SYNOPSIS
Remarkable progress has been made in the treatment of bradykinin mediated angioedema with the advent of multiple new angioedema specific therapies over the last decade, largely studied in patients with hereditary angioedema (HAE) due to C1 esterase inhibitor (C1-INH) deficiency. While affected individuals previously had little treatment available, patients in many parts of the world now have effective medications available for prophylaxis and treatment of acute angioedema attacks. Despite these advances, patients are still faced with challenges of a burdensome disease that can lead to debilitating and dangerous angioedema episodes associated with significant costs for individuals and society. The burden of treatment must also be addressed with regard to medication administration difficulties, treatment complications, and adverse side effects. Multiple new therapies with novel mechanisms of action are currently being investigated and may offer potential solutions to these challenges faced by patients and physicians. We herein review the emerging therapeutic options for the treatment of HAE.
Keywords: Angioedema, Emerging Therapies, C1 esterase inhibitor, Bradykinin, Kallikrein, Factor XII
INTRODUCTION
Angioedema occurs due to the transient movement of fluid from the vasculature into the interstitial space leading to subcutaneous or submucosal swelling, which can have life threatening consequences. Current evidence suggests that most angioedema conditions can be grouped into two categories: histamine mediated or bradykinin mediated angioedema. While effective therapies for histamine mediated angioedema have existed for decades, effective therapies for bradykinin mediated angioedema have only more recently been developed, studied rigorously, and approved by regulatory agencies. As such, the treatment options for hereditary angioedema (HAE) have increased substantially over the last decade.
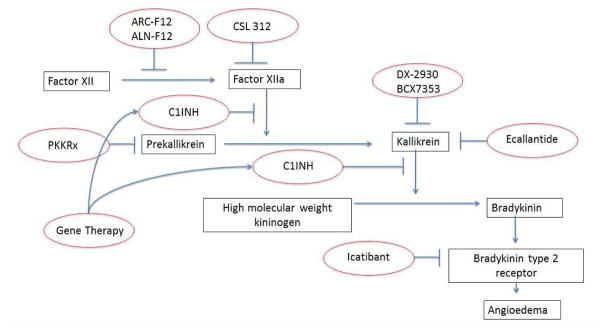
In the United States, therapy for HAE angioedema attacks was largely supportive a decade ago – currently four effective HAE-specific acute treatment options are available.1 In addition, advances in HAE-specific prophylactic treatment have been realized and continue to evolve. This review will largely focus on emerging treatments for bradykinin mediated angioedema, specifically HAE due to C1-INH deficiency, as the majority of recent research and therapeutic development has focused on improved prevention of HAE symptoms. To provide context for therapeutic strategies, we provide a cursory review of the pathophysiology of angioedema. (Figure 1)
Figure 1.
Pathogenesis of bradykinin mediated angioedema with targets for existing and developing therapies.
Histaminergic versus bradykinin pathways
As detailed in other articles of this issue, angioedema is generally caused by one of two mechanisms: through a mast cell mediated pathway (histaminergic angioedema) or through a non-histaminergic pathway. Current evidence strongly supports bradykinin as the predominant mediator responsible for non-histaminergic forms of angioedema. Clinically distinguishing between these two pathways is paramount in selecting the appropriate agents for both acute and preventative treatment as these two categories respond to completely different classes of medications.
Histaminergic angioedema is mediated by mast-cell activation with release of histamine, leukotrienes, and other mast-cell associated mediators. This form of angioedema is often accompanied by urticaria or pruritus and is seen in IgE-mediated allergic reactions due to food, medication or venom allergy, though a substantial portion of recurrent histaminergic angioedema is idiopathic in nature.
Non-histaminergic angioedema appears to be primarly mediated by bradykinin dysregulation wherein symptoms result from the overproduction of bradykinin which causes vasodilatation and vascular permeability by binding to the bradykinin B2 receptor on endothelial cells.2 Bradykinin is generated through the activation of the kallikrein-kinin (contact) system, although the precise mechanisms are still poorly understood. Angioedema episodes are believed to be initiated by activation of the contact system, prekallikrein and factor XII, forming factor XIIa and kallikrein. Bradykinin is formed by cleavage of high molecular weight kininogen by plasma kallikrein. C1-INH is a serine protease that inhibits proteases involved in this pathway. HAE due to C1-INH deficiency occurs with mutations in the SERPING1 gene. Bradykinin mediated angioedema can be due to HAE with C1-INH deficiency or with normal C1-INH, acquired C1-INH deficiency or ACE inhibitor induced angioedema. HAE is classically diagnosed through C1-INH deficiency, though a subset of patients who behave similarly to patients with classic HAE have normal levels of C1-INH.
