Abstract
The role of nitric oxide (NO) in nociceptive transmission at the spinal cord level remains uncertain. Increased activity of spinal N-methyl-D-aspartate (NMDA) receptors contributes to development of chronic pain induced by peripheral nerve injury. In this study, we determined how endogenous NO affects NMDA receptor activity of spinal cord dorsal horn neurons in control and spinal nerve-ligated rats. Bath application of the NO precursor L-arginine or the NO donor S-nitroso-N-acetylpenicillamine (SNAP) significantly inhibited NMDA receptor currents of spinal dorsal horn neurons in both sham control and nerve-injured rats. Inhibition of neuronal nitric oxide synthase (nNOS) or blocking the S-nitrosylation reaction with N-ethylmaleimide abolished the inhibitory effects of L-arginine on NMDA receptor currents recorded from spinal dorsal horn neurons in sham control and nerve-injured rats. However, bath application of the cGMP analog 8-bromo-cGMP had no significant effects on spinal NMDA receptor currents. Inhibition of soluble guanylyl cyclase also did not alter the inhibitory effect of L-arginine on spinal NMDA receptor activity. Furthermore, knockdown of nNOS with siRNA abolished the inhibitory effects of L-arginine, but not SNAP, on spinal NMDA receptor activity in both groups of rats. Additionally, intrathecal injection of L-arginine significantly attenuated mechanical or thermal hyperalgesia induced by nerve injury, and the L-arginine effect was diminished in rats treated with a nNOS inhibitor or nNOS-specific siRNA. These findings suggest that endogenous NO inhibits spinal NMDA receptor activity through S-nitrosylation. NO derived from nNOS attenuates spinal nociceptive transmission and neuropathic pain induced by nerve injury.
Keywords: synaptic plasticity, synaptic transmission, signal transduction, ion channel, neuropathic pain, dorsal horn neurons
INTRODUCTION
Chronic neuropathic pain induced by nerve injury and trauma remains difficult to treat owing to our limited understanding of the cellular and molecular mechanisms involved. The spinal cord dorsal horn is a critical site for nociceptive transmission and modulation. Glutamate is the most predominant excitatory neurotransmitter involved in synaptic transmission from primary sensory nerves to spinal dorsal horn neurons (Pan and Pan, 2004; Yoshimura and Jessell, 1990). The glutamate N-methyl-D-aspartate receptor (NMDAR) activity in the spinal dorsal horn is markedly increased after peripheral nerve injury and is essential for synaptic plasticity associated with development of chronic neuropathic pain (Chen et al., 2014b; Isaev et al., 2000; Li et al., 2016; Zhou et al., 2012). However, it remains unclear how the NMDAR activity of dorsal horn neurons is regulated under physiological and neuropathic pain conditions.
Nitric oxide (NO) is a freely diffusible, soluble gas and is synthesized by the nitric oxide synthase (NOS) from L-arginine and different cofactors. NO is produced stoichiometrically after the conversion of L-arginine to citrulline in a process requiring NADPH and other cofactors (Marletta, 1993; Stuehr et al., 2004). The three NOS isoforms, including neuronal NOS (nNOS, encoded by Nos1), endothelial NOS (eNOS, encoded by Nos3), and inducible NOS (iNOS, encoded by Nos2), have distinct structures and functions (Lipton et al., 1994; Stuehr, 1997). Both nNOS and eNOS are expressed constitutively in the spinal dorsal horn, and their activity is controlled by calcium and calmodulin (Alderton et al., 2001; Infante et al., 2007). Some studies reported that pain hypersensitivity induced by nerve or tissue injury is reduced by intrathecal administration of nNOS inhibitors and in nNOS-knockout mice (Chu et al., 2005; Guan et al., 2007; Tanabe et al., 2009). However, other studies showed that intrathecal injection of L-arginine reduces the mechanical nociception in rats (Jin et al., 2011; Zhuo et al., 1993). Consistent with the antinociceptive role of NO, spinally administered NO donors inhibit the firing activity of spinal dorsal horn neurons, whereas inhibition of spinal NOS increases the background firing rate of these neurons (Hoheisel et al., 2000; Pehl and Schmid, 1997). At the present time, the precise role of NO in regulating nociceptive transmission at the spinal cord level remains to be defined.
Neuronal NOS and NMDAR subunits are highly expressed in the spinal dorsal horn (Chen et al., 2014a; Sardella et al., 2011). Although calcium influx via activation of NMDARs is a key trigger for NO production in the central nervous system (Esplugues, 2002), little is known about how endogenously released NO affects spinal NMDAR activity in neuropathic pain. In the present study, we determined the role of NO in the control of NMDAR activity in the spinal dorsal horn in a rat model of neuropathic pain. We also examined the signaling mechanism involved in regulating spinal NMDAR activity by endogenous NO. Our findings provide new insight into the underlying signaling mechanisms of NO in the inhibition of nociceptive transmission at the spinal cord level in neuropathic pain.
MATERIALS AND METHODS
Animal neuropathic pain model
All surgical preparation and experimental protocols were approved by the Animal Care and Use Committee of The University of Texas MD Anderson Cancer Center and conformed to the National Institutes of Health guidelines for the ethical use of animals. Male Sprague-Dawley rats (10-weeks-old; Harlan, Indianapolis, IN) were used in this study. Spinal nerve ligation (SNL) was used as an experimental model of neuropathic pain in our study (Chen et al., 2014b; Kim and Chung, 1992). In brief, we induced anesthesia with 2–3% isoflurane and then isolated the left L5 and L6 spinal nerves under a surgical microscope. Both nerves were tightly ligated with 5–0 silk suture. Control animals underwent a sham surgical procedure without nerve ligation. In this SNL model, stable pain hypersensitivity is typically developed 10–14 days after SNL and lasts for at least 8 weeks. Final behavioral and electrophysiological recordings were done 3 weeks after surgery.
Implantation of intrathecal catheters
Intrathecal catheters were implanted in rats anesthetized with 2–3% isoflurane. Briefly, a small incision was made at the back of the animal’s neck. A small opening was then made in the atlanto-occipital membrane of the cisterna magna, and a PE-10 catheter (~8.0 cm) was inserted such that the caudal tip reached the lumbar spinal cord level (Chen et al., 2001). The rats were allowed to recover for 5 days after catheter implantation. Animals displaying signs of motor or neurological dysfunction were promptly killed with an overdose of anesthetics.
Nociceptive behavioral tests
To measure the mechanical nociceptive threshold of the hindpaw, the paw pressure (Randall-Selitto) test was used (Chen et al., 2014a; Chen et al., 2001). The test was performed by applying a pressure stimulus to the hindpaw of conscious rats with use of the Ugo Basile Analgesimeter (Varese, Italy). When the animal displayed pain by either paw withdrawal or vocalization, the pedal was immediately released, and the nociceptive threshold was read on the scale. A maximum of 400 g of pressure was used as a cutoff to avoid potential tissue injury to the rats.
To assess heat nociception, rats were placed on the glass surface of a thermal testing apparatus (IITC Life Sciences, Woodland Hills, CA). The temperature of the glass surface was maintained at a constant 30 °C. The rats were first allowed to acclimate inside a chamber placed on the testing apparatus for 30–40 min. A mobile radiant heat source located directly under the glass was focused onto the hindpaw of each rat. After the radiant heat was turned on, the paw withdrawal latency was automatically recorded with a timer. A cut-off of 30 s was used to prevent potential tissue damage (Chen and Pan, 2001). The hindpaw was tested twice to obtain the average.
