Abstract
Introduction: Previous studies have reported an association between classic galactosemia (CG) and decreased bone mass. The primary objective of this systematic review with meta-analysis was to determine the extent of bone mineral density (BMD) Z-score reduction. Low BMD was defined as a Z-score ≤−2 standard deviations (SD). The secondary objective was to evaluate other indicators of bone status through a descriptive analysis.
Methods: Systematic search strategies were developed by an experienced clinical librarian. Selection of relevant manuscripts, risk of bias assessment, and data extraction were performed independently by two investigators.
Results: Four studies were included in the meta-analysis. BMD Z-scores in children and adults with CG measured at the lumbar spine (LBMD; 4 studies; n = 112), total hip (HBMD; 2 studies; n = 58), and femoral neck (FBMD; 2 studies; n = 73) were assessed. Mean BMD Z-scores in the CG population were LBMD −0.70 (95% CI: −0.88, −0.52); HBMD −0.89 (95% CI: −1.14, −0.64); and FBMD −0.63 (95% CI −1.29, 0.02). Results from studies included in the descriptive analysis (n = 7) show that vitamin D levels were frequently in the low reference range, whereas serum calcium levels were within reference range.
Conclusion: The mean BMD Z-score in the CG population is −0.7, which is lower than in the general population, though still within two SD of the reference mean of zero. This indicates that bone health is mildly affected in CG and that more patients, compared to the general population, are at risk for a BMD Z-score ≤−2 SD. In conclusion, clinicians should ensure appropriate preventive and therapeutic measures for CG patients.
Electronic supplementary material: The online version of this chapter (doi:10.1007/8904_2016_28) contains supplementary material, which is available to authorized users.
Keywords: Bone mass, Bone mineral density, Bone turnover markers, Classic galactosemia, DXA, GALT deficiency, Vitamin D
Introduction
Classic galactosemia (CG, MIM 230400), a genetic disorder of galactose metabolism due to deficiency of galactose-1-phosphate uridyltransferase (GALT; EC 2.7.7.12), is characterized by the occurrence of late complications in spite of early diagnosis and lifelong dietary treatment. The first report of an association between CG and decreased bone mass dates back to 1993 (Kaufman et al. 1993) and a bone mineral density (BMD) Z-score more than two standard deviations (SD) below the mean was found in 25–30% of adult patients (Waisbren et al. 2012; Batey et al. 2013). Thorough evaluation of the frequency and severity of impaired bone health in CG patients, compared to the non-galactosemia population, is crucial for determining its extent and relevance for patient care.
CG patients could be at risk for compromised bone health due to diet restrictions, ovarian insufficiency in women, limited physical activity in some cases, and possibly unknown intrinsic factors associated with the disease. Sufficient intake of calories, protein, and micronutrients is essential for acquiring an optimal bone mass, and the lifelong galactose restriction may predispose patients to nutritional deficiencies (Wiesmann et al. 1995; Rutherford et al. 2002; El-Bassyouni et al. 2006; Waisbren et al. 2012). Furthermore, ovarian damage resulting in low estrogen concentrations is present in over 80% of female patients with CG (Fridovich-Keil et al. 2011), which increases their susceptibility to the development of low bone mass. Remarkably, supplementation of calcium, vitamins, and estrogen only seems to partially improve bone mass (Kaufman et al. 1993; Renner et al. 1999; Panis et al. 2006), which may point to the presence of an underlying intrinsic bone defect. Aberrant glycosylation of collagen and other glycoproteins related to bone metabolism, which is also seen in patients with other glycosylation defects such as phosphomannomutase 2 deficiency (PMM2-CDG) (Bons et al. 2008), has been suggested as a potential intrinsic abnormality (Kaufman et al. 1993; Coss et al. 2013) and the decreased IGF-I and IGFBP-3 concentrations that are found in patients might reflect this (Panis et al. 2007). Furthermore, reduced physical activity due to motor abnormalities (Rubio-Agusti et al. 2013) and cognitive impairment (Schweitzer et al. 1993; Rasmussen et al. 1996; Shield et al. 2000; Hoffmann et al. 2011) may affect bone health as well. Clinical practice, focused on reducing the risk of impaired bone health in those with CG, includes routine monitoring of BMD, which is an important determinant of bone strength and has predictive value in assessing fracture risk (Marchall et al. 1996), and optimization of exogenous factors affecting bone mass (nutrition, estrogen concentrations, physical activity) (Van Erven et al. 2014).