Treatment of histamine vs bradykinin mediated angioedema
Historically, histamine mediated angioedema has been more successfully managed given the availability of effective medications for mast cell mediated conditions (i.e. antihistamines, corticosteroids, epinephrine, omalizumab, etc.), as well as health care providers’ familiarity with the “allergic” pathway as a cause of angioedema symptoms. Most treatment deficits and unmet need have involved the bradykinin-mediated angioedema conditions, most prominently HAE. Thus, the vast majority of research and development efforts in angioedema therapy have focused on the bradykinin pathway. Recent clinical work in histamine-mediated angioedema has largely confirmed the efficacy of existing histamine-targeted therapies.3, 4 As such, the remainder of this review will focus on therapeutic development efforts in bradykinin-mediated angioedema.
Recent Developments in HAE Therapy
HAE THERAPY FOR PEDIATRICS
Pediatric HAE is currently an area of unmet need requiring additional therapeutic advances. Many HAE patients will experience their first attack in childhood and pediatric patients currently have limited treatment options due to a lack of pediatric efficacy and safety data with most HAE medications. Plasma-derived C1INH concentrate (Berinert) is to date the only FDA approved treatment for acute attacks in HAE patients younger than 12. There are currently no FDA approved drugs for long-term prophylaxis in pediatric HAE patients. Pediatric patients may often be treated with HAE-specific medications in an “off label” approach with limited controlled data to guide these decisions.5 Data from uncontrolled trials support the use of C1INH concentrate (Cinryze) and tranexamic acid for prophylaxis, and icatibant for on demand treatment in children. Data from uncontrolled trials suggests the efficacy of danazol prophylaxis for children but use is not recommended given the numerous potentially serious adverse effects. A number of clinical trials are ongoing to evaluate the efficacy of HAE specific medications for the pediatric population.
Clinical Trials
A phase 2, multicenter, open-label study was conducted to investigate the safety and efficacy of ecallantide in the treatment of children and adolescents experiencing an acute HAE attack (NCT01832896). Patients between the ages of 2 and 15 years of age were included in the study. Ecallantide is currently FDA approved for patients 12 years and older. Pediatric patients experiencing an acute attack were treated with varying doses subcutaneously depending on weight. The estimated primary completion date was July 2015, though no results have been published.
Recombinant C1INH (Ruconest) is currently being studied in a phase 2, open-label, single arm study to evaluate the safety, immunogenicity and pharmacology in the treatment of acute HAE attacks in children between the ages of 2 and 13 (NCT01359969). Recombinant C1INH is currently FDA approved for adolescents and adults for treatment of acute attacks. Within the study, patients experiencing an acute HAE attack who weigh less than 84kg will receive 50units/kg of Ruconest in a one time IV injection; patients who weigh more than 84 kg will receive 4200 units. The estimated date of completion for collection of primary data is December 2017.
A phase 3, multicenter, open-label, non-randomized study is ongoing to assess the safety and pharmacology of icatibant in children older than 2 and adolescents younger than 18 in the treatment of acute HAE attacks (NCT01386658). Icatibant is currently FDA approved for adults 18 years and older. Pediatric patients experiencing an acute HAE attack will receive a single dose of icatibant 0.4mg/kg SC up to a maximal dose of 30mg and will be followed for 90 days. The estimated study completion date is September 2017.
A phase 3, multicenter, randomized, single blinded, dose-ranging, crossover study is in progress to evaluate the safety and efficacy of Cinryze for long-term HAE prophylaxis in children between the ages of 6 and 11 (NCT 02052141). This study will evaluate the relative efficacy of Cinryze 500 units or 1000 units IV every 3–4 days to prevent angioedema attacks in a 12 week treatment period. Patients will receive the alternate dose the following 12 weeks. The estimated completion of primary data collection is in May 2017.
EMERGING THERAPIES FOR LONG TERM PROPHYLAXIS
Treatments in Human Studies
C1 Inhibitor Concentrates
Currently, three intravenous (IV) C1INH concentrates are available for the treatment of HAE with 1 product licensed by the FDA for long term prophylactic therapy. However, frequently patients have limited or difficult vascular access, which complicates use of repeated IV medication. In some cases, the placement of a long term catheter is required for therapy which poses additional risks, such as infection and thrombosis. A subcutaneous route of administration would obviate these challenges and provide an additional prophylactic option for patients.
