Abstract
Introduction
Pulmonary congestion is a common finding of heart failure (HF), but it remains unclear how pulmonary and heart blood volumes (Vp and Vh) and extravascular lung water (EVLW) change in stable HF and impact lung function.
Methods
Fourteen patients with HF (age: 68±11 y; LVEF: 33±8%) and 12 controls (age: 65±9 y) were recruited. A pulmonary function test, thoracic CT scan, and contrast perfusion scan were performed. From the thoracic scan, a histogram of CT attenuation of lung tissue was generated and skew, kurtosis, and full-width half-max were calculated as surrogates of EVLW. Blood volumes were calculated from the transit time of the contrast through the great vessels of the heart.
Results
Patients with HF had greater Vp and Vh (Vp: 0.55±0.21 l v.s 0.41±0.13 l; Vh: 0.53±0.33 l vs. 0.40±0.15 l) and EVLW (skew: 3.2±0.5 vs. 3.7±0.7; kurtosis: 19.4±6.6 vs. 25.9±9.4; FWHM: 73±13 vs 59±9Hu). Spirometric measures were decreased in HF (%-predicted FVC: 86±17% vs. 104±9%; FEV1: 83±20% vs. 105±11%; FEF25–75: 82±42% vs. 115±43%). Vp was associated with decreased expiratory flows, while EVLW was associated with decreased lung volumes.
Conclusions
Congestion in stable patients with HF includes expanded Vp and increased EVLW associated with reductions in lung volumes and expiratory flows.
Keywords: pulmonary function, computed tomography, thoracic fluid volumes
Introduction
Pulmonary congestion is a common clinical complication of heart failure (HF) as a result of the close functional relationship between the heart and lungs. The failing left ventricle causes altered hemodynamics that lead to accumulation of blood centrally [1]. This accumulated blood may contribute to elevated pulmonary wedge pressure, engorgement of the pulmonary vasculature, bronchial vasculature, and capillaries, and increased transudation of fluid into the interstitial space and increased extravascular lung water (EVLW) [2, 3]. As the lung has the ability to clear fluid efficiently away from the gas exchanging region of the alveoli and prevent the formation of edema, it remains unclear the degree to which EVLW persists in stable disease [4, 5]. Overall, the degree to which these blood volumes and extravascular fluid are affected in stable, chronic disease and contribute to the congestive state is poorly defined.
Pulmonary function deteriorates in HF with significant heterogeneity between individuals. Patients with HF often exhibit changes that are both restrictive, reductions in forced vital capacity (FVC) and total lung capacity (TLC), and obstructive, reductions in peak expiratory flow (PEF) and maximal mid-expiratory flow (FEF25–75) [6–8]. Patients with HF also exhibit changes in lung compliance, altered ventilatory control, and poor exertional tolerance [2, 9, 10]. These functional limitations contribute to the symptoms of dyspnea and are associated with the development of pulmonary congestion.
It has long been understood that the accumulation of fluid in the lungs results in symptoms associated with HF. In patients with HF who have become acutely decompensated, fluid accumulation in both the lung extravascular space and the lymphatics causes symptoms of dyspnea and exercise intolerance that requires in-patient diuretic treatment to return fluid levels to baseline conditions[11, 12]. Constriction of the bronchial vasculature with methoxamine was shown to improve exercise tolerance in subjects with chronic, stable HF highlighting the importance of bronchial circulation in this population [13, 14]. In healthy subjects, rapid fluid loading has been shown to reduce lung volumes and forced expiratory flows, while heart transplant in patients with severe disease, subsequently reducing pulmonary pressures, improves these same measures, suggesting that either thoracic blood volumes or EVLW may contribute to changes in lung function [15, 16]. Additionally, the heart and lungs must compete for space within the thoracic cavity, where cardiomegaly and/or other changes in vascular beds may impinge on the ability of the lungs to expand [17].
Modulation of thoracic fluid volumes affect pulmonary function in HF; however, it is not clear the degree to which pulmonary congestion persists in the stable HF population and how various fluid compartments within the thorax impact lung function. There are a number of compartments that have been implicated as contributors, including the larger pulmonary vessels, the capillaries, the heart, and extravascular space. Some of these central fluid volumes have been shown to be elevated in chronic heart failure, but their link to changes in pulmonary function has not been clearly investigated.
The purpose of the present study was to a) define how various thoracic fluid volumes, specifically the thoracic, pulmonary, and heart blood volumes and EVLW, are impacted in patients with chronic, stable systolic HF and b) determine the relationship between these volumes and changes in lung function in chronic, stable patients with HF. We hypothesized that chronic, stable patients with HF would have increased thoracic, pulmonary, and heart blood volumes and greater levels of EVLW than the control group resulting in decreased pulmonary function. Further, we hypothesized that the pulmonary blood volume would be associated with obstructive changes in lung function, while EVLW would be associated with restrictive changes in lung function.
