, and 113% and 101% for 25-OHD3-G at 1 and 18 nM, respectively. The mean extraction recovery for the internal standards d6-25-OHD3-S and d6-25-OHD3-G was 98% and 100%, respectively. The high extraction recovery of the conjugates likely contributes to the reproducibility and sensitivity of the quantification method.
Table 2.
Estimated recovery of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide and their deuterated internal standards following solid phase extraction
| 25-OHD3-3-sulfate | d6-25-OHD3-3-sulfate | 25-OHD3-3-glucuronide | d6-25-OHD3-3-glucuronide | |||
|---|---|---|---|---|---|---|
| 10 nM | 180 nM | ~ 20 nM | 1 nM | 18 nM | ~ 8 nM | |
| 99.6 | 91.2 | 95.9 | 114 | 98.4 | 107 | |
| 99.8 | 88.8 | 91.8 | 115 | 98.0 | 72.3 | |
| Recovery (%)a | 94.8 | 91.6 | 98.0 | 111 | 100 | 111 |
| 96.2 | 89.3 | 96.2 | 113 | 102 | 104 | |
| 98.3 | 92.4 | 106 | 111 | 104 | 90.4 | |
| 93.1 | 93.6 | 101 | 117 | 103 | 116 | |
|
| ||||||
| N | 6 | 6 | 6 | 6 | 6 | 6 |
| Average | 97.0 | 91.2 | 98.2 | 113 | 101 | 100 |
| SD (%) | 2.7 | 1.9 | 4.9 | 2.4 | 2.5 | 16.2 |
Recovery (%) was calculated as the percentage of the peak area of samples post-extraction to the peak area without extraction.
Table 3 shows the effects of serum matrix on the mass spectrometric response of the analytes. Matrix enhancement effects were observed for both 25-OHD3-S and 25-OHD3-G, ranging from an average of 159% to 223% in the presence of blank human serum matrix. The matrix effects on both metabolites seemed to be more evident at the lower analyte concentrations than at the higher analyte concentrations. However, the deuterated internal standards, which demonstrated the same mass spectrometric behavior as the analytes, were able to correct for variations caused by the matrix.
Table 3.
Matrix effects of vitamin D3-free human serum on 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide and their deuterated internal standards
| 25-OHD3-3-sulfate
|
25-OHD3-3-glucuronide
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Analyte (10 nM) | IS | Analyte (180 nM) | IS | Analyte (1 nM) | IS | Analyte (18 nM) | IS | |
| 228 | 207 | 171 | 204 | 182 | 202 | 154 | 207 | |
| Matrix effect (%)a | 217 | 218 | 176 | 148 | 196 | 165 | 158 | 124 |
| 223 | 189 | 178 | 164 | 194 | 210 | 164 | 140 | |
|
| ||||||||
| N | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| Average | 223 | 205 | 175 | 172 | 191 | 192 | 159 | 159 |
| SD (%) | 5.1 | 14.6 | 4.0 | 28.8 | 7.6 | 24.1 | 5.0 | 5.0 |
The serum matrix effect (%) was calculated as the percentage of the peak area in the presence of matrix to the peak area in the absence of matrix.
3.3.5. Stability
The stability of the analytes subjected to various treatment conditions was evaluated using three replicates of the QCs at two concentrations. As shown in Table 4, the accuracy of all determinations was within 15%, indicating that the analytes in plasma were stable when left at room temperature for 2 hours, stored at −80°C for 50 days, and following three freeze-thaw cycles. The analytes in prepared samples were also stable when stored in the autosampler at 4°C for 24 hours prior to analysis.
Table 4.