Subcutaneous C1INH
Two subcutaneous (SC) plasma-derived C1INH concentrates are currently being studied for long-term prevention of HAE attacks.
Early studies conducted by CSL Behring with SC C1INH demonstrated the drug to be well tolerated subcutaneously with sufficient bioavailability to be a viable treatment option. The PASSION study was a prospective, randomized, open-label study with patients receiving Berinert either via IV or SC infusion using 1000 U in 20mL of solution.6 Administration of the IV solution occurred over 3 minutes, whereas the SC administration occurred over 15 minutes at 2 separate abdominal sites (500 U at each site).
The investigators reported 15 out of 24 subjects (62.5%) receiving the SC formulation had a total of 32 adverse events as compared to 7 of the 24 subjects (29.2%) who received the IV administration with a total of 14 adverse events. All adverse events reported in the IV group were reported unlikely or not caused by the drug, while 23 of the 32 adverse events in the SC group were possibly or probably related to the drug. All of the reactions in the SC group were classified as mild and consisted predominantly of irritation and swelling at the infusion site. SC administration of Berinert resulted in 39.7% bioavailability compared to IV administration. C1-INH functional levels were lower after SC as compared to IV administration but the mean half life of C1-INH was longer with SC compared to IV administration (120 hours vs. 62 hours). While SC administration resulted in reduced bioavailability, pharmacodynamic studies revealed this level could exert an appreciable increase in C4 antigen and a decrease in cleaved high molecular weight kininogen (ClHK).
The COMPACT phase II study was an open-label, dose-ranging, cross over study examining the pharmacology and safety of a SC C1INH, CSL830.7 Eighteen patients first received a single dose of Berinert 20U/kg IV within 2–7 days of receiving CSL830. The patients then received twice weekly subcutaneous injections of CSL830, a highly concentrated, volume-reduced C1-INH, for 4 weeks followed by a washout of 4 weeks and then received a different concentration of CSL830 for another 4 weeks. Patients received two of three doses (1500, 3000 or 6000 IU). The trough functional C1-INH activity level and C4 antigen level were found to increase in a dose dependent manner with CSL830 treatment. Patients in the 3000 and 6000 IU dose groups reached and maintained a C1-INH activity level greater than 40% of normal, postulated as a level adequate to prevent angioedema attacks. CSL830 also demonstrated a more consistent level of exposure compared to IV administration with a lower peak-to-trough ratio. Safety data for CSL830 was reassuring with no serious related adverse events, deaths or thromboembolic events. CSL830 was generally well tolerated; the most common adverse event was local pain and swelling at the infusion site. Moderate site swelling was reported in 5/12 patients receiving the 6000 IU dose compared to 1/12 patients in the 3000 IU dose group and 2/12 patients in the 1500 IU dose group. Seven patients experienced 29 angioedema related events, with 11 events occurring during weeks of drug exposure and 18 events occurring outside of drug exposure periods. During the drug exposure period, 2 patients in the 1500 IU and 2 patients in the 3000 IU groups experienced all of the attacks while zero patients in the 6000 IU groups had an attack.
A phase III double-blinded, randomized, placebo-controlled, cross-over study was recently completed to assess the efficacy of SC C1-INH compared to placebo in the prevention of HAE angioedema attacks. Final data collection for the primary outcome measure was completed in October 2015 (NCT01912456). Preliminary results were presented at the 2016 American College of Allergy, Asthma, and Immunology (ACAAI) conference.8 Ninety patients were randomized in a 1:1:1:1 fashion to 1of 4 treatment arms. Each treatment arm included two 16 week treatment periods. Patients received either 40IU/kg or 60IU/kg of CSL830 in twice weekly SC injections followed by placebo, or vice versa. 79/90 patients completed the study. The primary endpoint was time-normalized number of HAE attacks. Both doses of the study drug significantly reduced the number of HAE attacks compared to placebo with a p value of <0.001. The lower dose group had a mean difference of −2.42 attacks/month (95% CI −3.38 to −1.46) representing an 89% median reduction. The higher dose group had a mean difference of −3.51 attacks/month (95% CI −4.21 to −2.81) representing a 95% median reduction. Secondary endpoints included response rate (defined as subjects with at least a 50% reduction in time-normalized number of HAE attacks as compared to placebo), and time-normalized number of rescue medications required, as well as adverse events. The lower dose group had a 76% response rate (95% CI 62 to 87%) and the higher dose group had a 90% response rate (95% CI 77 to 96%). The number of rescue medications used in the placebo group was 5.55 uses/month as compared to 1.13 in the lower dose group, and 3.89 in the placebo group as compared to 0.32 in the higher dose group. Consistent with previous trials, the majority of adverse events were mild injection site reactions. This study data has been submitted to the FDA for review with publication of the full study results forthcoming.
