Abstract
Chronic pelvic pain is a frustrating symptom for patients with endometriosis and is frequently refractory to hormonal and surgical management. While these therapies target ectopic endometrial lesions, they do not directly address pain due to central sensitization of the nervous system and myofascial dysfunction, which can continue to generate pain from myofascial trigger points even after traditional treatments are optimized. This article provides a background for understanding how endometriosis facilitates remodeling of neural networks, contributing to sensitization and generation of myofascial trigger points. A framework for evaluating such sensitization and myofascial trigger points in a clinical setting is presented. Treatments that specifically address myofascial pain secondary to spontaneously painful myofascial trigger points and their putative mechanisms of action are also reviewed, including physical therapy, dry needling, anesthetic injections, and botulinum toxin injections.
Keywords: endometriosis, chronic pelvic pain, sensitization, myofascial trigger points, botulinum toxin
Chronic pelvic pain (CPP), defined as pain below the umbilicus for at least 6 months in duration, is one of the most common clinical manifestations of endometriosis. Between 71 and 87% of women with CPP have laparoscopically proven endometriosis lesions,1 but lesion location correlates poorly with the locations that patients identify as their most intense areas of pain.2,3 Treatments for endometriosis have focused on hormonal therapies and surgery, both of which target ectopic endometrial lesions. This approach can control the extent of disease but often fails to provide a durable solution for associated pelvic pain. Central sensitization and myofascial pain secondary to active (i.e., spontaneously painful) myofascial trigger points (MTrPs) likely constitute another source of initiation, amplification, and perpetuation of pain. Either could easily propagate pain-related symptoms in women even after surgical and medical/hormonal treatment for endometriosis has been optimized.
Unfortunately, both central sensitization and myofascial dysfunction are frequently overlooked in the evaluation, diagnosis, and treatment of CPP associated with endometriosis. In addition, many gynecologists have not received training in the assessment of myofascial dysfunction, instead evaluating pelvic pain according to standard gynecological practice. In this review, we will examine CPP related to endometriosis from a pain-centered perspective, and discuss how sensitization and MTrPs are crucial components in the chronic pain women experience that warrant a more comprehensive evaluation and targeted treatment.
Neural Mechanisms in Endometriosis-Associated Chronic Pelvic Pain: The Dynamic Role of Sensitization
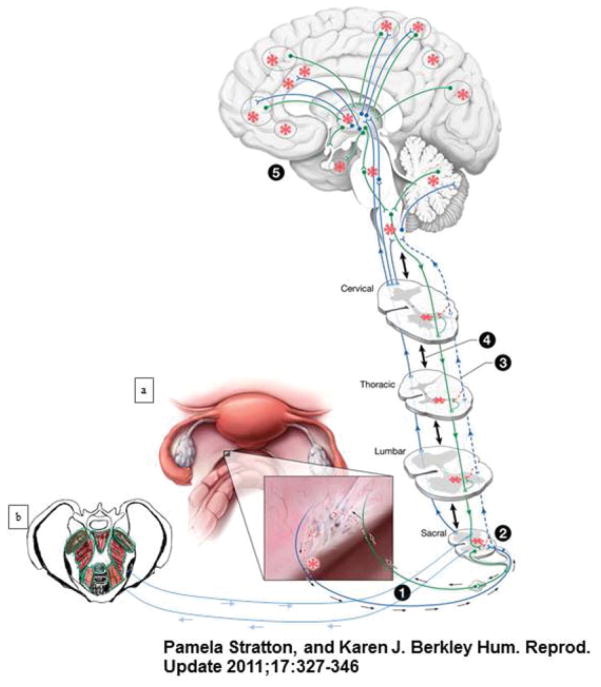
Pain is an unpleasant, subjective experience arising from the central nervous system (CNS) that normally alerts and protects the body from potentially noxious stimuli.4 Chronic pain, however, is pathological in itself, and often persists well after an inciting stimulus or injury has resolved. It is a result of functional and structural rearrangements of the CNS that both sustain the perception of pain and facilitate its expansion to distant regions.5 Given that endometriosis is a disease in which hormonally dependent, inflammatory, ectopic endometrial lesions engage the reproductive, endocrine, vascular, musculoskeletal, and neuronal systems, there are several factors that may contribute to CPP. This section will provide a model for CNS engagement in pain with endometriosis, and explain how myofascial dysfunction may develop from and reinforce this relationship6 (Fig. 1).
Fig. 1.
Nervous system engagement by endometrial lesions gives rise to different types of pain. (a) This figure illustrates how endometrial lesions can engage the nervous system to give rise to different types of pain associated with endometriosis and comorbid conditions. (1) Typical laparoscopic view of pelvic organs from the umbilicus. Inset demonstrates a deeply infiltrating lesion on the left uterosacral ligament. Both peptidergic sensory (blue) and sympathetic nerve fibers (green) sprout axon branches (red dashed lines) toward this lesion. Estradiol and sympathetic-sensory coupling drive peripheral sensitization (red asterisk) of new sensory fibers within the lesion. (2) Central sensitization (red asterisk) is propagated at synapses between sensitized peripheral nerve fibers and neurons in the sacral spine. This central sensitization is modulated differently from and can eventually become independent of peripherally sensitizing signals. (3) Although input from pelvic peripheral afferent fibers typically synapse with dorsal root ganglia in the sacral spine, branches of the fibers extend to other segments (blue dashed lines), and can propagate sensitization at distant spinal cord segments (red dashed lines). (4) Normally, multiple intersegmental spinal synaptic connections exist to coordinate bodily functions (double-arrowed black lines). In pathological pain conditions, this communication can alter processing of nociceptive and non-nociceptive sensory information in remote segments (“remote central sensitization, red asterisks). Via (3) and (4), increased nociception propagates into distant spinal segments. (5) Multiple afferent (blue) and efferent (green) pathways exist between the CNS and PNS with terminal connections in the brain. Input from sensitized spinal neurons can affect activity throughout the neuroaxis (red asterisks), altering normal processing of nociceptive and non-nociceptive information. Alterations in processing can occur on the medial cortical surface; the lateral prefrontal, frontal, and parietal lobes; and within the temporal lobe (dotted black ellipses). These influences can propagate signals independent of peripheral sensitization associated with lesions. (b) Muscles of the pelvic floor feed into sensitization pathways in the same manner as endometrial lesions. All mechanisms outlined can cause endometriosis-associated pain in the pelvis and at distant sites. (Reprinted with permission from Stratton and Berkley.3)
Few hypotheses address the question of how ectopic endometrial lesions activate the nervous system. One likely mechanism involves the innervation of lesions through neural sprouting of sensory and sympathetic fibers that innervate nearby blood vessels.7 Since endometrial lesions must be vascularized to survive and grow,8 the branching of blood vessels during lesion development permits the simultaneous invasion of nerves, as the same factors that act on sprouting blood vessels act on nerve fibers.9 Nerve growth factor (NGF), in particular, promotes neurite outgrowth from sensory neurons and nociceptors10; is found in high levels in peritoneal, deep adenomyotic, and ovarian endometriosis lesions11; and is associated with greater nerve fiber density in peritoneal endometriosis lesions compared with normal peritoneum.12 Newly sprouted nerve fibers may facilitate direct communication between the endometrial growths and the CNS, establishing a bridge for central integration of visceral sensory input.