Stability of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide in human serum under various treatment conditions
| Accuracy (%)a | 25-OHD3-3-sulfate | 25-OHD3-3-glucuronide | ||
|---|---|---|---|---|
|
| ||||
| 10 nM | 180 nM | 1 nM | 18 nM | |
| Prepared sample in autosampler at 4°C, 24 hr | 96.5 ± 3.3 | 92.6 ± 3.5 | 114 ± 3 | 95.9 ± 3.1 |
| Plasma at room temperature, 2 hr | 93.8 ± 2.6 | 89.3 ± 3.4 | 109 ± 38 | 89.1 ± 3.8 |
| Plasma following three freeze-thaw cycles | 89.8 ± 0.9 | 96.1 ± 4.0 | 92.8 ± 12.4 | 90.9 ± 2.2 |
| Plasma stored at −80°C, 50 days | 90.8 ± 1.6 | 91.7 ± 0.6 | 92.4 ± 0.2 | 90.6 ± 1.0 |
Accuracy (%) was calculated as the percentage of the determined concentration after the respective treatment to the theoretical concentration. Shown are mean ± SD.
3.3.6. Applicability for plasma analysis
To demonstrate whether this method is applicable to human plasma samples, we determined accuracy and precision of the method before and after adding known amounts of 25-OHD3-S and 25-OHD3-G to three human plasma samples containing endogenous vitamin D3 and metabolites. As summarized in Table 5, the measured concentrations of 25-OHD3-S and 25-OHD3-G were consistent with the summed concentrations of the baseline levels and spiked metabolites in the plasma samples. For each of the three donors, variations in the measurements were less than 10% (n=3) for both analytes and the accuracy ranged from 101% to 111%. These results confirm that this method (including both sample preparation and LC-MS/MS analysis) was applicable to simultaneous quantification of 25-OHD3-S and 25-OHD3-G in human plasma using vitamin D3-free human serum as the blank matrix for the calibration curves.
Table 5.
Validation of the quantification of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide in human plasma. Plasma samples from 3 donors were spiked with a standard mixture containing 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide. The native and spiked plasma samples were prepared and analyzed at the same time.
| Baseline (nM) a | Spikedb | |||
|---|---|---|---|---|
|
| ||||
| Calculated (nM) c | Measured (nM) | Accuracy % | ||
| 25-OHD3-3-glucuronide | ||||
| Donor 1 | 2.78 ± 0.12 | 10.8 | 11.6 ± 0.3 | 108 |
| Donor 2 | 4.47 ± 0.17 | 12.5 | 13.2 ± 0.2 | 106 |
| Donor 3 | 1.86 ± 0.07 | 9.86 | 10.6 ± 0.2 | 108 |
| 25-OHD3-3-sulfate | ||||
| Donor 1 | 70.3 ± 2.1 | 150 | 164 ± 5 | 109 |
| Donor 2 | 77.1 ± 0.7 | 157 | 158 ± 9 | 101 |
| Donor 3 | 53.5 ± 2.0 | 133 | 147 ± 3 | 111 |
Baselines represented the original metabolite concentrations in human plasma before spiking with standards.
Each plasma sample (200 µL) was spiked with metabolite standards which theoretically increased concentrations of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide by 8 and 80 nM, respectively.
Calculated concentration = baseline concentration + spiked concentration.