A SC formulation of the plasma-derived C1-INH concentrate, Cinryze, is being developed for prophylaxis of HAE attacks. In 2010, a phase II, open-label, multiple dose study evaluated the safety and pharmacology of SC versus IV administration of Cinryze (NCT01095497). Results from clinicaltrials.gov show 26 patients first received 1000 units of IV Cinryze twice weekly for 18 days followed by a 14 day washout period and then received one of two doses of subcutaneous Cinryze. Patients either received 1000 units of Cinryze SC twice weekly for two weeks or 2000 units of Cinryze SC twice weekly for two weeks. One patient in the lower dose SC group withdrew from the study. 12/26 patients in the IV group reported adverse events, 1 of whom reported an injection site reaction. 10/13 patients in the lower dose SC group and 11/12 in the higher dose SC group reported adverse events, all of which were injection site reactions. There were no serious adverse events.
A phase II, open-label, multiple dose study evaluated the safety and pharmacology of Cinryze with recombinant human hyaluronidase (rHuPH20) in HAE patients in 2011 (NCT01426763). The results on clinicaltrial.gov show 12 patients received either 1000 units of Cinryze with 20,000 units of rHuPH20 or 2000 units of Cinryze with 40,000 units of rHuPH20 twice weekly for two weeks. 11/12 patients experienced adverse events, which were largely local site reactions manifested with erythema, swelling and/or pain. Pharmacology results were not reported.
A phase II, randomized, double-blinded, multicenter, dose-ranging, crossover study examined the safety and efficacy of subcutaneous Cinryze with rHuPH20 in 2012.9 Patients received either 1000 units of Cinryze with 24,000 units of rHuPH20 or 2000 units of Cinryze with 48,000 units of rHuPH29 as a single 20mL SC injection twice weekly for 8 weeks. The primary outcome measure was normalized number of angioedema attacks during the treatment period. Patients in the lower dose treatment group had a normalized number of angioedema attacks of 1.58 (95% CI 0.88–2.29) as compared to 0.97 (0.41–1.53) in the higher dose treatment group with a p value of 0.0523. Secondary outcome measures showed a statistically significant lower severity of attacks and lower number of attacks that required acute treatment in the higher dose treatment group. There were no serious adverse events in either group; almost all patients experienced local injection site reactions. Of note, 45% of patients had detectable non-neutralizing antibodies to rHuPH20. None of the antibodies to rHuPH20 were associated with adverse effects but the study was terminated early as a precaution. The pharmacokinetic data were variable, but there appeared to be no significant bioavailability advantage with the use of hyaluronidase.
Based on this study data, a phase III, randomized, double-blind, placebo-controlled, two-period, three-sequence, partial crossover study to evaluate the safety and efficacy of a subcutaneous liquid formulation of C1-INH without the use of recombinant human hyaluronidase is currently ongoing (NCT02584959). Target completion of primary data collection is in December 2017.
Recombinant human C1INH
Recombinant human C1INH (rhC1INH) is being investigated for long-term prophylaxis for HAE. Recently, a phase II, randomized, double-blind, placebo controlled trial was completed with data presented at the ACAAI 2016 meeting.10 Patients 13 years and older were randomized to 3 separate 4 week treatment periods separated by a 1 week washout period. Patient were assigned to one of six treatment sequences with all arms including treatment periods with rhC1INH 50IU/kg IV twice weekly, rhC1INH 50IU/kg IV once weekly + saline IV once weekly, and saline IV twice weekly. Study results demonstrated that IV rhC1INH treatment significantly reduced the number of HAE attacks as compared to placebo. Patients in the 50IU/kg twice weekly arm experienced a mean of 2.7 attacks over the 4 week period and patients in the 50IU/kg once weekly arm experienced a mean of 4.4 attacks over 4 weeks compared to 7.2 attacks over 4 weeks in the placebo arm (p<0.0001 and 0.0004 as compared to placebo, respectively). A clinical response (defined as ≥50% reduction in number of HAE attacks) was observed in 95.7% of patients during the 50IU/kg twice weekly period and 56.5% of patients during the 50IU/kg once weekly period. Overall, the drug was well tolerated with no drug-related serious adverse events reported.