Spinal cord slice preparation and electrophysiological recordings
The lumbar spinal cord at the L5-L6 level was removed through laminectomy after rats were anesthetized with 2–3% isoflurane. The spinal cord tissues were immediately placed in an ice-cold sucrose artificial cerebrospinal fluid (aCSF) presaturated with 95% O2 and 5% CO2. The aCSF contained (in mM) 234 sucrose, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 12.0 glucose, and 25.0 NaHCO3. The spinal cord tissue was then glued onto the stage of a vibratome, and transverse slices (350 μm) of spinal cords were cut in ice-cold sucrose aCSF and preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34°C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (in mM) 117.0 NaCl, 3.6 KCl, 1.2 MgCl2, 2.5 CaCl2, 1.2 NaH2PO4, 11.0 glucose, and 25.0 NaHCO3. The spinal cord slice was placed in a glass-bottomed chamber and continuously perfused with Krebs solution at 5.0 ml/min at 34°C maintained by an inline solution heater. The spinal lamina II, a translucent region in the superficial dorsal horn, was identified on an upright fixed- stage microscope with differential interference contrast/infrared illumination. Lamina II outer zone neurons were visualized and selected for whole-cell patch-clamp recordings because they are predominantly excitatory interneurons and receive nociceptive input from unmyelinated sensory nerves (Pan and Pan, 2004; Santos et al., 2007).
We used a glass pipette (5–10 MΩ) filled with internal solution containing (in mM) 135.0 potassium gluconate, 5.0 TEA, 2.0 MgCl2, 0.5 CaCl2, 5.0 HEPES, 5.0 EGTA, 5.0 ATP-Mg, 0.5 Na-GTP, and 10 lidocaine N-ethyl bromide (adjusted to pH 7.2–7.4 with 1 M KOH; 290–300 mOsm). NMDAR currents were elicited by puff application of 100 μM NMDA using a positive pressure system (4 psi, 15 ms; Toohey Company, Fairfield, NJ). The tip of the puff pipette was place 150 μm away from the recorded neuron, and the recordings were performed in the extracellular solution containing 0.1 mM Mg2+, 10 μM glycine, and 1 μM tetrodotoxin at a holding potential of −60 mV and using the pipette internal solution containing (in mM) 110.0 Cs2SO4, 2.0 MgCl2, 0.1 CaCl2, 1.1 EGTA, 10.0 HEPES, 2.0 MgATP, and 0.3 Na2GTP (pH was adjusted to 7.25 with 1.0 M CsOH; 280–300 mOsm). The low concentration of Mg2+ in the extracellular solution was used to minimize the Mg2+ block of NMDARs at negative holding potentials (Chen et al., 2014a; Chen et al., 2014b). The input resistance was monitored, and the recording was abandoned if it changed more than 15%. All signals were recorded with use of an amplifier (MultiClamp700B; Axon Instruments Inc., Union City, CA), filtered at 1–2 kHz, and digitized at 10 kHz. In electrophysiological experiments, only one neuron was recorded from each slice, and 4–5 rats were used for each protocol.
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin -1-one (ODQ) and S-nitroso-N-acetylpenicillamine (SNAP) were obtained from Ascent Scientific (Princeton, NJ). 8-Bromo-cGMP and 1,2-trifluoromethylphenyl imidazole (TRIM) were purchased from Tocris Bioscience (Ellisville, MO). TRIM and ODQ stock solutions were first dissolved in dimethyl sulfoxide and were then diluted in artificial cerebrospinal fluid to the final concentration. Tetrodotoxin was purchased from Alomone Laboratories (Jerusalem, Israel).
siRNA knockdown of nNOS
For intrathecal injection, siRNA was first mixed with iFect to a final concentration 400 mg/l (Laumet et al., 2015). The nNOS-specific siRNA (4 μg; siRNA ID, SASI_Rn01_00047014, sequence start 535) and the universal negative control siRNA (Sigma, St. Louis, MO) were administered intrathecally for 4 consecutive days in rats. Twenty-four h after the last injection, the dorsal spinal cords at L5 and L6 levels were removed and used for real-time PCR and Western blotting analyses.
Quantitative PCR
Rats were deeply anesthetized with sodium pentobarbital (60 mg/kg, i.p.) and dorsal spinal cord tissues at the L5 and L6 levels were then rapidly removed. Total RNA was extracted from the tissue using the Trizol/chloroform and treated with DNase I (Invitrogen, Carlsbad, CA). cDNA was prepared by using the Superscript III first-strand synthesis kit and treated by RNase H (Invitrogen). Quantitative PCR was performed using the iQ5 real-time PCR detection system with the SYBR green PCR mix (Bioline, Taunton, MA). The primer sequence used for rat nNOS (Nos1) corresponded to 5′-GGC ACT GGC ATC GCA CCC TT-3′ (sense, base pairs 4096 to 4115) and 5′-CTT TGG CCT GTC CGG TTC CC-3′ (antisense, base pairs 4308 to 4289). The primer pairs for rat eNOS (Nos3) corresponded to 5′-CTG CTG CCC GAG ATA TCT TC-3′ (sense, base pairs 255 to 274) and 5′-CAG GTA CTG CAG TCC CTC CT-3′ (antisense, base pairs 482 to 465). The relative mRNA amount of target genes in each sample was first normalized to the level of a housekeeping gene, Gapdh (forward 5′-TGA TTC TAC CCA CGG CAA GTT-3′; reverse 5′-TGA TGG GTT TCC CAT TGA TGA-3′) and then normalized to its expression level in control siRNA-treated rats. The PCR product specificity was verified by melting-curve analysis and agarose gel electrophoresis.
Western immunoblotting
Dorsal spinal cord tissues at the L5 and L6 levels were removed, dissected, and homogenized in 300 μl RIPA buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% Na-deoxycholate, 150 mM NaCl, 1 mM EDTA, 1 mM Na3VO4, and 1 mM NaF in the presence of the protease inhibitor cocktail. Samples were then put on ice for 30 min with shaking. Lysates were centrifuged at 13,000 g for 30 min at 4°C and the supernatant was collected. The protein concentration was quantified using a DC protein assay kit (Bio-Rad, Hercules, CA). Thirty μg of total proteins of each sample was loaded and separated on 4–12% Bis-Tris SDS-PAGE (Invitrogen). The resolved proteins were transferred to nitrocellulose membranes. The membranes were treated with 5% bovine serum albumin in Tris buffer containing Tween 20 for 2 h and then incubated with a rabbit anti-nNOS antibody (Cat. #07-571-I, EMD Millipore) overnight at 4°C. The membrane was washed three times and then incubated with horseradish peroxidase–conjugated goat anti-rabbit secondary antibody (Jackson ImmunoResearch, West Grove, PA) for 1 h at room temperature. The protein band was revealed with an ECL Plus detection kit (ThermoFisher, Rockfort, IL), and the protein band intensity was quantified by using the ImageJ software program. The amounts of proteins were normalized by GAPDH (Cell Signaling Technology), which was used as a protein loading control.
Statistical analysis
Data are presented in means ± s.e.m. The amplitude of NMDAR currents in spinal dorsal horn neurons was analyzed with Clampfit 9.2 (Axon Instruments). Two-tailed Student’s t-test was used to determine the effect of siRNA treatment on the mRNA and protein levels in the spinal cord. We used one-way analysis of variance (with Dunnett’s or Tukey’s post hoc test) to compare the treatment effects on NMDAR currents and nociceptive withdrawal thresholds. The level of significance was set at P < 0.05.