Direct innervation of ectopic lesions by sensory and sympathetic nerve fibers has been confirmed in studies using a rat model13 and in women with endometriosis.14 Furthermore, their presence correlates with the severity of pelvic pain and dysmenorrhea.15 Sensory fibers that innervate endometrial lesions are calcitonin gene-related peptide (CGRP)-positive,13,14 indicating that they include C-fiber nociceptors.16 Nociceptors respond to noxious stimuli in the periphery, and are especially sensitive to immune and inflammatory factors which are prevalent in endometriosis.
The peritoneal fluid of women with endometriosis contains elevated levels of tumor necrosis factor-α, interleukin (IL)-1, IL-6, IL-8, IL-10, RANTES, monocyte chemotactic protein-1, and prostaglandins E2 and F, all of which directly sensitize or activate nociceptors or trigger the release of activating substances from nearby cells.17 NGF found near endometriosis lesions also activates nociceptors and recruits mast cells, which release inflammatory molecules with degranulation.10,18
Repeated or prolonged activation of nociceptors results in lowering of their activation threshold, a state known as peripheral sensitization.19 Once activated, nociceptors themselves can facilitate sensitization by secreting neuropeptides such as substance P and CGRP that are produced (by the dorsal root ganglion) and released antidromically into peripheral tissue following repeated stimulation.20,21 These substances induce vasodilation, increase local vascular permeability, and recruit and activate immune cells, causing neurogenic inflammation.22 Changes also occur along the peripheral terminals of nociceptors that permit further sensitization, such as the addition of new receptors to the cell membrane and the increased expression of existing receptors.19 In addition, although many visceral nociceptors are initially functionally silent even with intense stimulation, they may be activated after being sufficiently sensitized by inflammation.23 Thus, peripheral sensitization ultimately increases the excitability of nociceptors.
Ongoing nociceptor activation generates an afferent bombardment of noxious information into the dorsal horn of the spinal cord.19 This process, in turn, induces structural and functional changes throughout the spinal cord and more rostral structures, which ultimately lead to central sensitization and evoked exaggerated responses to peripheral stimuli (Fig. 1).5 Furthermore, these dynamic alterations within the central circuitry can amplify and perpetuate the perception of pain long after the initiating pathology resolves.5 Central sensitization is manifested clinically as allodynia (pain to a non-noxious stimulus), hyperalgesia (increased pain to a noxious stimulus), and referred pain (perceived pain outside of the area of noxious stimulation).
Two aspects of central sensitization that are especially relevant to women with CPP and endometriosis are viscerosomatic convergence and the viscerosomatic reflex. Visceral afferents constitute only 2 to 7% of all afferent fibers that pass through each dorsal root ganglion and synapse onto the spinal cord.24,25 As a result, almost all spinal neurons that receive visceral input also receive somatosensory input from the muscle and skin, through a process known as viscerosomatic convergence.26 Convergence of inputs hinders precise localization and discrimination of sensory information.27 It is also the basis for referred pain, and explains why visceral pathologies are commonly felt as pain in somatic structures (particularly muscles) innervated by the same spinal segment. Furthermore, since visceral afferent fibers terminate over several spinal segments above and below the segment level of input, referred pain may be present in areas remote from the affected visceral organ,26,28 an effect demonstrated in a rat model and in humans.29,30 Overall, viscerosomatic convergence explains how ongoing noxious visceral input can sensitize multiple areas of the spinal cord, generating the broad areas of allodynia, hyperalgesia, and referred pain seen with somatic dysfunction.31
Another consequence of central integration of visceral input is the development of the viscerosomatic reflex. Visceral nociceptors converge with somatic nociceptors onto interneurons in the spinal cord that activate both alpha and gamma motor neurons innervating skeletal muscle.32 Ongoing visceral input can produce increased muscle tone and spasm, usually in the area of pain referral. In addition, “guarding reflexes,” which involve heightened sacral reflexes that are triggered by visceral pain and inflammation, could contribute to muscle tightening and result in pelvic floor dysfunction.33 These mechanisms may create an environment prone to generating or activating MTrPs.
The Contribution of Active Myofascial Trigger Points to Chronic Pelvic Pain
Myofascial pain arises from dysfunction in the muscle and surrounding connective tissue. Despite being a common clinical problem with a lifetime prevalence of up to 85% in the general population,34 myofascial pain is an underdiagnosed and often overlooked component of nonarticular musculoskeletal pain. A hallmark of its diagnosis is the presence of MTrPs in the symptomatic region, which are small, palpable, hyperirritable nodules located on taut bands of skeletal muscle and that are in a sustained state of contracture.35 They can be spontaneously painful (i.e., active) or painful only upon perturbation (i.e., latent), and they often refer pain in predictable patterns. MTrPs may also cause motor and autonomic disturbances, and affect the function of visceral organs.35 MTrPs are commonly found in many chronic pain conditions, and, when active, typically present as a regional pain syndrome.