3.3.7. Application of this method to a clinical study
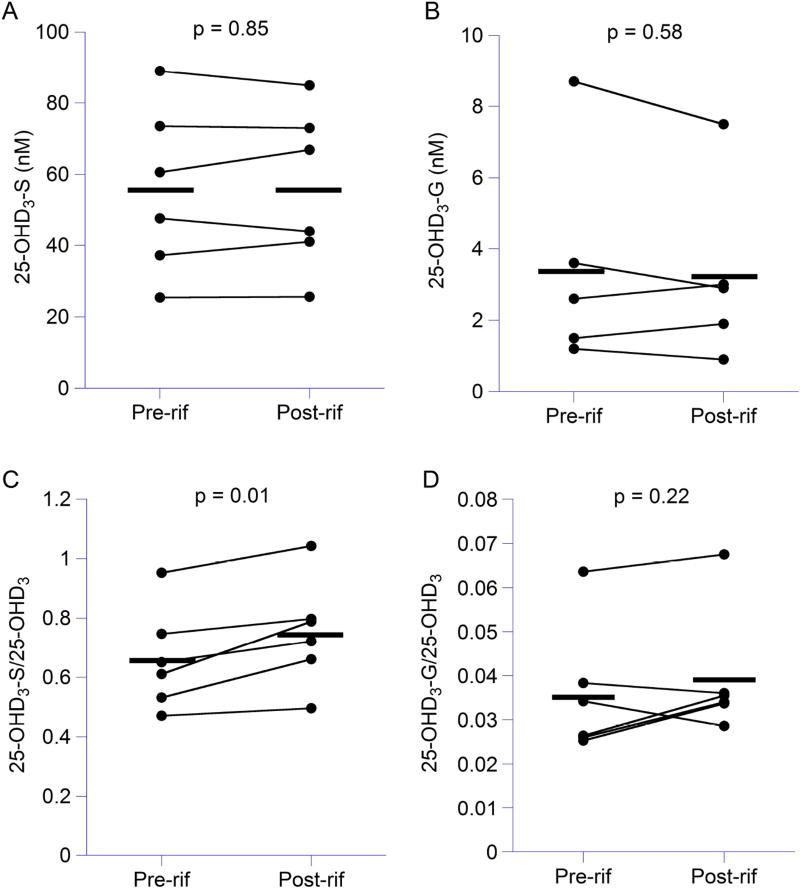
Finally, we used our validated analytical method to simultaneously quantify 25-OHD3-S and 25-OHD3-G in human serum samples obtained from six healthy volunteers who we reported on in a previous study [23]. On average, serum concentrations of 25-OHD3-S and 25-OHD3-G were 55.6 ± 23.6 nM (Fig. 4A) and 3.4 ± 2.8 nM (Fig. 4B) at baseline. Plamsa concentrations of 25-OHD3 in these subjects were significantly reduced by 11% following rifampin treatment (p < 0.05) as we previously reported [23]. Serum concentrations of 25-OHD3-S and 25-OHD3-G were not significantly changed following rifampin treatment (Fig. 4A and Fig. 4B). However, when normalized to 25-OHD3 plasma concentrations using data from the previous study [23], the ratio of 25-OHD3-S/25-OHD3 was significantly increased following rifampin treatment (pre-rifampin: 0.66 ± 0.17 vs. post-rifampin: 0.75 ± 0.18, p = 0.01) (Fig. 4C), whereas the ratio of 25-OHD3-G/25-OHD3 was not changed (pre-rifampin: 0.036 ± 0.015 vs. post-rifampin: 0.039 ± 0.014) (Fig. 4D). While the inducibility of UGT1A4 and SULT2A1, the primary enzymes catalyzing the formation of 25-OHD3-G [12] and 25-OHD3-S [27], is still unclear based on in vitro experimentation [28], our results suggest that sulfonation of 25-OHD3 may be increased by rifampin treatment, possibly due to induction of SULT2A1. In addition, we hypothesized that rifampin may induce other pathways of 25-OHD3 metabolism (e.g., 4β,25(OH)2D3 formation via CYP3A4) as we previously described [23].
Figure 4. Serum concentrations of 25-OHD3-3-sulfate and 25-OHD3-3-glucuronide following rifampin treatment.
Serum concentrations of 25-OHD3-3-sulfate (A) and 25-OHD3-3-glucuronide (B) and ratios of 25-OHD3-3-sulfate/25-OHD3 (C) and 25-OHD3-3-glucuronide/25-OHD3 (D) in healthy volunteers (n = 6) before and after rifampin treatment were compared by a paired Student’s t-test. A p-value < 0.05 was considered significant. Each individual is represented by a dot and the bar represents the means of each group.