RESULTS
Endogenous NO inhibits NMDAR activity of spinal dorsal horn neurons in sham and SNL rats
To determine how endogenous NO affects NMDAR currents of dorsal horn neurons, we tested the effect of the NO precursor L-arginine on currents elicited by puff application of 100 μM NMDA directly onto the recorded lamina II neuron. In 13 neurons from sham control rats, bath application of L-arginine at 300 μM for 5–6 min caused a rapid and readily reversible inhibition of whole-cell NMDAR currents (Fig. 1). As reported previously (Chen et al., 2014b; Li et al., 2016), the amplitude of currents of spinal dorsal horn neurons elicited by puff NMDA was much larger in SNL than in sham control rats. L-arginine produced a similar inhibitory effect on NMDAR currents of 12 dorsal horn neurons recorded from SNL rats (Fig. 1).
Figure 1. Endogenous NO or NO donor inhibits NMDAR activity of spinal dorsal horn neurons in sham control and SNL rats.
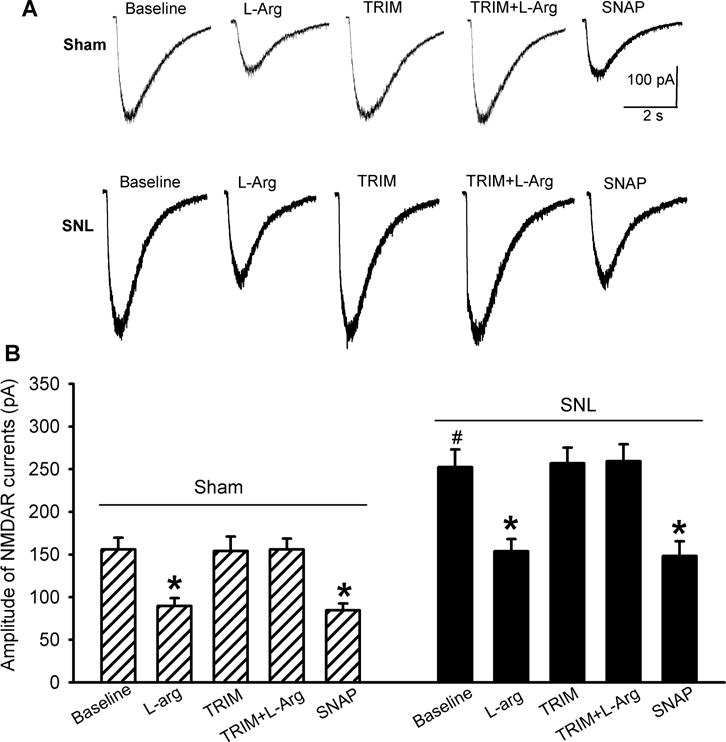
Representative recording traces (A) and mean effects (B) of 300 :M L-arginine, 100 :M TRIM, TRIM plus L-arginine, and 100 :M SNAP on currents elicited by puff application of 100 :M NMDA onto spinal dorsal horn neurons in rats that had undergone sham surgery (n = 13 neurons) or SNL (n = 12 neurons) 3 weeks ago. Data are means ∀ s.e.m. *P < 0.05 (versus respective baseline controls). #P < 0.05 (versus baseline control in the sham group). One-way ANOVA analysis followed by Tukey’s post hoc test.
In these neurons, bath application of TRIM (100 μM), a nNOS-preferring inhibitor (Handy et al., 1996; Jin et al., 2011; Li et al., 2004), alone had no significant effect on the amplitude of puff NMDA-elicited currents. In the presence of TRIM, L-arginine failed to reduce the amplitude of puff NMDA currents in dorsal horn neurons from sham and SNL rats (Fig. 1). However, bath application of an NO donor, SNAP (100 μM) (Jin et al., 2011; Zhou et al., 2015), still significantly reduced puff NMDA-elicited currents (Fig. 1).
NO inhibits NMDAR activity of dorsal horn neurons independent of sGC-cGMP signaling
Both nNOS and soluble guanylyl cyclase (sGC) are present in the superficial dorsal horn (Ding and Weinberg, 2006; Terenghi et al., 1993). sGC-cGMP signaling mediates some of the NO effects in the central nervous system (Calabrese et al., 2007; Li et al., 2004). To determine whether cGMP-mediated signaling is involved in the inhibition of NMDAR activity by NO, we tested the effect of a membrane-permeable cGMP analogue, 8-bromo-cGMP (Li et al., 2004; Sekhar et al., 1992), on currents elicited by puff application of 100 μM NMDA directly onto the dorsal horn neuron. In dorsal horn neurons from both sham and SNL rats, bath application of 60 μM 8-bromo-cGMP had no significant effect on the amplitude of NMDAR currents (n = 12 neurons per group, Fig. 2).
Figure 2. NO inhibits NMDAR activity in spinal dorsal horn neurons independent of sGC-cGMP signaling.
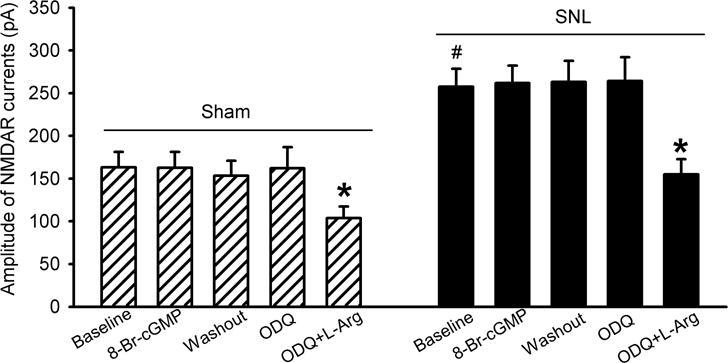
Summary data show the mean effects of 60 :M 8-bromo-cGMP (8-Br-cGMP), 10 :M ODQ, and ODQ plus 300 :M L-arginine on currents elicited by puff application of 100 :M NMDA onto spinal dorsal horn neurons in rats that had undergone sham surgery or SNL 3 weeks ago (n = 12 neurons per group). Data are means ∀ s.e.m. *P < 0.05 (versus respective baseline controls). #P < 0.05 (versus baseline control in the sham group). One-way ANOVA analysis followed by Tukey’s post hoc test.
To determine if endogenous NO inhibits NMDAR activity through sGC, a specific sGC inhibitor, ODQ (Li et al., 2004; Zhao et al., 2000), was used. Bath application of 10 μM ODQ alone for 6 min had no effect on puff NMDA currents in dorsal horn neurons. In the presence of ODQ, subsequent application of 300 μM L-arginine still significantly reduced NMDAR currents of dorsal horn neurons recorded from both sham and SNL rats (n = 12 neurons per group, Fig. 2).
Endogenous NO inhibits NMDAR activity of dorsal horn neurons through S-nitrosylation
We have shown that NO inhibits voltage-activated calcium channels through S-nitrosylation (Jin et al., 2011; Zhou et al., 2015). In a cell line expressing NMDARs, S-nitrosylation is involved in the inhibition of NMDAR activity by NO (Choi et al., 2000). We thus determined whether S-nitrosylation plays a role in the inhibitory effect of NO on NMDAR activity of dorsal horn neurons. N-ethylmaleimide (NEM), a specific alkylating agent of cysteine sulfhydryls, covalently modifies protein sulfhydryl groups thereby preventing subsequent S-nitrosylation of proteins (Bolotina et al., 1994; Broillet and Firestein, 1996). Bath application of 100 μM NEM alone did not significantly change the amplitude of puff NMDA currents in dorsal horn neurons recorded from sham and SNL rats. In the presence of NEM, L-arginine failed to significantly change the amplitude of puff NMDA currents in these neurons (n = 12 neurons per group, Fig. 3).