MTrPs can be found throughout the body, including the pelvic floor, where they may refer pain to the urethra, vagina, rectum, coccyx, sacrum, lower back, lower abdomen, and posterior thighs.36 MTrPs may also refer pain from these muscles back to the pelvic region,30,35–37 making myofascial pelvic pain difficult to localize. Dyspareunia, dyschezia, and dysuria are the most common symptoms among women with myofascial pelvic pain, though these symptoms can also reflect coincident gynecological, gastrointestinal, and urological conditions.36
In general, MTrPs are believed to occur secondary to muscle overload or overuse. They are also associated with a variety of medical conditions including those of metabolic, visceral, endocrine, infectious, and psychological origin.35,37 Within the pelvic floor, previous gynecological surgeries, childbirth, injury, sexual abuse, dyspareunia, and improper mechanics may all contribute to MTrP formation.37 MTrPs can also develop secondary to visceral disease.35 Studies have shown that MTrPs are associated with endometriosis38 and interstitial cystitis/painful bladder syndrome39 as well as other gynecologic, genitourinary, and gastrointestinal conditions, such as vulvodynia, irritable bowel syndrome, coccygodynia, and urethral syndrome.36,40 MTrPs compound the pain experienced with any of these conditions. Abdominal and pelvic MTrPs were commonly found in women with CPP and current biopsy-proven endometriosis, and, adjusting for any history of endometriosis, women with MTrPs were most likely to present with signs of sensitization in one clinical study.38
A myofascial component to pelvic pain adds another dimension to a patient’s disease and requires its own diagnosis and treatment. Once formed, MTrPs can become a self-sustaining source of pain even after the visceral insult has resolved.41 Active MTrPs, in particular, serve as a source of ongoing nociception; they can reduce pain thresholds, enhance visceral and referred pain, and sensitize the nervous system.42 In regard to endometriosis, MTrPs that develop secondary to disease could sustain the pain and dysfunction despite lesion removal and hormonal management.
Clinical Evaluation for Endometriosis-Related Pelvic Pain and Sensitization
Given the multifaceted nature of CPP associated with endometriosis, clinical evaluation benefits from a systematic, interdisciplinary approach that includes a targeted pelvic examination and a broader neuro-musculoskeletal pain assessment. Table 1 presents a summary of this clinical assessment and complements the standard approach for a gynecological assessment by focusing on evaluating pain related to endometriosis rather than the lesions themselves or other noted pathology.
Table 1.
Clinical evaluation of endometriosis-related pain and sensitization
| Patient History |
| History of endometriosis |
| Method of diagnosis |
| Previous and current treatments with their outcomes |
| Gynecologic and obstetric history |
| Menses: duration of flow, cramping, spotting, regularity, infertility, desire for future children |
| Gynecologic conditions, history of sexually transmitted infections |
| Current hormonal therapy |
| Surgery (type, findings, and treatment) |
| General medical history |
| Medical conditions |
| Headaches/migraines, depression, anxiety |
| Bowel and bladder symptoms suggestive of irritable bowel and painful bladder |
| Current medications/allergies |
| History of chronic pelvic pain |
| Duration, frequency (intermittent, constant) |
| Any temporal relationship to menstrual cycle (ovulation, menstruation, etc.) |
| Alleviating and exacerbating factors |
| Other pain conditions |
| Previous and current treatments with their outcomes |
|
|
| Gynecological Examination – Evoked Pain Assessment |
| Abdominal wall |
| Location and pattern of tenderness (diffuse, focal, not localized) |
| Allodynia |
| Myofascial trigger points (number, location, severity of pain) |
| Perineum |
| Improper positioning, trauma, scars |
| Observe pelvic floor muscle contraction and relaxation |
| Pelvic region |
| Superficial perineal muscles for muscle spasm and tenderness |
| Pelvic floor muscles: myofascial trigger points, taut bands, tenderness |
| Bladder and urethral tenderness |
| Uterosacral, forniceal, and vaginal tenderness and nodularity |
| Central uterine tenderness |
| Pattern of pelvic tenderness (diffuse, focal, not localized) |
| Location of worst pain |
|
|
| Neuro-Musculoskeletal Examination |
| Dermatome: |
| Allodynia via pinch and roll techniquea |
| Hyperalgesia via Wartenberg pinwheela |
| Myotomeb |
| Regional/abdominopelvic muscles: rectus abdominis, external oblique, iliacus and gluteus maximus |
| General muscles: temporalis, masseter, upper trapezius, supraspinatus, adductors, vastus medialis |
Assess spinal segments paraspinally, bilaterally in the region of pain. Record which segments have allodynia and hyperalgesia. If most segments are affected, then add segments above and below to assess the extent of sensitization.
Palpate bilaterally for myofascial trigger points in region of pain and throughout the body; identify as pain free or painful (latent or active) by region or in general. Integrate the pelvic and neuro-musculoskeletal examination findings with the clinical presentation.
When taking a medical history, practitioners focus on the details regarding the history of endometriosis and CPP. Often, having the patient complete a pain calendar for a menstrual cycle can help reveal patterns of pain associated with menstruation and hormonal fluctuations. Past and current treatments (pharmaceutical, hormonal, surgical, etc.) for endometriosis or pain and the outcomes for each treatment aid to inform treatment options.
The pelvic examination for active MTrPs explores the external and internal pelvic areas as well as the abdomen using only a single digit or one hand. This approach isolates areas of tenderness and allows for assessment of myofascial dysfunction and sensitization. Since abdominal wall muscle pain can refer to the pelvis,35 all regions of the abdominopelvic region are examined for allodynia and diffuse tenderness, which can be signs of sensitization.41 The abdomen is assessed for the number and location of abdominal muscle MTrPs, and the severity of pain elicited with palpation. Sacroiliac (SI) joint tenderness is also assessed.
The pelvic exam begins with an external assessment of the pelvic floor muscles for improper positioning, trauma, or scars, followed by having the patient contract and relax her pelvic floor muscles to assess for myofascial pain or hypertonicity. Using a single digit, the examiner then individually palpates the pelvic floor muscles, first externally and then internally, starting anteriorly with the superficial perineal muscles and moving posteriorly to the coccyx, noting MTrPs, taut bands, or generalized tenderness that reproduce the patient’s pain. The sphincter ani, levator ani, coccygeus, and obturator internus most commonly harbor MTrPs.43 MTrPs in these muscles are not usually nodules, but instead are taut bands that are tender, span the distance of the fiber, and are noticeably in spasm.30 Performing the exam slowly and pausing when pain is elicited often gives the best insights into the degree and extent of MTrP activation and local spasticity.
Bladder and urethral tenderness as well as uterosacral, forniceal, and vaginal tenderness and nodularity are also examined using a single digit. A bimanual exam can then be performed to assess central uterine tenderness. Recording the pattern of abdominopelvic tenderness as well as the location and pattern of worst pain provides a framework for considering pelvic pain triggers.