Methods
Participants
Fourteen patients with a history of HF and 12 age-matched controls recruited for this study and characteristics are reported in Table 1. Patients had a minimum of 1 year of disease history and on stable medication for at least one month, left ventricular ejection fraction (LVEF) of less than 40%, a body mass index (BMI) of less than 35 kg·m−2, and no history of pulmonary or renal disease. Subjects had a range of clinical severity with New York Heart Association (NYHA) functional class of I, II, and III. Control subjects had no history of cardiovascular, respiratory or metabolic disease and a BMI of less than 35 kg·m−2. The study protocol obtained Mayo Clinic institutional review board approval and informed written consent was obtained from subjects prior to participation.
Table 1.
Participant characteristics in control and heart failure groups. Subjects were well matched for age, but the patients with HF were taller and heavier than the control subjects. No differences were observed between mean arterial pressure, hemoglobin, or estimated glomerular filtration rate (eGFR) but patients with HF had greater levels of NT-proBNP. Data are mean plus or minus standard error of the mean.
| Control | Heart Failure | |
|---|---|---|
| N (female) | 12 (4) | 14 (2) |
| Age, y | 65.1 ± 2.6 | 67.6 ± 3.0 |
| Height, cm | 168.5 ± 2.8 | 175.7 ± 2.7* |
| Weight, kg | 70.8 ± 3.9 | 93.9 ± 3.8* |
| BMI, kg·m−2 | 24.7 ± 0.8 | 30.4 ± 1.1* |
| BSA, m2 | 1.8 ± 0.06 | 2.1 ± 0.05* |
| Mean arterial pressure, mmHg | 87.9 ± 2.0 | 83.6 ± 2.8 |
| Hemoglobin, g·dl−1 | 13.7 ± 0.3 | 13.7 ± 0.5 |
| eGFR, ml·min−1·1.73−1·m−2 | 77.1 ± 4.7 | 70.2 ± 4.8 |
| NT-proBNP, pg·ml−1 | 58.6 ± 13.1 | 1169 ± 298.9* |
| Left ventricular ejection fraction, % | – | 33.0 ± 2.0 |
| NYHA | ||
| I | – | 5 |
| II | – | 6 |
| III | – | 3 |
| Medications | ||
| Ace-Inhibitor | – | 7 |
| Angiotension 2 blocker | – | 5 |
| Beta blocker | – | 13 |
| Digitalis | – | 3 |
| Diuretic | – | 10 |
P<0.05 control vs heart failure
Overview of experimental procedures
All experimental procedures were conducted within a single day. A venipuncture blood draw was performed to obtain a measurement of hemoglobin, to rule out anemia, and creatinine, to ensure adequate renal function. Dynamic pulmonary function tests were performed to assess FVC, forced expiratory volume in 1 s (FEV1), FEV1/FVC, FEF25–75, and PEF according to standard methods [18]. Breath-by-breath measures of minute ventilation (VE), respiratory rate (RR), tidal volume, oxygen consumption (VO2), carbon dioxide production (VCO2), VE/VCO2 ratio, and respiratory exchange ratio (RER) were collected during six minutes of resting breathing. A thoracic and ECG-gated contrast perfusion computed tomography (CT) scan were obtained (see details below).
Computed Tomography Scanning
All CT scans were performed on the same scanner (SOMATOM Definition Force, Siemens, Erlangen, Germany). A thoracic CT scan was obtained for semi-quantitative measurement of EVLW and for alignment for the ECG-gated contrast perfusion scan. For the perfusion scan, a region of interest (ROI) was chosen that included the vena cava (VC), pulmonary artery (PA), pulmonary vein (PV), and aorta (AO). Iodinated contrast (0.33 ml per kg body weight) was infused intravenously at the antecubital fossa. Simultaneously, scanning was initiated, and a scan (11 slices per scan, 4.7 mm per slice) was taken during each diastole over a period of 30 seconds. All scans were performed while the subject was at total lung capacity as confirmed via the Medspira Breath Hold System (Medspira, Minneapolis, MN).