Figure 3. Endogenous NO inhibits NMDAR activity in spinal dorsal horn neurons through S-nitrosylation.
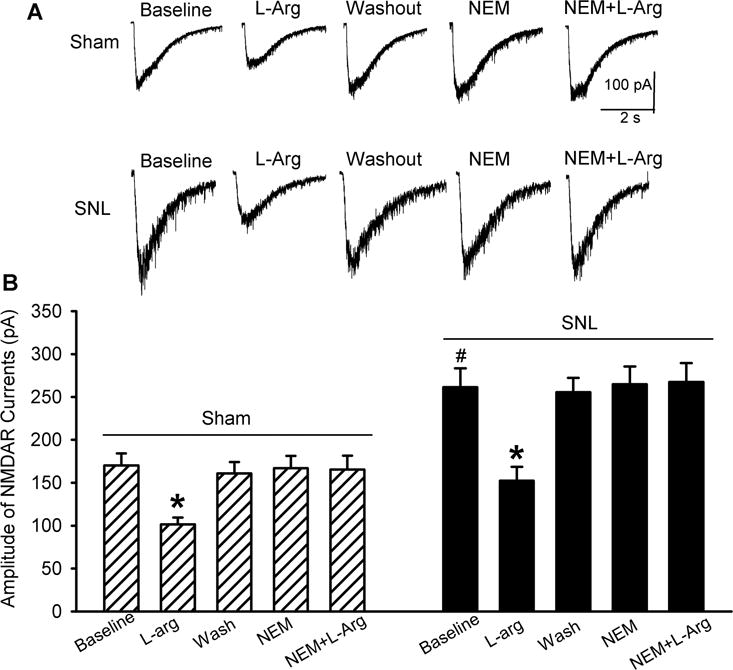
Original current traces (A) and mean effects (B) of 300 :M L-arginine, 100 :M NEM, and NEM plus L-arginine on currents elicited by puff application of 100 :M NMDA onto spinal dorsal horn neurons in rats that had undergone sham surgery or SNL 3 weeks ago (n = 12 neurons per group). Data are means ∀ s.e.m. *P < 0.05 (versus respective baseline controls). #P < 0.05 (versus baseline control in the sham group). One-way ANOVA analysis followed by Tukey’s post hoc test.
NO derived from nNOS inhibits NMDAR activity of dorsal horn neurons in sham and SNL rats
Because there is no highly specific agents for nNOS inhibition, we used siRNA to specifically knock down nNOS in the spinal cord. The nNOS-specific siRNA (4 μg/day) and the control siRNA were administered intrathecally for 4 consecutive days. Twenty-four h after the last injection, the dorsal spinal cord at L5 and L6 levels were removed and used for quantitative PCR and Western blot analyses. Treatment with nNOS-specific siRNA in sham and SNL rats induced a large reduction in the mRNA level of nNOS, but not eNOS, in the dorsal spinal cord (Fig. 4A,B). Also, treatment with nNOS-specific siRNA significantly reduced the protein level of nNOS in the dorsal spinal cord obtained from sham and SNL rats (Fig. 4C). Notably, intrathecal injection of nNOS-specific siRNA, but not control siRNA, over 4 days gradually reduced the mechanical nociceptive threshold in both sham and SNL rats (n = 8 rats per group, Fig. 4D).
Figure 4. Tonic nociceptive inhibition by nNOS at the spinal cord level in sham and SNL rats.
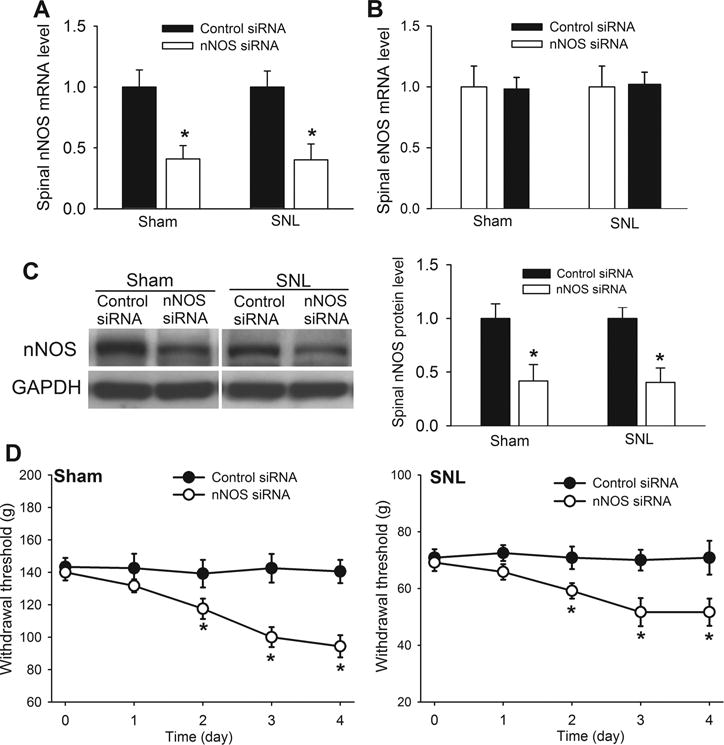
(A,B) Mean effects of intrathecal treatment with nNOS-specific siRNA (4 :g/day for 4 days) on the mRNA level of nNOS and eNOS in the dorsal spinal cord of sham control rats and SNL rats (n = 8 rats in each group). Data are presented as means ∀ s.e.m. *P < 0.05 (versus control siRNA-treated rats). Two-tailed Student’s t-test. (C) Western blot analysis and quantification of the nNOS protein level (~160 kDa) in the dorsal spinal cord of sham control rats and SNL rats (n = 6 rats in each group). GAPDH was used as a loading control. Data are means ∀ s.e.m. *P < 0.05 (versus control siRNA-treated rats). Two-tailed Student’s t-test. (D) Time course of the effects of intrathecal treatment with control siRNA or nNOS-specific siRNA (4 :g/day for 4 days) on the mechanical nociceptive withdrawal thresholds of sham and SNL rats (n = 8 rats in each group). Data are means ∀ s.e.m. *P < 0.05 (versus baseline at time 0). One-way ANOVA analysis followed by Dunnett’s post hoc test.
In spinal dorsal horn neurons from sham (n = 15 neurons) and SNL (n = 13 neurons) rats treated with control siRNA, bath application of 300 μM L-arginine for 5–6 min induced a large reduction in puff NMDA-elicited currents (Fig. 5A,B). However, L-arginine failed to reduce the amplitude of puff NMDA-elicited currents in dorsal horn neurons recorded from sham (n = 13 neurons) or SNL (n = 12 neurons) rats treated with nNOS-specific siRNA (Fig. 5C,D). Nevertheless, bath application of SNAP (100 μM) still significantly reduced the amplitude of puff NMDA-elicited currents in these neurons (Fig. 5C,D).
Figure 5. Endogenous NO derived from nNOS inhibits NMDAR activity of spinal dorsal horn neurons in sham and SNL rats.