To supplement the pelvic examination, a brief neuro-musculoskeletal pain assessment can identify signs of widespread pain, central sensitization, and myofascial dysfunction. Each dermatome and myotome is evaluated with the patient reporting when pain is evoked (Table 1). Dermatomes are assessed for allodynia and hyperalgesia bilaterally over the skin, approximately 2.5 cm lateral to each spinal process. Allodynia is assessed using the pinch-and-roll technique or by brushing the skin with a thin microfilament, while hyperalgesia is assessed by scratching the skin with the edge of a paper clip or Wartenberg pinwheel. The myotome is assessed for the presence of MTrPs. By examining a series of muscles spanning the length of the body, the examiner can evaluate the distribution of myofascial dysfunction and extent of overall sensitization.
These complementary exams together provide a more complete assessment of patients with CPP associated with endometriosis. The pelvic exam is valuable for identifying pelvic floor tenderness and spasm, which may be potential triggers for persistent pain and may warrant directed treatment. The neuro-musculoskeletal exam identifies alterations in pain perception beyond the pelvic muscles, placing the pelvic exam findings in the broader perspective of global neuromuscular and myofascial dysfunction.
Myofascial Release Techniques for Chronic Pelvic Pain
Treatment for pelvic pain associated with endometriosis warrants identification and therapy directed to the pathological findings that generate and sustain pain symptoms. Since a myofascial source may contribute to endometriosis-associated CPP even after hormonal and surgical treatment has been undertaken, a growing number of practitioners are exploring pain management methods that directly address myofascial pain.
One set of treatment is collectively known as myofascial release, which involves physical therapy and other manual techniques including deep pressure massage, stretching techniques, joint mobilization, and foam rollers, often in combination with teaching about strategies used to manage pain, including breathing and relaxation exercises.37,44 This dual approach addresses physiological and psychological components of chronic myofascial pain, alleviates MTrP-related pain, and furnishes patients with coping strategies to redirect their focus during a painful episode. Small studies tout the effectiveness of these techniques in the treatment of myofascial pelvic pain, including one retrospective study which showed that physical therapy benefits up to 63% of patients who attempt it.37,45 However, no randomized, controlled trials have compared physical therapy with the standard of care for endometriosis patients with MTrPs.
MTrP injection, with or without a topical anesthetic (wet or dry needling, respectively), is another common form of myofascial treatment being studied. The theoretical basis of MTrP injection is that entry into a hypercontracted muscle causes mechanical disruption that interrupts the aberrant sensory signals that cause MTrP formation.44 Direct injection of a local anesthetic such as lidocaine is thought to further blunt the ability to transmit pain signals through these hyperactive neural networks, providing additional pain relief that may be more durable than needling of MTrPs alone. Prospective studies using dry needling have not been performed in the pelvic region. However, dry needling in other body regions has been shown to reduce pain and is non-inferior to wet needling, though pain relief from MTrP anesthetic injections is longer lasting.44,46 For CPP patients with MTrPs in the abdominal wall, direct injections with lidocaine provided superior clinical response rates to physical therapy out to 12 weeks after treatment,47 and small studies of CPP patients who received lidocaine injections in the pelvic musculature report pain relief as well.48,49
Botulinum Toxin Type A: Alleviating Hypertonicity to Lessen Myofascial Pelvic Pain Associated with Endometriosis
Botulinum toxin (BTX) is the first-line therapy for focal dystonias, and is an increasingly common treatment for other neuromuscular conditions arising from excessive muscle activity.50,51 Randomized, controlled trials evaluating the effectiveness of BTX in relieving myofascial pain in the neck, cervicothoracic region, shoulder, and upper back have generated mixed results.52–54 There have been a limited number of studies to date, including two double-blind, placebo controlled trials, evaluating BTX injection into pelvic floor muscles for CPP. Further investigation is required to explore this approach, including optimization of dosage and injection technique; however, the current evidence suggests that BTX may be effective in treating endometriosis-associated CPP.
The clinical efficacy of BTX in treating spasticity, dystonia, hemifacial spasm, overactive bladder, and migraine has been well established. BTX is a neurotoxin produced by Clostridium botulinum that irreversibly blocks acetylcholine release at the neuromuscular junction.55 This action prevents the transmission of signals that stimulate muscle fibers. When the dose is carefully titrated, excessive muscle contraction and spasm can be eased without muscle paralysis. Clinically, a reduction of muscle spasm and associated pain results.55 Skeletal muscle strength decreases within 2 to 5 days after injection, reaches its nadir within 2 weeks, and recovers gradually after that. The effects of BTX on the neuromuscular junction are irreversible, requiring new motor axon regeneration and restoration of the neuromuscular junction. The period of clinical efficacy typically lasts 3 to 6 months for approved indications, so that reinjection is required to maintain benefit. The safety of repeated BTX injection has been established through over 20 years of clinical use. Two toxin serotypes (A and B) and several brands of toxin are currently commercially available.
The duration of therapeutic effects, well-characterized muscle relaxant activity, and evidence of direct analgesia make BTX an ideal candidate for treatment of myofascial CPP in the setting of endometriosis. Several case studies and small trials have examined the utility of BTX injections for treatment of various types of CPP (Table 2). The first documented gynecologic use for BTX in 1997 included electromyography (EMG) to identify hyperactive areas of the anterior vaginal wall in a woman with vaginismus. A series of two injections over 7 weeks resolved bladder, urethral, and vaginal pain symptoms.65 A subsequent series of studies from Australian researchers examined type A BTX for the treatment of levator ani spasm and showed a reduction in resting pelvic floor pressure by manometry as well as a statistically significant improvement in dyspareunia and nonmenstrual pelvic pain.56,59,61,66 A randomized placebo-controlled trial from this same group examining type A BTX for 60 women showed that only improvement in nonmenstrual pelvic pain was unique to the treatment group, implying that dry needling may contribute to pain relief at MTrPs.56 Other groups have observed benefits of BTX injections for dysmenorrhea and quality of life in patients with pelvic floor spasm.62–64 Unfortunately, not all studies have shown efficacy; in a small randomized controlled trial examining 60 women with vestibulodynia, a low dose of type A BTX into the vestibule did not significantly improve pain or quality-of-life measures at 3 or 6 months after injection compared with placebo.57 It is likely that differences in dose, dilution, muscle selection, and injection technique contributed to the differences seen in these studies’ outcomes. Further research is needed on each of these aspects of BTX treatment.
Table 2.