Monoclonal antibody inhibitor of kallikrein
DX2930/SHP643/lanadelumab
A fully human monoclonal antibody inhibitor of plasma kallikrein was developed by Dyax, now part of Shire. Initially known as DX2930, the drug has more recently been designated as SHP643 and named lanadelumab. A phase 1, single center, double-blinded, randomized study with 32 subjects studied subcutaneous administration in 4 sequential dose cohorts using 0.1, 0.3, 1.0 or 3.0mg/kg of DX-2930. Pharmacokinetic and pharmacodynamic studies revealed a dose and time dependent inhibition of kallikrein with a long acting effect favorable for long-term prophylaxis. No serious adverse events were observed.11 Subsequently, a phase 1b study has been completed with 37 subjects randomized to 4 doses of DX-2930 (30, 100, 300 or 400mg) or placebo.12 The 300mg treatment arm demonstrated a 100% reduction in HAE attacks as compared to placebo (p<0.0001) and the 400mg arm showed a 88% reduction HAE attacks (p=0.05) in the primary efficacy assessment period from days 8 to 50. Cohort numbers were small but 4/4 patients in the 300mg arm were attack free during this assessment period, and 9/11 patients in the 400mg arm were attack free as compared to 3/11 patients in the placebo arm (p=0.026 and p=0.030, respectively). No serious adverse events or safety signals were identified in the study.
Currently a phase III, multicenter, randomized, double-blinded, placebo-controlled clinical trial of lanadelumab for the long-term prevention of HAE attacks is underway (NCT02586805). The study will investigate the efficacy and safety of subcutaneous doses of lanadelumab at 150mg every 4 weeks, 300mg every 2 weeks, and 300mg every 4 weeks compared to placebo. Completion of data collection for the primary outcome is expected in early 2017.
Oral kallikrein inhibitors
Development of effective and safe oral medications for prophylaxis of HAE attacks would provide a potentially important alternative to injected/infused medications. The current oral options include antifibrinolytics, which lack relative efficacy and require multiple daily dosing, and attenuated androgens, which are complicated by significant long-term adverse effects for many patients. Development of oral small molecule plasma kallikrein inhibitors is being pursued by BioCryst and KalVista.
Avoralstat (BCX4161)
A first generation oral small molecule kallikrein inhibitor, Avoralstat (BCX4161) (NCT02670720), has been studied with preclinical, Phase I, and Phase II study data supporting safety and efficacy. However, a recently completed placebo-controlled Phase III study failed to show a significant reduction in the frequency of HAE attacks for the active drug vs. placebo. 110 patients were randomized to treatment with avoralstat (500mg or 300mg) or placebo three times daily for 12 weeks. No statistically significant differences were observed in the mean attack rate with either treatment arm as compared to placebo (mean attack rate of 0.63 per week in the 500mg arm, 0.71 per week in the 300mg arm and 0.61 per week in the placebo arm).13 Pharmacokinetic data collected during the trial indicated that drug exposure was suboptimal within treated patients due to low and variable bioavailability. Avoralstat required a three times daily dosing regimen during the Phase III study. Avoralstat clinical development has been halted based on these findings.
BCX7353
A second generation kallikrein inhibitor, BCX7353, is currently in development. Results from a Phase I clinical trial with 122 healthy subjects demonstrated BCX7353 to be safe and well tolerated. There were no reported serious adverse events, and 89% of adverse events were mild. The remaining adverse events were moderate and included, nausea, vomiting, hay fever and self limited diarrhea. 5% of subjects who received the drug for at least 7 days developed a self limited skin rash.14 Pharmacodynamic and pharmacokinetic studies supported a once-daily dosing regimen, unlike its predecessor Avoralstat. Part 1 of a phase II, randomized, double-blinded, placebo-controlled, dose-ranging, parallel-group study to evaluate the efficacy, safety and pharmacologic properties of BCX7353 is currently underway (NCT02870972). Twenty-four subjects will be randomized to BCX7353 350mg once daily or placebo daily for four weeks. If an interim analysis supports efficacy, part 2 of this trial will include randomization to additional doses (250mg daily and 125mg daily) to assess for dose responsiveness. Target completion of primary outcome data collection is in April 2017.15
KVD818
KVD818, an oral plasma kallikrein inhibitor, is currently in Phase I human clinical studies; no study data on the molecule has been published to date.16
Antisense targeting prekallikrein
Ionis PKKRx
Ionis Pharmaceuticals is developing antisense therapy for HAE prophylaxis, targeting the reduction of prekallikrein.17, 18 A phase 1, blinded, placebo-controlled, dose-escalation clinical trial completed in 2015 demonstrated a 95% reduction in prekallikrein in healthy volunteers. The drug was well tolerated and safe in both single and multiple dose arms. The company has indicated plans to move forward with this clinical development program for the prevention of HAE attacks.