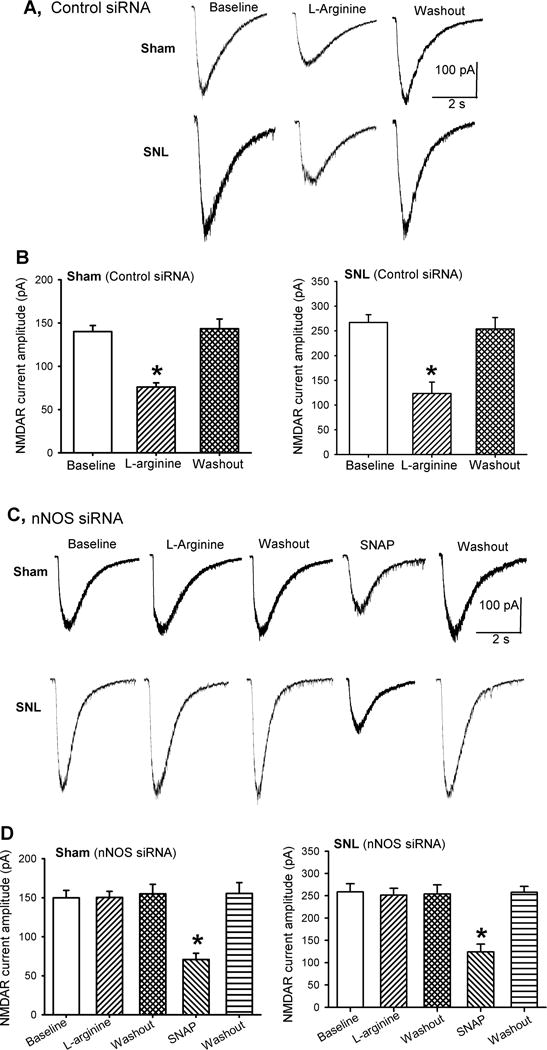
(A,B) Representative traces (A) and mean effects (B) of 300 :M L-arginine and 100 :M SNAP on currents elicited by puff application of 100 :M NMDA onto spinal dorsal horn neurons in sham (n = 15 neurons) and SNL (n = 13 neurons) rats treated with control siRNA. (C,D) Original recording traces (C) and mean effects (D) of 300 :M L-arginine and 100 :M SNAP on puff NMDA currents of spinal dorsal horn neurons in sham (n = 13 neurons) and SNL (n = 12 neurons) rats treated with nNOS-specific siRNA. Data are means ∀ s.e.m. *P < 0.05 (versus respective baseline controls). One-way ANOVA analysis followed by Dunnett’s post hoc test.
NO derived from nNOS at the spinal cord level tonically inhibits nociception in sham and SNL rats
We next determined whether NO generated from nNOS plays a role in regulating nociception at the spinal cord level. We did not use von Frey filaments for testing mechanical sensitivity in this particular study because (1) they produce a non-nociceptive (tactile) stimulus in sham control rats and (2) the von Frey tactile threshold in SNL rats is very low (generally < 2 g), which is difficulty to detect a further decrease in the tactile threshold produced by the NOS inhibitor or siRNA. Intrathecal injection of 300 μg L-arginine significantly increased the paw withdrawal threshold in response to a noxious pressure or heat stimulus in sham rats (n = 9 rats, Fig. 6A,B). Intrathecal pretreatment with 50 μg TRIM blocked the effect of L-arginine on the mechanical and thermal nociceptive threshold (Fig. 6A,B). In 11 SNL rats, intrathecal injection of L-arginine also significantly reversed the decrease in the paw pressure and thermal withdrawal thresholds caused by nerve injury. However, in SNL rats pretreated with TRIM, subsequent intrathecal injection of L-arginine produced no significant effect on the nociceptive withdrawal threshold (n = 10 rats, Fig. 6A,B). Notably, intrathecal injection of 50 μg TRIM alone significantly reduced the pressure and thermal withdrawal thresholds in both sham and SNL rats (Fig. 6A,B).
Figure 6. Endogenous NO derived from nNOS at the spinal level reduces mechanical and thermal nociception in sham and SNL rats.
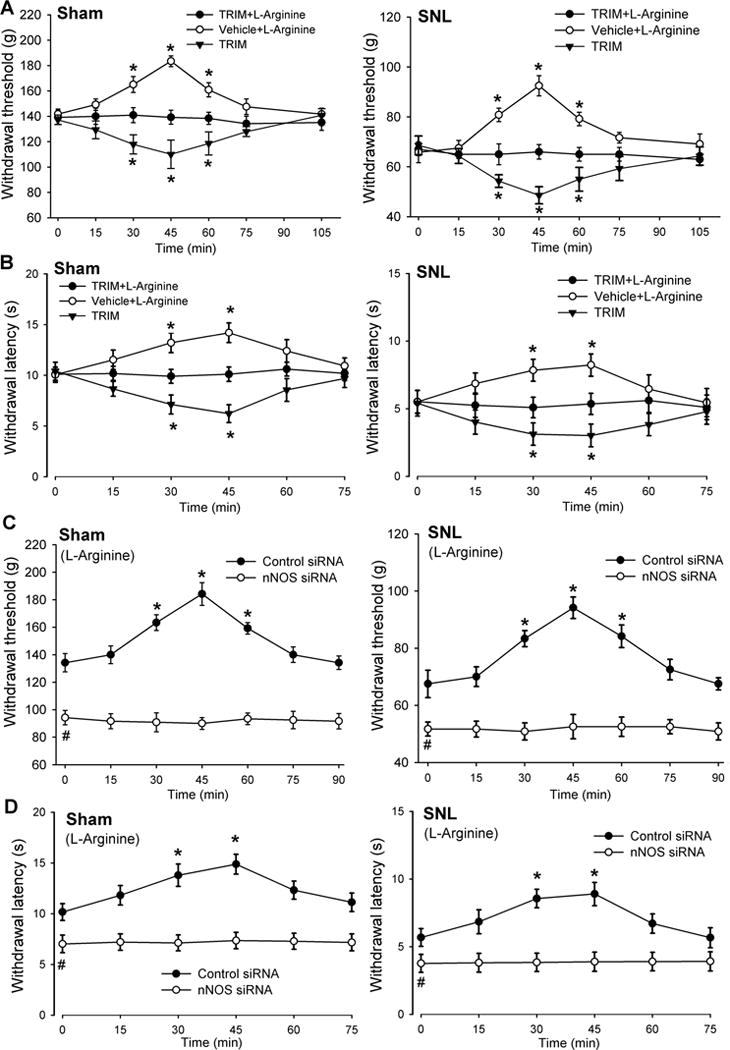
(A,B) Time course of the effects of intrathecal injection of 300 :g L-arginine with vehicle or 50 :g TRIM and TRIM alone on the paw withdrawal threshold in response to a noxious pressure or heat stimulus in sham control (n = 9 rats per group) and SNL (n = 10 rats per group) rats. (C,D) Time course of the effects of intrathecal injection of 300 :g L-arginine on the mechanical and thermal nociceptive withdrawal thresholds in sham and SNL rats treated with control siRNA (n = 11 rats per group) or nNOS-specific siRNA (n = 12 rats per group). Data are means ∀ s.e.m. *P < 0.05 compared with the baseline control (time 0). #P < 0.05 (versus baseline control in the control siRNA group). One-way ANOVA analysis followed by Dunnett’s post hoc test.