Clinical studies evaluating botulinum toxin type A treatment for chronic pelvic pain
| Study type | Authors (year) | N | Total BTX-A dose | Areas injected | Outcomes |
|---|---|---|---|---|---|
| Randomized controlled trial | Abbott et al56 (2006) | 60 | Onabotulinum-toxin A 80 U | Puborectalis and pubococcygeus bilaterally | Both treatment and placebo groups had reductions in dyspareunia and pelvic floor pressure BTX-A group alone showed improved nonmenstrual pain |
| Petersen et al57 (2009) | 64 | Onabotulinum-toxin A 20 U | Musculus bulbospongiosus | No improvement compared with placebo at 3 and 6 mo; both treatment and placebo groups had pain reduction at 6 mo | |
| Prospective open-label study | Ghazizadeh and Nikzad 58 (2004) | 23 | Abobotulinum-toxin A 150–400 U | Puborectalis at 3 points bilaterally | Dyspareunia and vaginismus subjectively improved |
| Jarvis et al59 (2004) | 12 | Onabotulinum-toxin A 40 U | Puborectalis and pubococcygeus bilaterally | Dyspareunia and dysmenorrhea significantly improved Significant reduction in resting pelvic floor muscle pressure | |
| Bertolasi et al60 (2009) | 39 | Type A toxin not otherwise specified up to 8 repeat cycles over 120 wk | Levator ani | Dyspareunia, levator ani hyperactivity by EMG improved Improvements in subjective pain, function, and quality of life by standardized pelvic pain surveys | |
| Nesbitt-Hawes et al61 (2012) | 37 | Onabotulinum-toxin A 100 U | Puborectalis and pubococcygeus bilaterally | 26 women received single injection 11 women received two or more injections Dyspareunia, nonmenstrual pelvic pain, and vaginal pressures significantly improved in both groups |
|
| Morrissey et al62 (2015) | 21 | Onabotulinum-toxin A Up to 300 U | Levator ani | Dyspareunia and sexual dysfunction significantly improved Significant reduction in pelvic floor muscle tenderness, resting pressures, and in maximum contraction pressures | |
| Retrospective cohort study | Adelowo et al63 (2013) | 29 | Onabotulinum-toxin A 100–300 U | Individualized tender and contracted points in the pelvic floor muscles | Levator tenderness on palpation significantly improved Self-reported urinary incontinence decreased significantly |
| Case series | Romito et al64 (2004) | 2 | Abobotulinum-toxin A 40–80U | Levator ani | Complete resolution of pelvic pain and spasm |
| Case report | Brin and Vapnek et al65 (1997) | 1 | Onabotulinum-toxin A Two 10 U injections then 40 U after 7 wk | Anterior vaginal wall muscles | Vaginal, bladder, and urethral spasms subjectively improved |
| Thomson et al66 (2005) | 1 | Onabotulinum-toxin A Two 40 U injections then two 80 U injections, ~20 wk apart | Puborectalis and pubococcygeus bilaterally | Dyspareunia, dysmenorrhea, and dyschezia improved after each injection Reduction in pelvic floor muscle manometry readings |
|
| Park and Paraiso67 (2009) | 1 | Type A toxin not otherwise specified. 40 U | Levator ani and pubococcygeus | Unresolved postsurgical dyspareunia improved |
At our center, we conducted a randomized placebo-controlled pilot study in which seven women with CPP, most of whom had laparoscopically confirmed endometriosis, received either 100 U onabotulinum toxin A diluted into 4 mL of saline or placebo injected transvaginally into the areas of pelvic floor spasm in the levator ani muscles under EMG guidance (data not published). The injections were part of a comprehensive approach to pelvic pain after surgical treatment of endometriosis in which patients could also choose to use hormonal contraceptives, progestin-releasing intrauterine devices (IUDs), analgesics, and have pain and palliative care therapy including massage, acupuncture, and mind–body therapies.
Women in both active drug and placebo groups experienced improvement in pain. The duration of pain improvement was longer in those who received BTX (3.5 weeks for BTX vs. 1 week for placebo). Women receiving BTX were more likely to be able to quantify the extent and duration of benefit from injection, whereas those who received placebo reported benefit in vague terms. These findings suggest, as in the Abbott et al study, that dry needling of MTrPs may have provided at least some brief pain relief to women in the placebo group.56 These preliminary data are difficult to interpret, as those receiving BTX had lower overall pain severity prior to injection than those in the placebo group (3.6 vs. 8.5 on VAS scale from 0 to 10). However, these data were promising enough that we are now conducting a double-blind, placebo-controlled study of BTX for CPP in women with surgically documented endometriosis (NCT01553201).
BTX is, thus, a promising treatment for endometriosis-associated CPP. With regard to the myofascial contribution to CPP characterized by MTrPs of muscle fibers that are in a prolonged state of contracture, BTX may be effective in relaxing the muscles of the pelvic floor that are unlikely to respond to surgery or hormonal therapy, and that may not respond to myofascial release techniques.
BTX may also have direct analgesic effects that complement the effects on muscle relaxation.68 Although BTX is highly specific for cholinergic neurons, it has shown additional effects on the secretion of peripheral and central neurotransmitters in culture and in mouse models.68 These studies have shown a decrease in the release of proinflammatory neuropeptides such as substance P and CGRP, a resultant decrease in inflammation, and alterations in intracellular trafficking patterns that, altogether, may contribute to an overall decrease in pain and inflammation.68
In addition, the effects of BTX on pain may not be limited to peripheral benefits. The inflammatory nature of the endometriosis lesions as well as the abnormal contracture of MTrPs all contribute to a feed-forward mechanism that eventually restructures how pain is centrally processed and experienced. Research studies have shown that the release of the MTrPs is followed by a corresponding decrease in the levels of substances associated with pain and inflammation around the area of treatment.69,70 Lowering the levels of these proinflammatory molecules reduces the barrage of noxious input from the periphery that cause maladaptive changes within the CNS.71 Thus, BTX may not only target the peripheral sources of CPP but can also work in conjunction with the usual gynecologic treatments in endometriosis to reduce peripheral and central sensitization and restore normal pain and sensory information processing.
Conclusion
It is important to consider CPP associated with endometriosis from a global pain-centered perspective, as MTrPs and sensitization appear to contribute significantly to the clinical manifestations. While direct innervation of endometrial lesions may set the stage for visceral nociception and peripheral sensitization, over time, central sensitization creates a process for pain sustention that is independent of the initial pathology and is potentially reversible.41 Viscerosomatic convergence may not only provide the means for pain referral to somatic structures but also govern the reflex that induces muscle spasm and the eventual formation of MTrPs. Painful MTrPs, in turn, may serve as an additional source of nociceptive input, and become a key component of CPP. Their deactivation through a targeted intervention may be a critical aspect to reversing central sensitization and improving pain associated with endometriosis.