4. Conclusion
We have developed and validated a LC-MS/MS method for simultaneous quantification of 25-OHD3-S and 25-OHD3-G in human serum and plasma. This method is accurate and reproducible, with quantification ranges of 5–200 nM and 0.5–20 nM for 25-OHD3-S and 25-OHD3-G, respectively. To the best of our knowledge, this is the first validated LC-MS/MS method for simultaneous measurement of 25-OHD3-S and 25-OHD3-G in biological matrices. This highly robust and accurate method for simultaneous quantification of 25-OHD3-S and 25-OHD3-G can be used to measure plasma concentrations of the two conjugative metabolites in clinical studies that assess the effects of drugs, disease, environmental factors and genetic variation on vitamin D3 disposition.
Highlights.
A method for simultaneous quantification of 25-hydroxyvitamin D3-3-sulfate and 25-hydroxyvitamin D3-3-glucuronide in human serum or plasma was developed and validated.
This is the first LC-MS/MS method published for reproducible quantitation of 25-hydroxyvitamin D3-3-glucuronide concentration in human plasma.
Derivatization using DAPTAD was adopted to improve the detection sensitivity.
In 6 healthy subjects, baseline serum 25-hydroxyvitamin D3-3-sulfate and 25-hydroxyvitamin D3-3-glucuronide concentrations ranged from 24.4 to 93.0 nM and 0.7 to 9.1 nM, respectively.
Acknowledgments
This work was supported in part by grants from the National Institutes of Health: R01 GM063666 (to K.E.T) and T32 GM007750 (to M.C.B and T.W.) and R01 DK099199 and P30 DK089507.
Abbreviations
- LC-MS/MS
liquid chromatography-tandem mass spectrometry
- DAPTAD
4-(4’.-dimethylaminophenyl)-1,2,4-triazoline-3,5-dione
- LLE
liquid-liquid extraction
- LLOQ
lower limit of quantification
- MRM
multiple reaction monitoring
- 25-OHD3
25-hydroxyvitamin D3
- 25-OHD3-S
25-hydroxyvitamin D3-3-sulfate
- 25-OHD3-G
25-hydroxyvitamin D3-3-glucuronide
- PAPS
3’-phosphoadenosine-5’-phosphosulfate
- UDPGA
uridine 5’-diphosphoglucuronic acid
- PTAD
4-phenyl-1,2,4-triazoline-3,5-dione
- SPE
solid phase extraction
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chemistry & biology. 2014;21:319–329. doi: 10.1016/j.chembiol.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pludowski P, Holick MF, Pilz S, Wagner CL, Hollis BW, Grant WB, Shoenfeld Y, Lerchbaum E, Llewellyn DJ, Kienreich K. Vitamin D effects on musculoskeletal health, immunity, autoimmunity, cardiovascular disease, cancer, fertility, pregnancy, dementia and mortality—a review of recent evidence. Autoimmunity reviews. 2013;12:976–989. doi: 10.1016/j.autrev.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 3.Pike JW, Lee SM, Meyer MB. Regulation of gene expression by 1, 25-dihydroxyvitamin D3 in bone cells: exploiting new approaches and defining new mechanisms. BoneKEy reports. 2014;3 doi: 10.1038/bonekey.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pike JW, Meyer MB. The vitamin D receptor: new paradigms for the regulation of gene expression by 1, 25-dihydroxyvitamin D 3. Rheumatic Disease Clinics of North America. 2012;38:13–27. doi: 10.1016/j.rdc.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haussler MR, Whitfield GK, Kaneko I, Haussler CA, Hsieh D, Hsieh J-C, Jurutka PW. Molecular mechanisms of vitamin D action. Calcified tissue international. 2013;92:77–98. doi: 10.1007/s00223-012-9619-0. [DOI] [PubMed] [Google Scholar]