We then used the siRNA approach to validate the findings with the nNOS inhibitor. Intrathecal treatment with nNOS-specific siRNA, but not control siRNA, significantly reduced the mechanical and thermal nociceptive thresholds in both sham and SNL rats (Fig. 6C,D). Furthermore, in both sham and SNL rats pretreated with control siRNA, intrathecal injection of 300 μg L-arginine significantly increased the nociceptive withdrawal thresholds (n = 11 rats per group, Fig. 6C,D). In contrast, intrathecal injection of L-arginine had no significant effect on the mechanical and thermal nociceptive thresholds in sham or SNL rats pretreated with nNOS-specific siRNA (n = 12 rats per group, Fig. 6C,D).
DISCUSSION
The major finding of our study is that NO derived from nNOS inhibits NMDAR activity in the spinal dorsal horn in both control and nerve-injured rats. We showed that the NO precursor L-arginine and the NO donor SNAP markedly suppressed the activity of NMDARs in spinal dorsal horn neurons in sham and SNL rats. Furthermore, we found that inhibition of nNOS with TRIM or knockdown of nNOS with siRNA abolished the inhibitory effect of L-arginine on spinal NMDARs in sham and SNL rats. In this study, we used NMDA puff application, but not synaptically-evoked responses, because NO inhibits voltage-activated calcium channels (Jin et al., 2011; Zhou et al., 2015), which can attenuate presynaptic glutamate release and the amplitude of evoked excitatory postsynaptic currents. It has been reported that exogenously applied NO can inhibit NMDAR currents in recombinant systems (Lei et al., 1992). Also, NO donors inhibit NMDA-elicited currents in cultured cortical neurons (Aizenman and Potthoff, 1999) and striatal neurons (Manzoni et al., 1992). In addition, SNAP reduces NMDAR activity of spinal dorsal horn neurons from naive control rats (Nicholson et al., 2004). In the central nervous system, nNOS is located at the postsynaptic density and is closely linked to NMDARs (Christopherson et al., 1999; Valtschanoff and Weinberg, 2001). Peripheral nerve injury causes a large increase in synaptic NMDAR activity of spinal dorsal horn neurons (Chen et al., 2014b; Li et al., 2016). Because increased NO synthesis and release often occur in the same neurons after NMDAR activation, endogenous NO may be involved in negative feedback inhibition of NMDARs and NMDAR-mediated nociceptive transmission in the spinal dorsal horn under physiological and neuropathic pain conditions.
Our study provides further evidence that endogenous NO inhibits NMDAR activity of spinal dorsal horn neurons primarily through S-nitrosylation. The NO signaling is commonly mediated through cGMP upon activation of NO-sensitive sGC, S-nitrosylation, and the interaction with superoxide to form peroxynitrite (Choi et al., 2000; Gow et al., 2004; Jin et al., 2011; Li et al., 2004; Rudkouskaya et al., 2010). We observed that inhibition of sGC with ODQ did not significantly alter the inhibitory effect of L-arginine on NMDAR activity of dorsal horn neurons. Also, 8-bromo-cGMP had no effect on spinal NMDAR activity in control and SNL rats. Thus, sGC-cGMP signaling is not involved in the inhibition of NMDARs by endogenous NO in the spinal dorsal horn. Consistent with our findings, it has been shown that the inhibitory effect of NO donors is not through cGMP in NMDAR-expressing cell lines (Lei et al., 1992). In cell lines expressing NMDARs, NO inhibits NMDAR activity via S-nitrosylation by reacting with thiol groups on GluN1 and GluN2 subunits (Choi et al., 2000). Hence, endogenous NO inhibits the activity of NMDARs in the spinal cord predominantly through covalent modification of thiol residues (i.e., S-nitrosylation). NEM covalently modifies sulfhydryl groups and prevents S-nitrosylation (Broillet and Firestein, 1996; Jin et al., 2011). We showed in this study that NEM abolished the inhibitory effects of L-arginine on NMDAR currents of spinal dorsal horn neurons in both sham and SNL rats. Interestingly, we showed that endogenous NO similarly inhibited NMDAR activity in both sham and SNL rats. In the SNL model, the increased NMDAR activity of spinal dorsal horn neurons is largely caused by potentiated CK2 activity (Chen et al., 2014b). Our data suggest that the NO effect in vivo is independent of NMDAR phosphorylation by CK2 and is likely through direct S-nitrosylation of NMDARs.
Previous studies report that NO is either pronociceptive or antinociceptive in various animal models (Chu et al., 2005; Li and Qi, 2010; Sousa and Prado, 2001; Tanabe et al., 2009; Zhuo et al., 1993). The discrepancy regarding the divergent role of NO in nociceptive processing may result from the use of different animal models of pain and/or the local levels of NO generated in various studies. For example, although intrathecal injection of low doses of L-arginine and NO donors reduce nociception, L-arginine and NO donors at the high doses increase painful responses to tissue and nerve damage (Li and Qi, 2010; Sousa and Prado, 2001). High concentrations of NO also stimulate TRPV1 and TRPA1 receptors (Miyamoto et al., 2009). Furthermore, many NOS inhibitors, such as L-NAME, inhibit acetylcholine muscarinic receptors (Buxton et al., 1993), which are tonically involved in the regulation of spinal nociceptive transmission (Cai et al., 2009; Chen et al., 2001; Zhuo and Gebhart, 1991). In addition, there is an increase in the expression of other NOS isoforms in the spinal cord in eNOS-, nNOS-, or iNOS-knockout mice (Boettger et al., 2007; Tao et al., 2003). This compensatory upregulation of other NOS subtypes in specific NOS isoform-knockout mice further confounds the interpretation of the results. In the present study, we found that intrathecal administration of L-arginine markedly increased the mechanical and thermal nociceptive thresholds in sham and SNL rats. Furthermore, pretreatment with TRIM, a nNOS inhibitor, or siRNA specifically targeting nNOS blocked the antinociceptive effect of L-arginine. Notably, treatment with nNOS-specific siRNA at the spinal cord level or intrathecal injection of TRIM alone reduced the nociceptive threshold in both sham and SNL rats, suggesting that endogenous NO derived from nNOS tonically modulates spinal nociceptive transmission under control and neuropathic pain conditions. The lack of tonic inhibitory effect of NO in electrophysiological recordings is uncertain. Thin tissue slices used in the in vitro recordings can cause the removal of normal synaptic input and/or reduced tonic neuronal activity, which likely result in diminished levels of endogenous NO and NO precursors. We have shown that NO also inhibits voltage-activated calcium channels present in primary afferent terminals through S-nitrosylation (Jin et al., 2011; Zhou et al., 2015). Thus, endogenous NO may reduce spinal nociceptive transmission through inhibition of both NMDARs and voltage-activated calcium channels.
In summary, our study provides new information that endogenous NO inhibits the activity of spinal NMDARs through S-nitrosylation. Our findings support the notion that NO functions as a negative feedback regulator of NMDARs and nociceptive transmission at the spinal cord level under physiological and neuropathic pain conditions. Interventions that promote endogenous NO production and release in the spinal dorsal horn may alleviate chronic neuropathic pain.
Highlights.
-
$
Nitric oxide (NO) affects synaptic transmission but its role in neuropathic pain is unclear.
-
$
Endogenous NO inhibits NMDA receptor activity in the spinal dorsal horn through S-nitrosylation.
-
$
NO reduces spinal cord NMDA receptor activity and pain hypersensitivity caused by nerve injury.
-
$
Understanding how NO controls pain transmission may improve treatments for neuropathic pain.