Treatments in Preclinical Studies
Factor XII
Factor XII is cleaved into Factor XIIa and is involved in the initiation of contact system activation that ultimately leads to bradykinin production and increased vascular permeability. C1-INH normally inhibits this signaling cascade but in the deficient state, excess Factor XII activation appears to be a critical factor for HAE symptoms, thus making it an attractive target for preventative therapy.
ALN-F12
Alnylam Pharmaceuticals is developing a RNA interference (RNAi) drug (ALN-F12) to knockdown Factor XII as a prophylactic treatment for HAE. Preclinical data was presented at the 2016 American Academy of Allergy, Asthma, and Immunology (AAAAI) annual meeting.19,20 ALN-F12 inhibits Factor XII gene expression through degradation of Factor XII mRNA. The drug is subcutaneously administered with preliminary studies completed in mice and non-human primates. Animal studies have demonstrated a significant reduction of Factor XII mRNA with drug treatment and a dose-dependent reduction of vascular permeability in both ACE inhibitor induced and mustard oil induced mouse models of bradykinin-induced vascular permeability. In Cynomolgus monkeys, a single dose of ALN-F12 exhibited a dose dependent decrease in plasma Factor XII that was maintained for 2 months. The highest dose studied was 3mg/kg with a greater than 85% reduction in plasma Factor XII levels at one month and more than a 75% reduction in plasma Factor XII levels at two months.
ARC-F12
Arrowhead Research Corporation is developing a RNA interference drug against factor XII (ARC-F12).21, 22 Studies in rat models have shown >90% knockdown of Factor XII with monthly injections of 4mg/kg of ARC-F12. Treatment with ARC-F12 significantly decreased paw swelling in a rat model of carageenan-induced paw edema.
Monoclonal antibody anti-Factor XIIa
A recombinant fully human monoclonal antibody blocking Factor XIIa (CSL 312) is in preclinical development. Preliminary data was presented at the AAAAI conference in February 2015.23 The initial compound, 3F7, selected from a human phage display antibody library, was shown to be a potent and specific inhibitor of Factor XIIa in enzymatic studies with binding capability to rabbit, mouse, and human activated Factor XII. Murine models have demonstrated attenuation of contact-system induced skin edema with 3F7 administration. Subsequently, a C1-INH deficient mouse model has demonstrated the ability of 3F7 to modulate captopril-induced vascular permeability. CSL312 is a 3F7-variant that has improved affinity and potency.
Gene therapy
Gene therapy for HAE due to C1INH deficiency remains an attractive conceptual treatment approach given the curative potential. Viral vector-based gene replacement and gene editing techniques are methods of considerable interest. Early preclinical work at Weill Medical College of Cornell School of Medicine in the department of Genetics (NIH Project number 1R03AI122040-01) has been instrumental in the development of adeno-associated virus (AAV) gene therapy in HAE. Adverum Biotechnologies is actively advancing clinical development of this technology: ADVM-053 is an AAV based gene transfer vector aimed at increasing plasma C1INH levels via C1INH gene therapy, with human studies planned for 2017. If successful, such therapy could prevent attacks as well as reduce the burden of repeated medication use.