Acknowledgments
This work was funded by the Intramural Research Program of the National Institutes of Health, the NIH Clinical Center, and the National Institute of Neurological Disorders and Stroke.
References
- 1.Practice bulletin no. 114: management of endometriosis. Obstet Gynecol. 2010;116(1):223–236. doi: 10.1097/AOG.0b013e3181e8b073. [DOI] [PubMed] [Google Scholar]
- 2.Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG. Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Hum Reprod. 2007;22(1):266–271. doi: 10.1093/humrep/del339. [DOI] [PubMed] [Google Scholar]
- 3.Hsu AL, Sinaii N, Segars J, Nieman LK, Stratton P. Relating pelvic pain location to surgical findings of endometriosis. Obstet Gynecol. 2011;118(2 Pt 1):223–230. doi: 10.1097/AOG.0b013e318223fed0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Institute of Medicine (US) Committee on Pain D, and Chronic Illness Behavior. The Anatomy and Physiology of Pain. In: Osterweis M, Kleinman A, Mechanic D, editors. Pain and Disability: Clinical, Behavioral, and Public Policy Perspectives. Washington, DC: National Academy Press; 1987. pp. 123–145. [PubMed] [Google Scholar]
- 5.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratton P, Berkley KJ. Chronic pelvic pain and endometriosis: translational evidence of the relationship and implications. Hum Reprod Update. 2011;17(3):327–346. doi: 10.1093/humupd/dmq050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burnstock G. Autonomic neurotransmission: 60 years since sir Henry Dale. Annu Rev Pharmacol Toxicol. 2009;49:1–30. doi: 10.1146/annurev.pharmtox.052808.102215. [DOI] [PubMed] [Google Scholar]
- 8.May K, Becker CM. Endometriosis and angiogenesis. Minerva Ginecol. 2008;60(3):245–254. [PubMed] [Google Scholar]
- 9.Raab S, Plate KH. Different networks, common growth factors: shared growth factors and receptors of the vascular and the nervous system. Acta Neuropathol. 2007;113(6):607–626. doi: 10.1007/s00401-007-0228-3. [DOI] [PubMed] [Google Scholar]
- 10.Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- 11.Anaf V, Simon P, El Nakadi I, et al. Hyperalgesia, nerve infiltration and nerve growth factor expression in deep adenomyotic nodules, peritoneal and ovarian endometriosis. Hum Reprod. 2002;17(7):1895–1900. doi: 10.1093/humrep/17.7.1895. [DOI] [PubMed] [Google Scholar]
- 12.Tokushige N, Markham R, Russell P, Fraser IS. Nerve fibres in peritoneal endometriosis. Hum Reprod. 2006;21(11):3001–3007. doi: 10.1093/humrep/del260. [DOI] [PubMed] [Google Scholar]
- 13.Berkley KJ, Dmitrieva N, Curtis KS, Papka RE. Innervation of ectopic endometrium in a rat model of endometriosis. Proc Natl Acad Sci U S A. 2004;101(30):11094–11098. doi: 10.1073/pnas.0403663101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkley KJ, Rapkin AJ, Papka RE. The pains of endometriosis. Science. 2005;308(5728):1587–1589. doi: 10.1126/science.1111445. [DOI] [PubMed] [Google Scholar]
- 15.Mechsner S, Kaiser A, Kopf A, Gericke C, Ebert A, Bartley J. A pilot study to evaluate the clinical relevance of endometriosis-associated nerve fibers in peritoneal endometriotic lesions. Fertil Steril. 2009;92(6):1856–1861. doi: 10.1016/j.fertnstert.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 16.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20(4):629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 17.Beste MT, Pfäffle-Doyle N, Prentice EA, et al. Molecular network analysis of endometriosis reveals a role for c-Jun-regulated macrophage activation. Sci Transl Med. 2014;6(222):222ra16. doi: 10.1126/scitranslmed.3007988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anaf V, Chapron C, El Nakadi I, De Moor V, Simonart T, Noël JC. Pain, mast cells, and nerves in peritoneal, ovarian, and deep infiltrating endometriosis. Fertil Steril. 2006;86(5):1336–1343. doi: 10.1016/j.fertnstert.2006.03.057. [DOI] [PubMed] [Google Scholar]
- 19.Willard F. Basic Mechanisms of Pain. In: Audette JF, Bailey A, editors. Integrative Pain Medicine: The Science and Practice of Complementary and Alternative Medicine in Pain Management. Totowa, NJ: Humana Press; 2008. pp. 19–61. [Google Scholar]
- 20.Sauer SK, Reeh PW, Bove GM. Noxious heat-induced CGRP release from rat sciatic nerve axons in vitro. Eur J Neurosci. 2001;14(8):1203–1208. doi: 10.1046/j.0953-816x.2001.01741.x. [DOI] [PubMed] [Google Scholar]
- 21.Saria A. Substance P in sensory nerve fibres contributes to the development of oedema in the rat hind paw after thermal injury. Br J Pharmacol. 1984;82(1):217–222. doi: 10.1111/j.1476-5381.1984.tb16461.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chiu IM, von Hehn CA, Woolf CJ. Neurogenic inflammation and the peripheral nervous system in host defense and immunopathology. Nat Neurosci. 2012;15(8):1063–1067. doi: 10.1038/nn.3144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gebhart GF, Bonica JJ. Lecture–2000: physiology, pathophysiology, and pharmacology of visceral pain. Reg Anesth Pain Med. 2000;25(6):632–638. doi: 10.1053/rapm.2000.18187. [DOI] [PubMed] [Google Scholar]
- 24.Cervero F, Tattersall JE. Somatic and visceral sensory integration in the thoracic spinal cord. Prog Brain Res. 1986;67:189–205. doi: 10.1016/s0079-6123(08)62763-6. [DOI] [PubMed] [Google Scholar]
- 25.Jänig W, Morrison JF. Functional properties of spinal visceral afferents supplying abdominal and pelvic organs, with special emphasis on visceral nociception. Prog Brain Res. 1986;67:87–114. doi: 10.1016/s0079-6123(08)62758-2. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz ES, Gebhart GF. Visceral pain. Curr Top Behav Neurosci. 2014;20:171–197. doi: 10.1007/7854_2014_315. [DOI] [PubMed] [Google Scholar]