Quantification of thoracic, pulmonary, and blood volumes
Perfusion scans were used for quantification of blood volumes. ROIs were selected in the VC, PA, PV, and AO, and the change in the CT attenuation while the contrast passed through each region was measured using Analyze software (Mayo Clinic, Rochester, MN). The CT attenuation was converted to contrast concentration (C) using equation 1:
| (1) |
Where t is time [19]. The gamma variate curve was fit to the curve for each ROI using equation 2:
| (2) |
Where a, b, and c are arbitrary fitted constants [20]. The cardiac output (Q) was then calculated from the ROI in the PA using equation 3:
| (3) |
Where M is the mass of contrast agent injected [21]. The mean transit time (MTT) for each ROI was calculated using equation 4 [22]:
| (4) |
The thoracic (Vt), pulmonary (Vp), and heart (Vh) blood volumes were calculated using equation 5 through 7:
| (5) |
| (6) |
| (7) |
Semi-quantitative measurement of EVLW
Thoracic CT scans were used for quantification of EVLW. Lung tissue was segmented from surrounding tissue and large blood vessels automatically using MATLAB built-in active contour algorithms (Mathworks, Inc, Natick, MA). Only pixels within the range of −1000 to 0 HU were included in the analysis. The values for the mean, skewness, and kurtosis were calculated from the distribution of CT attenuation within the segmented areas. The CT attenuation of volume within the lung varies from −1000 HU (corresponding to pure air) to 0 HU (corresponding to pure water), with most voxels having a value between these extremes as they contain a combination of air and water. If more of the voxels within the lung volume have an CT attenuation closer to −1000 HU the lung volume is relatively drier, while as more water accumulates in the lung, more voxels will move towards 0 HU. This is quantified through the mean, the skewness, the kurtosis, and full-width half-max (FWHM), where the curve will be skewed to the left, have a high kurtosis, and be narrow when the lung is dry. Conversely, as EVLW accumulates the mean shifts to the right, the skew decreases, the kurtosis decreases, and the FWHM increases as more voxels within the lung space are closer to 0 HU [23, 24].
Statistical Analysis
Statistical analysis was carried out in SPSS V20 (IBM, Chicago, IL). The Independent samples Mann Whitney U Test was used to compare subject demographics, gas exchange, blood volumes, EVLW measurements, and pulmonary function between patients with HF and age-matched control subjects. Additionally, the Cohen’s d was computed for blood volumes and EVLW to show effect size of the respective differences [25]. The interrelationships between blood volumes, EVLW and indices of pulmonary function were assessed via linear regression. Corrections for multiple comparisons have not been made. Demographic and gas exchange data are presented as mean and standard error of the mean, pulmonary function measures are expressed as mean and standard error of the mean, and blood volumes and extravascular lung water measurements are expressed median and interquartile range. Statistical significance was accepted if P < 0.05.
RESULTS
Pulmonary Gas-exchange
Measurements of resting gas-exchange and breathing pattern are reported in Table 2. VE was greater in patients with HF compared to healthy control subjects (P<0.05); all other resting ventilatory and gas exchange indices were similar between groups.
Table 2.
Gas exchange and breathing pattern measurements for control and heart failure groups. No differences were observed between the groups except patients with HF had a greater minute ventilation. Data are mean plus or minus standard error of the mean.
| Control | Heart Failure | |
|---|---|---|
| VO2, ml*min−1 | 239 ± 15 | 303 ± 30 |
| VCO2, ml*min−1 | 188 ± 12 | 253 ± 25 |
| RER | 0.79 ± 0.02 | 0.84 ± 0.07 |
| RR, breaths*min−1 | 15.1 ± 0.6 | 16.2 ± 1.6 |
| Tidal volume, ml | 546 ± 41 | 699 ± 88 |
| VE, l*min−1 | 7.9 ± 0.4 | 10.6 ± 1.0* |
| VE/VCO2 | 44.0 ± 1.2 | 44.6 ± 3.6 |
P<0.05
Thoracic Blood Volumes
Thoracic, pulmonary, and heart blood volumes were greater in the HF group in absolute terms (Vt: 1090±530 ml vs 810±250 ml; Vp: 550±210 ml vs 410±130 ml; Vh: 540±350 ml vs 400±150 ml; P<0.05). To account for differences in body size, Vt, Vp, and Vh normalized to body surface area (BSA) are reported in Table 3. All three blood volumes were greater in the patients with HF when normalized to BSA. The effect size is considered large for the Vt and Vp and medium for Vh suggesting that there is a consistent difference in these blood volumes for the two populations [25].
Table 3.
Thoracic, pulmonary, and heart blood volumes for control and heart failure groups normalized to BSA. Patients with HF had a greater level of all three blood volumes. Data are median and interquartile range.
| Control | Heart Failure | P value | Cohen’s d | |
|---|---|---|---|---|
| Thoracic blood volume, ml*m−2 | 430 (360, 530) | 640 (420, 670) | 0.015 | 0.91 |
| Pulmonary blood volume, ml*m−2 | 210 (190, 260) | 340 (220, 360) | 0.006 | 1.12 |
| Heart blood volume, ml*m−2 | 220 (180, 270) | 270 (210, 360) | 0.131 | 0.68 |
CT Quantitative Indices
The average CT attenuation distribution for both groups is shown in Figure 1. The mean, skew, kurtosis, and FWHM are shown in Table 4. The HF group had distributions that were less negative mean, less skewed, and wider suggesting greater levels of EVLW. Additionally, the effect size for all four measurements was large suggesting that these measurements differentiate between groups well.