ACUTE THERAPY – CONCEPTUAL
Oral Kallikrein Inhibitor
As discussed, oral kallikrein inhibitors are currently in development for long term prophylaxis. Pharmacokinetic studies have suggested a maximal plasma drug level within two to five hours for BCX7353 (and previously one to two hours for failed drug Avoralstat). This has raised consideration of oral kallikrein inhibitor use for acute therapy to treat HAE attacks. Oral on demand therapy would provide HAE patients with a convenient option and provide greater treatment flexibility in their daily lives as all current acute therapies require intravenous or subcutaneous injections. Enthusiasm for investigating the use of oral kallikrein inhibitors in acute attacks has been tempered somewhat by the potential challenges of administration and absorption for attacks involving the mouth, throat, and GI tract. In addition, the pharmacokinetic and pharmacodynamic parameters may not provide rapid symptom relief comparable to that seen with currently approved acute HAE medications. Phase I pharmacokinetic studies of BCX7353 showed that absorption was slowed in the setting of food consumption, which could be an additional concern. Thus, the clinical utility of an oral acute treatment approach for HAE remains undefined until future studies provide additional data.
FUTURE CONSIDERATIONS/SUMMARY
The landscape of therapeutic options for patients with bradykinin-mediated angioedema has changed dramatically in the last decade. Ongoing research promises even greater change in the foreseeable future. Given the economic and psychosocial burdens for patients living with angioedema, effective therapies with novel mechanisms will offer more choices for patients and physicians, as well provide greater flexibility in routes of administration. Ultimately, gene therapy strategies may offer a more definitive durable treatment obviating the need for chronic repeated medication use, though the safety and tolerability of such approaches remains largely unknown.
KEY POINTS.
While significant therapeutic progress has been made in the field of hereditary angioedema, current treatments are still limited by access, cost, and side effects.
Multiple new therapies are being investigated for the treatment of hereditary angioedema due to C1 esterase inhibitor deficiency.
Novel mechanisms of action and drug delivery include subcutaneous C1 esterase inhibitor concentrates, a monoclonal antibody inhibitor of kallikrein, oral kallikrein inhibitors, RNA targeted antisense against pre-kallikrein, RNA interference drugs against factor XII, monoclonal antibody inhibitor of factor XIIa, and gene therapy.
Studies are ongoing to expand the number of drugs available for pediatric patients with hereditary angioedema due to C1 esterase inhibitor deficiency.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bork K. A decade of change: recent developments in pharmacotherapy of hereditary angioedema (HAE) Clinic Rev Allerg Immunol. 2016;51:183–192. doi: 10.1007/s12016-016-8544-9. [DOI] [PubMed] [Google Scholar]
- 2.Zuraw BL, Christiansen SC. HAE pathophysiology and underlying mechanisms. Clinic Rev Allerg Immunol. 2016;51:216–229. doi: 10.1007/s12016-016-8561-8. [DOI] [PubMed] [Google Scholar]
- 3.von Websky A, Reich K, Steinkraus V, Breuer K. Complete remission of severe chronic recurrent angioedema of unknown cause with omalizumab. J Dtsch Dermatol Ges. 2013;11:677–8. 46. doi: 10.1111/ddg.12075. [DOI] [PubMed] [Google Scholar]
- 4.Sands MF, Blume JW, Schwartz SA. Successful treatment of 3 patients with recurrent idiopathic angioedema with omalizumab. J Allergy Clin Immunol. 2007;120:979–81. doi: 10.1016/j.jaci.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 5.Frank MM, Zuraw B, Banerji A, Bernstein JA, Craig T, Busse P, Christiansen S, Davis-Lorton M, Li HH, Lumry WR, Riedl M US Hereditary Angioedema Association Medical Advisory Board. Management of Children With Hereditary Angioedema Due to C1 Inhibitor Deficiency. Pediatrics. 