- 6.Christakos S. Mechanism of action of 1, 25-dihydroxyvitamin D3 on intestinal calcium absorption. Reviews in Endocrine and Metabolic Disorders. 2012;13:39–44. doi: 10.1007/s11154-011-9197-x. [DOI] [PubMed] [Google Scholar]
- 7.Jones G, Prosser DE, Kaufmann M. Cytochrome P450-mediated metabolism of vitamin D. Journal of lipid research. 2014;55:13–31. doi: 10.1194/jlr.R031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christakos S, Ajibade DV, Dhawan P, Fechner AJ, Mady LJ. Vitamin D: metabolism. Rheumatic Disease Clinics of North America. 2012;38:1–11. doi: 10.1016/j.rdc.2012.03.003. [DOI] [PubMed] [Google Scholar]
- 9.Feldman D, Krishnan AV, Swami S, Giovannucci E, Feldman BJ. The role of vitamin D in reducing cancer risk and progression. Nature reviews cancer. 2014;14:342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 10.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D 3-1α-hydroxylase in human health and disease. The Journal of steroid biochemistry and molecular biology. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 11.Axelson M. 25-Hydroxyvitamin D3 3-sulphate is a major circulating form of vitamin D in man. FEBS letters. 1985;191:171–175. doi: 10.1016/0014-5793(85)80002-8. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z, Wong T, Hashizume T, Dickmann LZ, Scian M, Koszewski NJ, Goff JP, Horst RL, Chaudhry AS, Schuetz EG, Thummel KE. Human UGT1A4 UGT1A3 conjugate 25-hydroxyvitamin D3: metabolite structure, kinetics, inducibility and interindividual variability. Endocrinology. 2014;155:2052–2063. doi: 10.1210/en.2013-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shimada K, Nakatani I, Saito K, Mitamura K. Separation and characterization of monoglucuronides of vitamin D3 and 25-hydroxyvitamin D3 in rat bile by high-performance liquid chromatography. Biological and Pharmaceutical Bulletin. 1996;19:491–494. doi: 10.1248/bpb.19.491. [DOI] [PubMed] [Google Scholar]
- 14.Higashi T, Homma S, Iwata H, Shimada K. Characterization of urinary metabolites of vitamin D 3 in man under physiological conditions using liquid chromatography-tandem mass spectrometry. Journal of pharmaceutical and biomedical analysis. 2002;29:947–955. doi: 10.1016/s0731-7085(02)00135-8. [DOI] [PubMed] [Google Scholar]
- 15.Ogawa S, Ooki S, Shinoda K, Higashi T. Analysis of urinary vitamin D3 metabolites by liquid chromatography/tandem mass spectrometry with ESI-enhancing and stable isotope-coded derivatization. Analytical and bioanalytical chemistry. 2014;406:6647–6654. doi: 10.1007/s00216-014-8095-y. [DOI] [PubMed] [Google Scholar]
- 16.Shimada K, Mitamura K, Kitama N. Quantitative determination of 25 - hydroxyvitamin D3 3 - sulphate in human plasma using high performance liquid chromatography. Biomedical Chromatography. 1995;9:229–232. doi: 10.1002/bmc.1130090508. [DOI] [PubMed] [Google Scholar]
- 17.Higashi T, Goto A, Morohashi M, Ogawa S, Komatsu K, Sugiura T, Fukuoka T, Mitamura K. Development and validation of a method for determination of plasma 25-hydroxyvitamin D 3 3-sulfate using liquid chromatography/tandem mass spectrometry. Journal of Chromatography B. 2014;969:230–234. doi: 10.1016/j.jchromb.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 18.Higashi T, Miura K, Kikuchi R, Shimada K, Hiyamizu H, Ooi H, Iwabuchi Y, Hatakeyama S, Kubodera N. Characterization of new conjugated metabolites in bile of rats administered 24, 25-dihydroxyvitamin D 3 and 25-hydroxyvitamin D 3. Steroids. 