Figure 1.
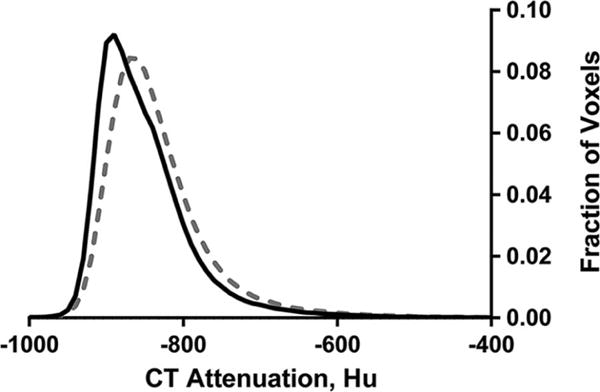
Histogram of CT attenuation of the lung tissue for control (solid line) and heart failure (dashed line). Confidence intervals are not shown for clarity.
Table 4.
Quantitative CT indices for the control and heart failure groups. Less negative means, lower skew and kurtosis, and higher full width half max suggest greater levels of extravascular lung water. Patients with HF had greater levels of EVLW as indicated by the difference in skew, kurtosis, and full width half max. Data are median and interquartile range.
| Control | Heart Failure | P value | Cohen’s d | |
|---|---|---|---|---|
| Mean, Hu | −860 (−869, −822) | −821 (−845, −802) | 0.17 | 0.98 |
| Skew | 3.44 (3.16, 4.44) | 2.99 (2.86, 3.43) | 0.27 | 0.94 |
| Kurtosis | 22.6 (18.6, 33.4) | 17.0 (15.1, 23.1) | 0.60 | 0.86 |
| FWHM, Hu | 57.0 (50.8, 69.0) | 71.1 (63.1, 86.1) | 0.001 | 1.64 |
Pulmonary Function
Pulmonary function measurements for both groups are shown in Figure 2. Absolute and percent predicted forced vital capacity (FVC), forced expiratory volume in 1 second (FEV1), FEF25–75, and peak expiratory flow (PEF) were lower in patients with HF vs. healthy control subject (p<0.05). By contrast, FEV1/FVC was not different between subject groups.
Figure 2.
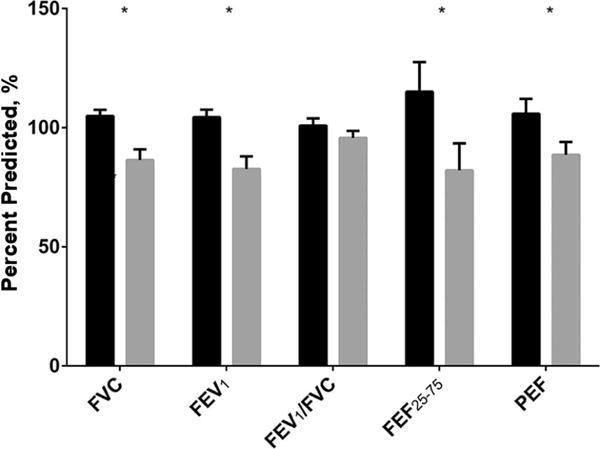
Spirometric measurements for control (solid black) and heart failure (dashed). Error bars are standard error of the mean. *-P<0.05.
Relationship between Thoracic Blood Volumes and EVLW and Pulmonary Function
Correlations between thoracic blood volumes and EVLW and pulmonary function measurements are reported in Table 5. In the control group, only thoracic blood volume was significantly correlated with FEV1. In the HF group, FVC was significantly related to the mean, skew, kurtosis, and FWHM, and FEV1 was related to the skew and kurtosis, where greater levels of EVLW were associated with decreased lung volumes. FEV1/FVC was inversely and significantly correlated with Vt, Vp, and Vh, while PEF was inversely correlated with Vt and Vp. Greater volumes of blood were associated with decreased pulmonary function. FEF25–75 was not associated with any measures of thoracic blood volume or EVLW in either group.
Table 5.