2016;138(5) doi: 10.1542/peds.2016-0575. [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Saguer I, Cicardi M, Suffritti C, Rusicke E, Aygören-Pürsün E, Stoll H, Rossmanith T, Feussner A, Kalina U, Kreuz W. P harmacokinetics of plasma-derived C1- INH after SC versus intravenous (IV) administration in subjects with mild or moderate hereditary angioedema: the PASSION study. Transfusion. 2014;54(6):1552–61. doi: 10.1111/trf.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zuraw BL, Cicardi M, Longhurst HJ, Bernstein JA, Li HH, Magerl M, Martinez-Saguer I, Rehman SM, Staubach P, Feuersenger H, Parasrampuria R, Sidhu J, Edelman J, Craig T. Phase II study results of a replacement therapy for hereditary angioedema with subcutaneous C1-inhibitor concentrate. Allergy. 2015;70(10):1319–28. doi: 10.1111/all.12658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuraw BL, Cicardi M, Longhurt H, Craig T, et al. A randomized trial of subcutaneous C1-inhibitor for the prevention of hereditary angioedema attacks. ACAAI Annual Meeting. 2016 [Google Scholar]
- 9.Riedl MR, Lumry WR, Li HH, Banerji A, Bernstein JA, Bas M, Bjorkander J, Magerl M, Maurer M, Rockich K, Chen H, Schranz J. Subcutaneous administration of human C1 inhibitor with recombinant human hyaluronidase in patients with hereditary angioedema. Allergy Asthma Proc. 2016;37:489–500. doi: 10.2500/aap.2016.37.4006. [DOI] [PubMed] [Google Scholar]
- 10.Riedl MR, Panovska VG, Moldovan D, Baker J, Yang WH, Reshef A, Andrejevic S, Lockey RF, Hakl R, MD, Kivity S, Bellizzi L, Harper JR, Relan A, Cicardi M. Randomized, double-blind placebo-controlled trial of recombinant human C1 inhibitor for prophylaxis of hereditary angioedema attacks. ACAAI. 2016 [Google Scholar]
- 11.Chyung Y, Vince B, Iarrobino R, Sexton D, Kenniston J, Faucette R, TenHoor C, Stolz LE, Stevens C, Biedenkapp J, Adelman B. A phase 1 study investigating DX-2930 in healthy subjects. Ann Allergy Asthma Immunol. 2014;113(4):460–6.e2. doi: 10.1016/j.anai.2014.05.028. [DOI] [PubMed] [Google Scholar]
- 12.Banerji A, Busse P, Shennak M, Lumry W, Davis-Lorton M, Wedner HJ, Jacobs J, Baker J, Bernstein J, Lockey R, Li H, Craig T, Cicardi M, Riedl MR, Al-Ghazawi A, Soo C, Iarrobino R, Sexton D, TenHoor C, Kenniston JA, Faucette R, Still JG, Kushner H, Mensah R, Stevens C, Biedenkapp J, Chyung Y, Adelman B. Inhibiting Plasma Kallikrein for Hereditary Angioedema Prophylaxis. N Engl J Med. 2017;376(8):717–728. doi: 10.1056/NEJMoa1605767. [DOI] [PubMed] [Google Scholar]
- 13.http://investor.shareholder.com/biocryst/releasedetail.cfm?ReleaseID=953694.
- 14.https://globenewswire.com/news-release/2016/08/11/863629/0/en/BioCryst-Announces-Initiation-of-the-APeX-1-Clinical-Trial-of-BCX7353-for-Hereditary-Angioedema.html.
- 15.http://www.biocryst.com/bcx_4161.
- 16.http://www.kalvista.com/hae.html.
- 17.Bhattacharjee G, et al. Inhibition of vascular permeability by antisense-mediated inhibition of plasma kallikrein and coagulation factor 12. Nucleic Acid Ther. 2013;23:175–187. doi: 10.1089/nat.2013.0417. [DOI] [PubMed] [Google Scholar]
- 18.Revenko AS, Gao D, Crosby JR, et al. Selective depletion of plasma prekallikrein or coagulation factor XII inhibits thrombosis in mice without increased risk of bleeding. Blood. 2011;118:5302–5311. doi: 10.1182/blood-2011-05-355248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J, Qin J, Castoreno A, Schlegel M, Maier MA, Fitzgerald K, Meyers R, Butler J, Akinc A. An Investigational RNAi Therapeutic Targeting Factor XII (ALN-F12) for the Treatment of Hereditary Angioedema. AAAAI 2016 Poster; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.http://www.alnylam.com/capella/presentations/new-program-aln-f12/.
- 21.Melquist S, Wakefield D, Hamilton H, Chu Q, Almeida A, Almeida L, Walters M, Montez J, Hegge J, Klein J, Hazlett C, Bertin S, Milarch T, Doss E, Schmidt R, Goth L, Ferger S, Rozema D, Hamilton J, Lewis D, Kanner S. Targeting Factor 12 (F12) with a novel RNAi delivery platform as a prophylactic treatment for Hereditary Angioedema (HAE). AAAAI 2016 Poster. [Google Scholar]
- 22.http://arrowheadpharma.com/pipeline/.
- 23.Cao Z, Biondo M, Rayzman V, Hardy M, McDonald A, Busfield S, Nolte MW, Wilson M, Nash A, Panousis C. Development and Characterization of an Anti-FXIIa Monoclonal Antibody for the Treatment of Hereditary Angioedema. AAAAI. 2015 [Google Scholar]