2000;65:281–294. doi: 10.1016/s0039-128x(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 19.Higashi T, Yokota M, Goto A, Komatsu K, Sugiura T, Ogawa S, Satoh M, Nomura F. A Method for Simultaneous Determination of 25-Hydroxyvitamin D3 and Its 3-Sulfate in Newborn Plasma by LC/ESI-MS/MS after Derivatization with a Proton-Affinitive Cookson-Type Reagent. Mass Spectrometry. 2016;5:S0051–S0051. doi: 10.5702/massspectrometry.S0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Axelson M, Christensen NJ. Vitamin D metabolism in human pregnancy. Concentrations of free and sulphated 25-hydroxyvitamin D3 in maternal and fetal plasma at term. Journal of steroid biochemistry. 1988;31:35–39. doi: 10.1016/0022-4731(88)90202-6. [DOI] [PubMed] [Google Scholar]
- 21.Wang Z, Schuetz EG, Xu Y, Thummel KE. Interplay between vitamin D and the drug metabolizing enzyme CYP3A4. The Journal of steroid biochemistry and molecular biology. 2013;136:54–58. doi: 10.1016/j.jsbmb.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ogawa S, Ooki S, Morohashi M, Yamagata K, Higashi T. A novel Cookson - type reagent for enhancing sensitivity and specificity in assessment of infant vitamin D status using liquid chromatography/tandem mass spectrometry. Rapid Communications in Mass Spectrometry. 2013;27:2453–2460. doi: 10.1002/rcm.6708. [DOI] [PubMed] [Google Scholar]
- 23.Wang Z, Lin YS, Zheng XE, Senn T, Hashizume T, Scian M, Dickmann LJ, Nelson SD, Baillie TA, Hebert MF, Blough D, Davis CL, Thummel KE. An inducible cytochrome P450 3A4-dependent vitamin D catabolic pathway. Molecular pharmacology. 2012;81:498–509. doi: 10.1124/mol.111.076356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding S, Schoenmakers I, Jones K, Koulman A, Prentice A, Volmer DA. Quantitative determination of vitamin D metabolites in plasma using UHPLC-MS/MS. Analytical and bioanalytical chemistry. 2010;398:779–789. doi: 10.1007/s00216-010-3993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higashi T, Awada D, Shimada K. Determination of 24, 25 - dihydroxyvitamin D3 in human plasma using liquid chromatography - mass spectrometry after derivatization with a Cookson - type reagent. Biomedical Chromatography. 2001;15:133–140. doi: 10.1002/bmc.43. [DOI] [PubMed] [Google Scholar]
- 26.Wang Z, Senn T, Kalhorn T, Zheng XE, Zheng S, Davis CL, Hebert MF, Lin YS, Thummel KE. Simultaneous measurement of plasma vitamin D 3 metabolites including 4β, 25-dihydroxyvitamin D 3, using liquid chromatography–tandem mass spectrometry. Analytical biochemistry. 2011;418:126–133. doi: 10.1016/j.ab.2011.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wong ZW Timothy, Claw Katrina, Gao Chunying, Foti Robert, Prasad Bhagwat, Chapron Alenka, Chapron Brian, Suzuki Mizuki, Chaudhry Amarjit, Schuetz Erin G, Koszewski Nicholas J, Goff Jesse P, Horst Ronald L, Mao Qingcheng, de Boer Ian, Thornton Timothy, Thummel Kenneth E. Unpublished Article. University of Washington; Seattle, WA, United States: 2017. Polymorphic Human Sulfotransferase 2A1 Mediates the Formation of 25-Hydroxyvitamin D3-3-sulfate, A Major Circulating Vitamin D Metabolite in Humans. DOI. [Google Scholar]
- 28.Fang H-L, Strom SC, Ellis E, Duanmu Z, Fu J, Duniec-Dmuchowski Z, Falany CN, Falany JL, Kocarek TA, Runge-Morris M. Positive and negative regulation of human hepatic hydroxysteroid sulfotransferase (SULT2A1) gene transcription by rifampicin: roles of hepatocyte nuclear factor 4α and pregnane X receptor. Journal of Pharmacology and Experimental Therapeutics. 2007;323:586–598. doi: 10.1124/jpet.107.124610. [DOI] [PubMed] [Google Scholar]