Pearson correlation coefficients between spirometric variables and thoracic, pulmonary, and heart blood volumes and CT quantitative indices for control and heart failure. Blood volumes were associated with obstructive changes in expiratory flows and EVLW with restrictive changes in lung volumes in the patients with HF, but not in the control subjects.
| Thoracic blood volume | Pulmonary blood volume | Heart blood volume | Mean | Skew | Kurtosis | FWHM | |
|---|---|---|---|---|---|---|---|
| Control | |||||||
| FVC | .27 | .48 | −.05 | −.13 | .19 | .31 | −.15 |
| FEV1 | .51* | .47 | .40 | .25 | −.09 | .01 | .17 |
| FEV1/FVC | .31 | .08 | .46 | .35 | −.22 | −.22 | .28 |
| FEF25–75 | .20 | .09 | .25 | .23 | −.02 | −.01 | .10 |
| PEF | .17 | −.02 | .33 | .32 | −.30 | −.24 | .32 |
| Heart Failure | |||||||
| FVC | .39 | .29 | .42 | −.55* | .51* | .49* | −.53* |
| FEV1 | −.04 | −.04 | −.04 | −.37 | .49* | .47* | −.38 |
| FEV1/FVC | −.64* | −.50* | −.67* | .16 | .09 | .10 | .11 |
| FEF25–75 | −.33 | −.25 | −.35 | −.19 | .40 | .40 | −.31 |
| PEF | −.46* | −.51* | −.38 | .13 | −.20 | −.27 | .03 |
P<0.05
DISCUSSION
Pulmonary congestion is a common, complicated manifestation of the pathophysiology of HF. In the present study, we sought to quantify thoracic, pulmonary, and heart blood volumes using a CT contrast perfusion method as well as to semi-quantitatively determine EVLW, to understand each compartment’s contribution to “pulmonary congestion” in a stable, chronic HF population, and understand their respective effects on lung function. We found that the HF population had increases in thoracic, pulmonary, and heart blood volumes, increased EVLW, and impaired lung function relative to controls. Greater pulmonary blood volume was associated with obstructive changes in the lungs, decreases in FEV1/FVC and PEF, while the increased EVLW was related to restrictive lung changes, reductions in FVC and FEV1. Thus even in stable, chronic HF, there are expanded thoracic, pulmonary, and heart blood volumes as well as evidence for increased EVLW consistent with subclinical edema. Each of these changes appears to negatively impact and contribute to the symptomology of HF.
Thoracic blood volumes
Traditionally, EVLW has been thought to be the main contributor to pulmonary congestion [26]. In our view, the expansion of the pulmonary vascular blood volume also contributes to the loss of pulmonary function. Thoracic blood volumes have been quantified in a number of ways to investigate if these compartments change with the progression of HF and other diseases. To our knowledge, we are the first to quantify these fluid compartments using the CT contrast perfusion technique described here, which is less invasive than previously used invasive indicator dilution based methods. Our findings are quantitatively similar to other groups using invasive indicator dilution methods, and qualitatively similar to positron emission tomography (PET) methods [27, 28].
We found that increased thoracic, pulmonary, and heart blood volumes were associated with reductions in FEV1/FVC ratio and PEF. There are several possible explanations for this result. Increases in pulmonary vascular volume may cause compression of the airways. Various studies in animals and humans after rapid fluid loading have shown that small vessels near small airways become engorged and edema forms in the airway walls, but that airways cross sectional area was maintained [29–31]. One study in dogs using CT scanning found approximately a 20% reduction in airway area with saline infusion [32]. A previous study in our lab found small reductions in the luminal airway of the large airways after fluid loading, but no differences in large airways in patients with HF [15, 33]. These divergent findings confound the understanding of the relationship between pulmonary function and thoracic blood volumes that were found in this study. It is possible that edema formation in the small bronchioles changes their structural properties making them more susceptible to collapse or causes changes in the luminal walls leading to increased airway resistance. In either case, the equal pressure point would move distally and create greater flow limitations.
It has been previously shown that heart volume can be a factor associated with reduced lung volumes; however, we did not observe this relationship in the present study [17, 34]. This relationship was likely not found due to only the blood volume within the heart being measured as opposed to the entire heart volume. Radiographically determined measures of heart volume are more predictive than echocardiographically determined measures due incorporation of the entire cardiac mass versus assessing individual chambers [35]. Given that the measure described here only measures the blood within the heart chambers, it would be expected to have poor predictive value for the lung volumes.
EVLW
Extravascular lung water is traditionally thought of as the main contributor to pulmonary congestion in HF. A histogram technique to semi-quantitatively measure differences in EVLW was used [23, 33, 36]. A decreased skew and kurtosis and increased FWHM as found here suggest the presence of additional EVLW in stable, chronic patients with HF compared with control subjects. This qualitatively matches other laboratories that have used direct measures, such as indicator dilution and PET techniques, and have found increased EVLW in patients with HF [37, 38]. This increased water likely forms due to the increased pulmonary wedge pressure. No differences were found in the resting gas-exchange measures suggesting the increased EVLW is not present in the alveoli and is being diverted to the thick side of the alveoli-capillary membrane to the lymphatic system.