- 27.Rogers JR. Basic pelvic neuroanatomy. In: Steege JF, Metzger DA, Levy BS, editors. Chronic Pelvic Pain: An Integrated Approach. Philadelphia: W.B. Saunders Company; 1998. pp. 31–58. [Google Scholar]
- 28.Sugiura Y, Tonosaki Y. Spinal organization of unmyelinated visceral afferent fibers in comparison with somatic afferent fibers. In: Gebhart GF, editor. Visceral Pain: Progress in Pain Research and Management Series. Vol. 5. Seattle, WA: IASP Press; 1995. pp. 41–59. [Google Scholar]
- 29.Nagabukuro H, Berkley KJ. Influence of endometriosis on visceromotor and cardiovascular responses induced by vaginal distention in the rat. Pain. 2007;132(Suppl 1):S96–S103. doi: 10.1016/j.pain.2007.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jarrell J. Myofascial dysfunction in the pelvis. Curr Pain Headache Rep. 2004;8(6):452–456. doi: 10.1007/s11916-004-0066-0. [DOI] [PubMed] [Google Scholar]
- 31.Willard FH. Nociception, the neuroendocrine immune system, and osteopathic medicine. In: Ward RC, Hruby RJ, Jerome JA, editors. Foundations for Osteopathic Medicine. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 137–156. [Google Scholar]
- 32.Patterson MM, Wurster RD. Neurophysiologic mechanisms of integration and disintegration. In: Ward RC, Hruby RJ, Jerome JA, editors. Foundations for Osteopathic Medicine. 2. Philadelphia, PA: Lippincott Williams & Wilkins; 2003. pp. 1370–1156. [Google Scholar]
- 33.Butrick CW. Discordant urination and defecation as symptoms of pelvic floor dysfunction. In: Howard FM, editor. Pelvic Pain: Diagnosis and Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 280–299. [Google Scholar]
- 34.Simons DG. Clinical and etiological update of myofascial pain from trigger points. J Musculoskeletal Pain. 1996;4(1–2):93–122. [Google Scholar]
- 35.Simons DG, Travell JG, Simons LS. Myofascial Pain and Dysfunction: The Trigger Point Manual. 2. Vol. 1. Baltimore, MD: Williams & Wilkins; 1999. [Google Scholar]
- 36.Simons DG, Travell JG, Simons LS. Myofascial Pain and Dysfunction: The Trigger Point Manual. Vol. 2. Baltimore, MD: Williams & Wilkins; 1992. [Google Scholar]
- 37.Pastore EA, Katzman WB. Recognizing myofascial pelvic pain in the female patient with chronic pelvic pain. J Obstet Gynecol Neonatal Nurs. 2012;41(5):680–691. doi: 10.1111/j.1552-6909.2012.01404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stratton P, Khachikyan I, Sinaii N, Ortiz R, Shah J. Association of chronic pelvic pain and endometriosis with signs of sensitization and myofascial pain. Obstet Gynecol. 2015;125(3):719–728. doi: 10.1097/AOG.0000000000000663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moldwin RM, Fariello JY. Myofascial trigger points of the pelvic floor: associations with urological pain syndromes and treatment strategies including injection therapy. Curr Urol Rep. 2013;14(5):409–417. doi: 10.1007/s11934-013-0360-7. [DOI] [PubMed] [Google Scholar]
- 40.Gerwin RD. Myofascial and visceral pain syndromes: visceralsomatic pain representations. J Musculoskeletal Pain. 2002;10(1–2):165–175. [Google Scholar]
- 41.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah JP, Thaker N, Heimur J, Aredo JV, Sikdar S, Gerber L. Myofascial trigger points then and now: a historical and scientific perspective. PM R. 2015;7(7):746–761. doi: 10.1016/j.pmrj.2015.01.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter JE. Abdominal wall and pelvic myofascial trigger points. In: Howard FM, editor. Pelvic Pain: Diagnosis & Management. Philadelphia, PA: Lippincott Williams & Wilkins; 2000. pp. 314–358. [Google Scholar]
- 44.Desai MJ, Saini V, Saini S. Myofascial pain syndrome: a treatment review. Pain Ther. 2013;2(1):21–36. doi: 10.1007/s40122-013-0006-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bedaiwy MA, Patterson B, Mahajan S. Prevalence of myofascial chronic pelvic pain and the effectiveness of pelvic floor physical therapy. J Reprod Med. 2013;58(11–12):504–510. [PubMed] [Google Scholar]
- 46.Ay S, Evcik D, Tur BS. Comparison of injection methods in myofascial pain syndrome: a randomized controlled trial. Clin Rheumatol. 2010;29(1):19–23. doi: 10.1007/s10067-009-1307-8. [DOI] [PubMed] [Google Scholar]
- 47.Montenegro ML, Braz CA, Rosa-e-Silva JC, Candido-dos-Reis FJ, Nogueira AA, Poli-Neto OB. Anaesthetic injection versus ischemic compression for the pain relief of abdominal wall trigger points in women with chronic pelvic pain. BMC Anesthesiol. 2015;15:175. doi: 10.1186/s12871-015-0155-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langford CF, Udvari Nagy S, Ghoniem GM. Levator ani trigger point injections: An underutilized treatment for chronic pelvic pain. Neurourol Urodyn. 2007;26(1):59–62. doi: 10.1002/nau.20393. [DOI] [PubMed] [Google Scholar]
- 49.Kim DS, Jeong TY, Kim YK, Chang WH, Yoon JG, Lee SC. Usefulness of a myofascial trigger point injection for groin pain in patients with chronic prostatitis/chronic pelvic pain syndrome: a pilot study. Arch Phys Med Rehabil. 2013;94(5):930–936. doi: 10.1016/j.apmr.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 50.Karp BI. Botulinum toxin treatment of occupational and focal hand dystonia. Mov Disord. 2004;19(Suppl 8):S116–S119. doi: 10.1002/mds.20025. [DOI] [PubMed] [Google Scholar]