Acknowledgments
This work was supported by the National Institutes of Health (grant R01 GM120844) and the N.G. and Helen T. Hawkins Endowment to H.-L.P.
List of abbreviations
- NO
nitric oxide
- nNOS
neuronal nitric oxide synthase
- NMDAR
N-methyl-D-aspartate receptor
- ODQ
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
- sGC
soluble guanylyl cyclase
- SNL
spinal nerve ligation
- SNAP
S-nitroso-N-acetylpenicillamine
- TRIM
1,2-trifluoromethylphenyl imidazole
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST
The authors do not have any conflict of interest.
References
- Aizenman E, Potthoff WK. Lack of interaction between nitric oxide and the redox modulatory site of the NMDA receptor. BrJ Pharmacol. 1999;126:296–300. doi: 10.1038/sj.bjp.0702295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alderton WK, Cooper CE, Knowles RG. Nitric oxide synthases: structure, function and inhibition. Biochem J. 2001;357:593–615. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettger MK, Uceyler N, Zelenka M, Schmitt A, Reif A, Chen Y, Sommer C. Differences in inflammatory pain in nNOS-, iNOS- and eNOS-deficient mice. Eur J Pain. 2007;11:810–818. doi: 10.1016/j.ejpain.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. doi: 10.1038/368850a0. [DOI] [PubMed] [Google Scholar]
- Broillet MC, Firestein S. Direct activation of the olfactory cyclic nucleotide-gated channel through modification of sulfhydryl groups by NO compounds. Neuron. 1996;16:377–385. doi: 10.1016/s0896-6273(00)80055-0. [DOI] [PubMed] [Google Scholar]
- Buxton IL, Cheek DJ, Eckman D, Westfall DP, Sanders KM, Keef KD. NG-nitro L-arginine methyl ester and other alkyl esters of arginine are muscarinic receptor antagonists. Circ Res. 1993;72:387–395. doi: 10.1161/01.res.72.2.387. [DOI] [PubMed] [Google Scholar]
- Cai YQ, Chen SR, Han HD, Sood AK, Lopez-Berestein G, Pan HL. Role of M2, M3, and M4 muscarinic receptor subtypes in the spinal cholinergic control of nociception revealed using siRNA in rats. J Neurochem. 2009;111:1000–1010. doi: 10.1111/j.1471-4159.2009.06396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese V, Mancuso C, Calvani M, Rizzarelli E, Butterfield DA, Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- Chen SR, Hu YM, Chen H, Pan HL. Calcineurin inhibitor induces pain hypersensitivity by potentiating pre-and postsynaptic NMDA receptor activity in spinal cords. J Physiol. 2014a;592:215–227. doi: 10.1113/jphysiol.2013.263814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SR, Khan GM, Pan HL. Antiallodynic effect of intrathecal neostigmine is mediated by spinal nitric oxide in a rat model of diabetic neuropathic pain. Anesthesiology. 2001;95:1007–1012. doi: 10.1097/00000542-200110000-00033. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HL. Spinal endogenous acetylcholine contributes to the analgesic effect of systemic morphine in rats. Anesthesiology. 2001;95:525–530. doi: 10.1097/00000542-200108000-00039. [DOI] [PubMed] [Google Scholar]
- Chen SR, Zhou HY, Byun HS, Chen H, Pan HL. Casein kinase II regulates N-methyl-D-aspartate receptor activity in spinal cords and pain hypersensitivity induced by nerve injury. J Pharmacol Exp Ther. 2014b;350:301–312. doi: 10.1124/jpet.114.215855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi YB, Tenneti L, Le DA, Ortiz J, Bai G, Chen HS, Lipton SA. Molecular basis of NMDA receptor-coupled ion channel modulation by S-nitrosylation. Nat Neurosci. 2000;3:15–21. doi: 10.1038/71090. [DOI] [PubMed] [Google Scholar]
- Christopherson KS, Hillier BJ, Lim WA, Bredt DS. PSD-95 assembles a ternary complex with the N-methyl-D-aspartic acid receptor and a bivalent neuronal NO synthase PDZ domain. J Biol Chem. 1999;274:27467–27473. doi: 10.1074/jbc.274.39.27467. [DOI] [PubMed] [Google Scholar]
- Chu YC, Guan Y, Skinner J, Raja SN, Johns RA, Tao YX. Effect of genetic knockout or pharmacologic inhibition of neuronal nitric oxide synthase on complete Freund’s adjuvant-induced persistent pain. Pain. 2005;119:113–123. doi: 10.1016/j.pain.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Ding JD, Weinberg RJ. Localization of soluble guanylyl cyclase in the superficial dorsal horn. J Comp Neurol. 2006;495:668–678. doi: 10.1002/cne.20901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esplugues JV. NO as a signalling molecule in the nervous system. British journal of pharmacology. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gow AJ, Farkouh CR, Munson DA, Posencheg MA, Ischiropoulos H. Biological significance of nitric oxide-mediated protein modifications. Am J Physiol Lung Cell Mol Physiol. 2004;287:L262–268. doi: 10.1152/ajplung.00295.2003. [DOI] [PubMed] [Google Scholar]
- Guan Y, Yaster M, Raja SN, Tao YX. Genetic knockout and pharmacologic inhibition of neuronal nitric oxide synthase attenuate nerve injury-induced mechanical hypersensitivity in mice. Mol Pain. 2007;3:29. doi: 10.1186/1744-8069-3-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handy RL, Harb HL, Wallace P, Gaffen Z, Whitehead KJ, Moore PK. Inhibition of nitric oxide synthase by 1-(2-trifluoromethylphenyl) imidazole (TRIM) in vitro: antinociceptive and cardiovascular effects. British journal of pharmacology. 1996;119:423–431. doi: 10.1111/j.1476-5381.1996.tb16003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoheisel U, Unger T, Mense S. A block of spinal nitric oxide synthesis leads to increased background activity predominantly in nociceptive dorsal horn neurones in the rat. Pain. 2000;88:249–257. doi: 10.1016/S0304-3959(00)00336-5. [DOI] [PubMed] [Google Scholar]
- Infante C, Diaz M, Hernandez A, Constandil L, Pelissier T. Expression of nitric oxide synthase isoforms in the dorsal horn of monoarthritic rats: effects of competitive and uncompetitive N-methyl-D-aspartate antagonists. Arthritis Res Ther. 2007;9:R53. doi: 10.1186/ar2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaev D, Gerber G, Park SK, Chung JM, Randik M. Facilitation of NMDA-induced currents and Ca2+ transients in the rat substantia gelatinosa neurons after ligation of L5-L6 spinal nerves. Neuroreport. 2000;11:4055–4061. doi: 10.1097/00001756-200012180-00030. [DOI] [PubMed] [Google Scholar]
- Jin XG, Chen SR, Cao XH, Li L, Pan HL. Nitric oxide inhibits nociceptive transmission by differentially regulating glutamate and glycine release to spinal dorsal horn neurons. J Biol Chem. 2011;286:33190–33202. doi: 10.1074/jbc.M111.270967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Laumet G, Garriga J, Chen SR, Zhang Y, Li DP, Smith TM, Dong Y, Jelinek J, Cesaroni M, Issa JP, Pan HL. G9a is essential for epigenetic silencing of K(+) channel genes in acute-to-chronic pain transition. Nat Neurosci. 2015;18:1746–1755. doi: 10.1038/nn.4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei SZ, Pan ZH, Aggarwal SK, Chen HS, Hartman J, Sucher NJ, Lipton SA. Effect of nitric oxide production on the redox modulatory site of the NMDA receptor-channel complex. Neuron. 1992;8:1087–1099. doi: 10.1016/0896-6273(92)90130-6. [DOI] [PubMed] [Google Scholar]