In the present study, a relationship was found between increased levels of EVLW as measured by the CT indices and restrictive changes in lung function as indicated by decreased FVC and FEV1. Increased EVLW has been linked to changes in pulmonary mechanics, specifically decreased compliance, which would limit lung volumes, and is related to the degree of EVLW present [39]. Overall, increased EVLW seems to be a factor in reduced lung volumes in stable, chronic HF.
Limitations
Four major points must be considered when interpreting the findings of the present study. First, the patients with HF were taller and weighed more than the control subjects. To account for these factors, we normalized our thoracic, pulmonary, and heart blood volumes to the subjects BSA and exclusively used percent predicted for pulmonary function measures. Second, the method used to measure EVLW does not provide a quantitative measure and may be susceptible to changes in tissue or blood volume. While we found increased pulmonary blood volume, most of this would be excluded by the segmentation process prior to histogram generation and calculation of the quantitative indices. Additionally, studies have found no difference in capillary blood volumes between HF and controls subjects; these factors suggest that this method is measuring primarily differences in water content [40]. Third, in some subjects, there was incomplete washout of the dye from the aorta during the 30 second scanning period. All subjects reached the downward slope of the contrast washout curve, but fitting errors could still exist as a result of extrapolation. However, the extrapolated curves were qualitatively similar to those fit to a complete dataset suggesting that the error is small. Finally, we have chosen not to correct for multiple comparisons due to a concern that false negatives could limit further investigation into these factors as causes of reduced function in heart failure, that a false discovery rate was not determined prior to data collection, and the relatively small sample size of the present study. Additionally, the large effects sizes suggest that these findings represent important, consistent differences between HF and control subjects.
Conclusions
In the present study, we sought to better define pulmonary congestion in stable patients with HF by examining both thoracic blood volumes and EVLW. The thoracic, pulmonary, and heart blood volumes were quantified using a novel CT contrast based approach. We determined that thoracic, pulmonary, and heart blood volumes and EVLW were increased in patients with chronic, stable HF. Symptoms consistent with pulmonary congestion such as decreased lung volumes and flows were observed. Overall, chronic, stable patients with HF had evidence of mild increases in EVLW that were associated with a reduction in lung volumes while thoracic blood volumes were linked to reduced forced expiratory flows. While EVLW is a recognized sequela of pulmonary congestion in a more acute decompensated state, our results suggest that vascular volumes are expanded in the thorax, that EVLW is elevated, and that both contribute to the altered lung function and symptoms of moderate, chronic HF.
Acknowledgments
The authors would like to thank Briana Ziegler, Erik Ritman, and the members of the CT Clinical Innovation Center.
FUNDING SOURCES
This study was funded by NIH Grant HL71478 and the Mayo Graduate School.
Abbreviations
- Vt
Thoracic blood volume
- Vp
Pulmonary blood volumes
- Vh
Heart blood volumes
- EVLW
Extravascular lung water
- VC
Vena cava
- PA
Pulmonary artery
- PV
Pulmonary vein
- AO
Aorta
- MTT
Mean transit time
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statements: The Authors have no conflicts of interest to disclose.