- 51.Münchau A, Bhatia KP. Uses of botulinum toxin injection in medicine today. BMJ. 2000;320(7228):161–165. doi: 10.1136/bmj.320.7228.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Göbel H, Heinze A, Reichel G, Hefter H, Benecke R Dysport myofascial pain study group. Efficacy and safety of a single botulinum type A toxin complex treatment (Dysport) for the relief of upper back myofascial pain syndrome: results from a randomized double-blind placebo-controlled multicentre study. Pain. 2006;125(1–2):82–88. doi: 10.1016/j.pain.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 53.Harden RN, Cottrill J, Gagnon CM, et al. Botulinum toxin a in the treatment of chronic tension-type headache with cervical myofascial trigger points: a randomized, double-blind, placebo-controlled pilot study. Headache. 2009;49(5):732–743. doi: 10.1111/j.1526-4610.2008.01286.x. [DOI] [PubMed] [Google Scholar]
- 54.Kwanchuay P, Petchnumsin T, Yiemsiri P, Pasuk N, Srikanok W, Hathaiareerug C. Efficacy and safety of single botulinum toxin type A (Botox®) injection for relief of upper trapezius myofascial trigger point: a randomized, double-blind, placebo-controlled study. J Med Assoc Thai. 2015;98(12):1231–1236. [PubMed] [Google Scholar]
- 55.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 56.Abbott JA, Jarvis SK, Lyons SD, Thomson A, Vancaille TG. Botulinum toxin type A for chronic pain and pelvic floor spasm in women: a randomized controlled trial. Obstet Gynecol. 2006;108(4):915–923. doi: 10.1097/01.AOG.0000237100.29870.cc. [DOI] [PubMed] [Google Scholar]
- 57.Petersen CD, Giraldi A, Lundvall L, Kristensen E. Botulinum toxin type A-a novel treatment for provoked vestibulodynia? Results from a randomized, placebo controlled, double blinded study. J Sex Med. 2009;6(9):2523–2537. doi: 10.1111/j.1743-6109.2009.01378.x. [DOI] [PubMed] [Google Scholar]
- 58.Ghazizadeh S, Nikzad M. Botulinum toxin in the treatment of refractory vaginismus. Obstet Gynecol. 2004;104(5 Pt 1):922–925. doi: 10.1097/01.AOG.0000141441.41178.6b. [DOI] [PubMed] [Google Scholar]
- 59.Jarvis SK, Abbott JA, Lenart MB, Steensma A, Vancaillie TG. Pilot study of botulinum toxin type A in the treatment of chronic pelvic pain associated with spasm of the levator ani muscles. Aust N Z J Obstet Gynaecol. 2004;44(1):46–50. doi: 10.1111/j.1479-828X.2004.00163.x. [DOI] [PubMed] [Google Scholar]
- 60.Bertolasi L, Frasson E, Cappelletti JY, Vicentini S, Bordignon M, Graziottin A. Botulinum neurotoxin type A injections for vaginismus secondary to vulvar vestibulitis syndrome. Obstet Gynecol. 2009;114(5):1008–1016. doi: 10.1097/AOG.0b013e3181bb0dbb. [DOI] [PubMed] [Google Scholar]
- 61.Nesbitt-Hawes EM, Won H, Jarvis SK, Lyons SD, Vancaillie TG, Abbott JA. Improvement in pelvic pain with botulinum toxin type A—single vs. repeat injections. Toxicon. 2013;63:83–87. doi: 10.1016/j.toxicon.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 62.Morrissey D, El-Khawand D, Ginzburg N, Wehbe S, O’Hare P, III, Whitmore K. Botulinum toxin A injections into pelvic floor muscles under electromyographic guidance for women with refractory high-tone pelvic floor dysfunction: a 6-month prospective pilot study. Female Pelvic Med Reconstr Surg. 2015;21(5):277–282. doi: 10.1097/SPV.0000000000000177. [DOI] [PubMed] [Google Scholar]
- 63.Adelowo A, Hacker MR, Shapiro A, Modest AM, Elkadry E. Botulinum toxin type A (BOTOX) for refractory myofascial pelvic pain. Female Pelvic Med Reconstr Surg. 2013;19(5):288–292. doi: 10.1097/SPV.0b013e3182989fd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Romito S, Bottanelli M, Pellegrini M, Vicentini S, Rizzuto N, Bertolasi L. Botulinum toxin for the treatment of genital pain syndromes. Gynecol Obstet Invest. 2004;58(3):164–167. doi: 10.1159/000079620. [DOI] [PubMed] [Google Scholar]
- 65.Brin MF, Vapnek JM. Treatment of vaginismus with botulinum toxin injections. Lancet. 1997;349(9047):252–253. doi: 10.1016/S0140-6736(05)64862-3. [DOI] [PubMed] [Google Scholar]
- 66.Thomson AJ, Jarvis SK, Lenart M, Abbott JA, Vancaillie TG. The use of botulinum toxin type A (BOTOX) as treatment for intractable chronic pelvic pain associated with spasm of the levator ani muscles. BJOG. 2005;112(2):247–249. doi: 10.1111/j.1471-0528.2004.00315.x. [DOI] [PubMed] [Google Scholar]
- 67.Park AJ, Paraiso MF. Successful use of botulinum toxin type a in the treatment of refractory postoperative dyspareunia. Obstet Gynecol. 2009;114(2 Pt 2):484–487. doi: 10.1097/AOG.0b013e3181998ce1. [DOI] [PubMed] [Google Scholar]
- 68.Pellett S, Yaksh TL, Ramachandran R. Current status and future directions of botulinum neurotoxins for targeting pain processing. Toxins (Basel) 2015;7(11):4519–4563. doi: 10.3390/toxins7114519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shah JP, Phillips TM, Danoff JV, Gerber LH. An in vivo microanalytical technique for measuring the local biochemical milieu of human skeletal muscle. J Appl Physiol (1985) 2005;99(5):1977–1984. doi: 10.1152/japplphysiol.00419.2005. [DOI] [PubMed] [Google Scholar]
- 70.Shah JP, Danoff JV, Desai MJ, et al. Biochemicals associated with pain and inflammation are elevated in sites near to and remote from active myofascial trigger points. Arch Phys Med Rehabil. 2008;89(1):16–23. doi: 10.1016/j.apmr.2007.10.018. [DOI] [PubMed] [Google Scholar]
- 71.Shah JP, Gilliams EA. Uncovering the biochemical milieu of myofascial trigger points using in vivo microdialysis: an application of muscle pain concepts to myofascial pain syndrome. J Bodyw Mov Ther. 2008;12(4):371–384. doi: 10.1016/j.jbmt.2008.06.006. [DOI] [PubMed] [Google Scholar]