- Li DP, Chen SR, Finnegan TF, Pan HL. Signalling pathway of nitric oxide in synaptic GABA release in the rat paraventricular nucleus. J Physiol. 2004;554:100–110. doi: 10.1113/jphysiol.2003.053371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li K, Qi WX. Effects of multiple intrathecal administration of L-arginine with different doses on formalin-induced nociceptive behavioral responses in rats. Neurosci Bull. 2010;26:211–218. doi: 10.1007/s12264-010-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Chen SR, Chen H, Wen L, Hittelman WN, Xie JD, Pan HL. Chloride Homeostasis Critically Regulates Synaptic NMDA Receptor Activity in Neuropathic Pain. Cell Rep. 2016;15:1376–1383. doi: 10.1016/j.celrep.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton SA, Singel DJ, Stamler JS. Nitric oxide in the central nervous system. Progress in brain research. 1994;103:359–364. doi: 10.1016/s0079-6123(08)61149-8. [DOI] [PubMed] [Google Scholar]
- Manzoni O, Prezeau L, Marin P, Desagher S, Bockaert J, Fagni L. Nitric oxide-induced blockade of NMDA receptors. Neuron. 1992;8:653–662. doi: 10.1016/0896-6273(92)90087-t. [DOI] [PubMed] [Google Scholar]
- Marletta MA. Nitric oxide synthase structure and mechanism. J Biol Chem. 1993;268:12231–12234. [PubMed] [Google Scholar]
- Miyamoto T, Dubin AE, Petrus MJ, Patapoutian A. TRPV1 and TRPA1 mediate peripheral nitric oxide-induced nociception in mice. PLoS One. 2009;4:e7596. doi: 10.1371/journal.pone.0007596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson R, Spanswick D, Lee K. Nitric oxide inhibits NMDA currents in a subpopulation of substantia gelatinosa neurons of the adult rat spinal cord. Neurosci Lett. 2004;359:180–184. doi: 10.1016/j.neulet.2004.01.057. [DOI] [PubMed] [Google Scholar]
- Pan YZ, Pan HL. Primary afferent stimulation differentially potentiates excitatory and inhibitory inputs to spinal lamina II outer and inner neurons. J Neurophysiol. 2004;91:2413–2421. doi: 10.1152/jn.01242.2003. [DOI] [PubMed] [Google Scholar]
- Pehl U, Schmid HA. Electrophysiological responses of neurons in the rat spinal cord to nitric oxide. Neuroscience. 1997;77:563–573. doi: 10.1016/s0306-4522(96)00495-2. [DOI] [PubMed] [Google Scholar]
- Rudkouskaya A, Sim V, Shah AA, Feustel PJ, Jourd’heuil D, Mongin AA. Long-lasting inhibition of presynaptic metabolism and neurotransmitter release by protein S-nitrosylation. Free Radic Biol Med. 2010;49:757–769. doi: 10.1016/j.freeradbiomed.2010.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SF, Rebelo S, Derkach VA, Safronov BV. Excitatory interneurons dominate sensory processing in the spinal substantia gelatinosa of rat. J Physiol. 2007;581:241–254. doi: 10.1113/jphysiol.2006.126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sardella TC, Polgar E, Watanabe M, Todd AJ. A quantitative study of neuronal nitric oxide synthase expression in laminae I-III of the rat spinal dorsal horn. Neuroscience. 2011;192:708–720. doi: 10.1016/j.neuroscience.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhar KR, Hatchett RJ, Shabb JB, Wolfe L, Francis SH, Wells JN, Jastorff B, Butt E, Chakinala MM, Corbin JD. Relaxation of pig coronary arteries by new and potent cGMP analogs that selectively activate type I alpha, compared with type I beta, cGMP-dependent protein kinase. Mol Pharmacol. 1992;42:103–108. [PubMed] [Google Scholar]
- Sousa AM, Prado WA. The dual effect of a nitric oxide donor in nociception. Brain Res. 2001;897:9–19. doi: 10.1016/s0006-8993(01)01995-3. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ. Structure-function aspects in the nitric oxide synthases. Ann Rev Pharmacol Toxicol. 1997;37:339–359. doi: 10.1146/annurev.pharmtox.37.1.339. [DOI] [PubMed] [Google Scholar]
- Stuehr DJ, Santolini J, Wang ZQ, Wei CC, Adak S. Update on mechanism and catalytic regulation in the NO synthases. J Biol Chem. 2004;279:36167–36170. doi: 10.1074/jbc.R400017200. [DOI] [PubMed] [Google Scholar]
- Tanabe M, Nagatani Y, Saitoh K, Takasu K, Ono H. Pharmacological assessments of nitric oxide synthase isoforms and downstream diversity of NO signaling in the maintenance of thermal and mechanical hypersensitivity after peripheral nerve injury in mice. Neuropharmacology. 2009;56:702–708. doi: 10.1016/j.neuropharm.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Tao F, Tao YX, Mao P, Zhao C, Li D, Liaw WJ, Raja SN, Johns RA. Intact carrageenan-induced thermal hyperalgesia in mice lacking inducible nitric oxide synthase. Neuroscience. 2003;120:847–854. doi: 10.1016/s0306-4522(03)00362-2. [DOI] [PubMed] [Google Scholar]
- Terenghi G, Riveros-Moreno V, Hudson LD, Ibrahim NB, Polak JM. Immunohistochemistry of nitric oxide synthase demonstrates immunoreactive neurons in spinal cord and dorsal root ganglia of man and rat. J Nurolog Sci. 1993;118:34–37. doi: 10.1016/0022-510x(93)90242-q. [DOI] [PubMed] [Google Scholar]
- Valtschanoff JG, Weinberg RJ. Laminar organization of the NMDA receptor complex within the postsynaptic density. J Neurosci. 2001;21:1211–1217. doi: 10.1523/JNEUROSCI.21-04-01211.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol. 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Brandish PE, DiValentin M, Schelvis JP, Babcock GT, Marletta MA. Inhibition of soluble guanylate cyclase by ODQ. Biochemistry. 2000;39:10848–10854. doi: 10.1021/bi9929296. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Chen SR, Byun HS, Chen H, Li L, Han HD, Lopez-Berestein G, Sood AK, Pan HL. N-methyl-D-aspartate receptor- and calpain-mediated proteolytic cleavage of K+-Cl- cotransporter-2 impairs spinal chloride homeostasis in neuropathic pain. J Biol Chem. 2012;287:33853–33864. doi: 10.1074/jbc.M112.395830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou MH, Bavencoffe A, Pan HL. Molecular basis of regulating high voltage-activated calcium channels by S-nitrosylation. J Biol Chem. 2015;290:30616–30623. doi: 10.1074/jbc.M115.685206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M, Gebhart GF. Tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1991;46:211–222. doi: 10.1016/0304-3959(91)90078-C. [DOI] [PubMed] [Google Scholar]
- Zhuo M, Meller ST, Gebhart GF. Endogenous nitric oxide is required for tonic cholinergic inhibition of spinal mechanical transmission. Pain. 1993;54:71–78. doi: 10.1016/0304-3959(93)90101-T. [DOI] [PubMed] [Google Scholar]