References
- 1.Schreiner BF, Jr, Murphy GW, James DH, Yu PN. Pulmonary blood volume in patients with congestive heart failure. Trans Assoc Am Physicians. 1966;79:250–61. [PubMed] [Google Scholar]
- 2.Sullivan MJ, Higginbotham MB, Cobb FR. Increased exercise ventilation in patients with chronic heart failure: intact ventilatory control despite hemodynamic and pulmonary abnormalities. Circulation. 1988;77:552–9. doi: 10.1161/01.cir.77.3.552. [DOI] [PubMed] [Google Scholar]
- 3.Borlaug BA, Kass DA. Invasive hemodynamic assessment in heart failure. Heart Fail Clin. 2009;5:217–28. doi: 10.1016/j.hfc.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.West JB, Dollery CT, Naimark A. Distribution of Blood Flow in Isolated Lung; Relation to Vascular and Alveolar Pressures. J Appl Physiol. 1964;19:713–24. doi: 10.1152/jappl.1964.19.4.713. [DOI] [PubMed] [Google Scholar]
- 5.Gehlbach BK, Geppert E. The pulmonary manifestations of left heart failure. Chest. 2004;125:669–82. doi: 10.1378/chest.125.2.669. [DOI] [PubMed] [Google Scholar]
- 6.Minasian AG, van den Elshout FJ, Dekhuijzen PN, Vos PJ, Willems FF, van den Bergh PJ, et al. Pulmonary function impairment in patients with chronic heart failure: lower limit of normal versus conventional cutoff values. Heart Lung. 2014;43:311–6. doi: 10.1016/j.hrtlng.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 7.Snyder EM, Turner ST, Johnson BD. Beta2-adrenergic receptor genotype and pulmonary function in patients with heart failure. Chest. 2006;130:1527–34. doi: 10.1378/chest.130.5.1527. [DOI] [PubMed] [Google Scholar]
- 8.Dimopoulou I, Daganou M, Tsintzas OK, Tzelepis GE. Effects of severity of long-standing congestive heart failure on pulmonary function. Respir Med. 1998;92:1321–5. doi: 10.1016/s0954-6111(98)90136-6. [DOI] [PubMed] [Google Scholar]
- 9.Mead J, Turner JM, Macklem PT, Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro JP. Periodic breathing in heart failure: bridging the gap between the sleep laboratory and the exercise laboratory. Circulation. 2006;113:9–10. doi: 10.1161/CIRCULATIONAHA.105.590265. [DOI] [PubMed] [Google Scholar]
- 11.Witte MH, Dumont AE, Clauss RH, Rader B, Levine N, Breed ES. Lymph circulation in congestive heart failure: effect of external thoracic duct drainage. Circulation. 1969;39:723–33. doi: 10.1161/01.cir.39.6.723. [DOI] [PubMed] [Google Scholar]
- 12.Warren JV, Stead EA., Jr Fluid dynamics in chronic congestive heart failure: An interpretation of the mechanisms producing the edema, increased plasma volume and elevated venous pressure in certain patients with prolonged congestive failure. Arch Intern Med. 1944;73:138–47. [Google Scholar]
- 13.Cabanes LR, Weber SN, Matran R, Regnard J, Richard MO, Degeorges ME, et al. Bronchial hyperresponsiveness to methacholine in patients with impaired left ventricular function. N Engl J Med. 1989;320:1317–22. doi: 10.1056/NEJM198905183202005. [DOI] [PubMed] [Google Scholar]
- 14.Cabanes L, Costes F, Weber S, Regnard J, Benvenuti C, Castaigne A, et al. Improvement in exercise performance by inhalation of methoxamine in patients with impaired left ventricular function. N Engl J Med. 1992;326:1661–5. doi: 10.1056/NEJM199206183262503. [DOI] [PubMed] [Google Scholar]
- 15.Ceridon ML, Snyder EM, Strom NA, Tschirren J, Johnson BD. Influence of rapid fluid loading on airway structure and function in healthy humans. J Card Fail. 2010;16:175–85. doi: 10.1016/j.cardfail.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lizak MK, Zakliczynski M, Jarosz A, Zembala M. The influence of chronic heart failure on pulmonary function tests in patients undergoing orthotopic heart transplantation. Transplant Proc. 2009;41:3194–7. doi: 10.1016/j.transproceed.2009.07.072. [DOI] [PubMed] [Google Scholar]
- 17.Olson TP, Beck KC, Johnson BD. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J Card Fail. 2007;13:100–7. doi: 10.1016/j.cardfail.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–38. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 19.Behrenbeck TR, McCollough CH, Miller WL, Williamson EE, Leng S, Kline TL, et al. Early changes in myocardial microcirculation in asymptomatic hypercholesterolemic subjects: as detected by perfusion CT. Ann Biomed Eng. 2014;42:515–25. doi: 10.1007/s10439-013-0934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson HK, Jr, Starmer CF, Whalen RE, McIntosh HD. Indicator Transit Time Considered as a Gamma Variate. Circ Res. 1964;14:502–15. doi: 10.1161/01.res.14.6.502. [DOI] [PubMed] [Google Scholar]
- 21.Geerts BF, Aarts LP, Jansen JR. Methods in pharmacology: measurement of cardiac output. Br J Clin Pharmacol. 2011;71:316–30. doi: 10.1111/j.1365-2125.2010.03798.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Fernandez JM. Theory of the measurement of the dispersion of an indicator in indicator-dilution studies. Circ Res. 1962;10:409–28. doi: 10.1161/01.res.10.3.409. [DOI] [PubMed] [Google Scholar]
- 23.Kato S, Nakamoto T, Iizuka M. Early diagnosis and estimation of pulmonary congestion and edema in patients with left-sided heart diseases from histogram of pulmonary CT number. Chest. 1996;109:1439–45. doi: 10.1378/chest.109.6.1439. [DOI] [PubMed] [Google Scholar]
- 24.Best AC, Lynch AM, Bozic CM, Miller D, Grunwald GK, Lynch DA. Quantitative CT indexes in idiopathic pulmonary fibrosis: relationship with physiologic impairment. Radiology. 2003;228:407–14. doi: 10.1148/radiol.2282020274. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. Statistical Power Analysis for the Behavioral Sciences. Taylor & Francis; 2013. [Google Scholar]
- 26.Pappas L, Filippatos G. Pulmonary congestion in acute heart failure: from hemodynamics to lung injury and barrier dysfunction. Rev Esp Cardiol. 2011;64:735–8. doi: 10.1016/j.recesp.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 27.Bossaller C, Schober O, Meyer GJ, Hundeshagen H, Lichtlen PR. Positron emission tomography of regional extravascular lung water and regional pulmonary blood volume in chronic heart failure. Z Kardiol. 1985;74:5–14. [PubMed] [Google Scholar]
- 28.Lewis ML, Gnoj J, Fisher VJ, Christianson LC. Determinants of pulmonary blood volume. J Clin Invest. 1970;49:170–82. doi: 10.1172/JCI106216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel RP, Zocchi L, Rossi A, Cardinal GA, Ploy-Song-Sang Y, Poulsen RS, et al. Does interstitial lung edema compress airways and arteries? A morphometric study. J Appl Physiol (1985) 1987;62:108–15. doi: 10.1152/jappl.1987.62.1.108. [DOI] [PubMed] [Google Scholar]
- 30.Michel RP, Meterissian S, Poulsen RS. Morphometry of the distribution of hydrostatic pulmonary oedema in dogs. Br J Exp Pathol. 1986;67:865–77. [PMC free article] [PubMed] [Google Scholar]
- 31.King LS, Nielsen S, Agre P, Brown RH. Decreased pulmonary vascular permeability in aquaporin-1-null humans. Proc Natl Acad Sci U S A. 2002;99:1059–63. doi: 10.1073/pnas.022626499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown RH, Zerhouni EA, Mitzner W. Airway Edema Potentiates Airway Reactivity. J Appl Physiol. 1995;79:1242–8. doi: 10.1152/jappl.1995.79.4.1242. [DOI] [PubMed] [Google Scholar]
- 33.Chase SC, Wheatley CM, Olson LJ, Beck KC, Wentz RJ, Snyder EM, et al. Impact of chronic systolic heart failure on lung structure-function relationships in large airways. Physiol Rep. 2016;4 doi: 10.14814/phy2.12867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Agostoni P, Cattadori G, Guazzi M, Palermo P, Bussotti M, Marenzi G. Cardiomegaly as a possible cause of lung dysfunction in patients with heart failure. Am Heart J. 2000;140:e24. doi: 10.1067/mhj.2000.110282. [DOI] [PubMed] [Google Scholar]
- 35.Olson TP, Frantz RP, Snyder EM, O’Malley KA, Beck KC, Johnson BD. Effects of acute changes in pulmonary wedge pressure on periodic breathing at rest in heart failure patients. Am Heart J. 2007;153:104 e1–7. doi: 10.1016/j.ahj.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hartley PG, Galvin JR, Hunninghake GW, Merchant JA, Yagla SJ, Speakman SB, et al. High-resolution CT-derived measures of lung density are valid indexes of interstitial lung disease. J Appl Physiol (1985) 1994;76:271–7. doi: 10.1152/jappl.1994.76.1.271. [DOI] [PubMed] [Google Scholar]
- 37.Lilienfield LS, Freis ED, Partenope EA, Morowitz HJ. Transcapillary migration of heavy water and thiocyanate ion in the pulmonary circulation of normal subjects and patients with congestive heart failure. J Clin Invest. 1955;34:1–8. doi: 10.1172/JCI103051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schober OH, Meyer GJ, Bossaller C, Creutzig H, Lichtlen PR, Hundeshagen H. Quantitative determination of regional extravascular lung water and regional blood volume in congestive heart failure. Eur J Nucl Med. 1985;10:17–24. doi: 10.1007/BF00261757. [DOI] [PubMed] [Google Scholar]
- 39.Frank NR, Lyons HA, Siebens AA, Nealon TF. Pulmonary compliance in patients with cardiac disease. Am J Med. 1957;22:516–23. doi: 10.1016/0002-9343(57)90106-7. [DOI] [PubMed] [Google Scholar]
- 40.Agostoni P, Bussotti M, Cattadori G, Margutti E, Contini M, Muratori M, et al. Gas diffusion and alveolar-capillary unit in chronic heart failure. Eur Heart J. 2006;27:2538–43. doi: 10.1093/eurheartj/ehl302. [DOI] [PubMed] [Google Scholar]