The primary aim of our study is to evaluate the extent of impaired bone health in patients with CG, and the need of monitoring and treatment. Few studies assessing bone health in CG are published. These studies have small patient sample sizes, and results vary from one study to another. As the current conjecture of low BMD in CG is based on single studies, further evaluation is needed to confirm the validity of this hypothesis (Haidich 2010). Meta-analysis is the most commonly used statistical technique to pool results from two or more separate studies. The added value of a meta-analysis may include increased power and improved precision of the results. Therefore, we performed a systematic review with meta-analysis of BMD in children and adults with CG. As recommended by the International Society for Clinical Densitometry (ISCD), low BMD is defined here as a BMD Z-score ≤−2.0 SD (Schousboe et al. 2013; Gordon et al. 2014). We also explored the usefulness of other indicators of bone status as potential diagnostic or monitoring tools.
Methods
Research Question
The primary outcome for our meta-analysis was bone mass reported as BMD (areal BMD), either as Z-score or as absolute measurement, assessed with dual-energy X-ray absorptiometry (DXA) since this is the preferred tool for evaluating BMD/bone mass in both children and adults (Schousboe et al. 2013; Gordon et al. 2014). BMD is a major determinant of bone strength and its assessment is considered the cornerstone in the diagnostics of low bone mass (Kanis et al. 2013). Only areal BMD measurements were included in the analysis since these are most commonly used in clinical practice; results of estimated volumetric BMD were not included.
The secondary outcomes for our systematic review, reported in a descriptive way, were bone mineral content (BMC), and parameters involved in bone metabolism such as vitamins, minerals, hormones, bone turnover markers (BTM), and fracture risk.
Inclusion and Exclusion Criteria for Study Selection
We included all original studies (cross-sectional study design, randomized controlled trial [RCT], cohort study, case-control study) as well as conference abstracts on bone health in children and adults with CG. If there was (potential) overlap between study cohorts, only the first/original article was included. Articles in a language other than English, and animal and cell studies were excluded.
Search Strategy
A computerized literature search was conducted in MEDLINE, EMBASE, and the Cochrane Library by one of the investigators (LW) and a trained clinical librarian from University of Amsterdam (see Supplementary data S1, Search strategies). Databases were searched initially in February 2015 and a final search was completed in July 2015 to ensure the inclusion of recently published articles. No limits were used in these searches.
Study Selection
Titles and abstracts generated by literature searches were screened by two separate researchers (BvE and LW) to select potentially eligible studies. Studies not relating to the research question were excluded. Selected conference abstracts and full text articles of selected studies were independently reviewed by two authors (BvE and LW) for inclusion in the systematic review. In case of exclusion, the reason was reported. Additionally, reference lists from included trials and excluded narrative or systematic reviews were hand-searched to identify additional relevant studies. Consensus was reached between the two authors regarding the eligibility assessments.
Data Extraction
Data were extracted by two separate researchers (BvE and LW) according to predefined criteria (see Supplementary data S2a and S2b, Summary of evidence tables). Outcome measures of interest were BMD at any site measured with DXA; BMC; incidence of fractures; BTM reflecting bone formation (bone-specific alkaline phosphatase, under-carboxylated osteocalcin [ucOC], carboxylated osteocalcin [cOC]), bone resorption (N-terminal telopeptide, C-terminal telopeptide) or bone modeling (insulin-like growth factor-1 [IGF-1]); and vitamins (vitamin D), minerals (calcium), and hormones (estradiol, parathormone) important for bone metabolism.
Data Collection Processes
If a mean BMD Z-score and/or standard deviation (SD) was not reported for the entire cohort in an article, the authors were requested to share either these variables together with mean age at testing and SD or individual patient data (IPD). IPD included individual mean BMD Z-score with SD, age at testing, and gender. The corresponding author was contacted first, and if there was no reply within 4 weeks, one of the other authors was contacted.
Individual Patient Data Integrity
IPD were checked for integrity by reviewing completeness; in case of incompleteness the data were not included in the systematic review. If more data were received from the authors than originally published, these were only included if patient characteristics matched those from the original article. In case of any discrepancies, the authors were contacted.
Assessment of Quality and Risk of Bias in Individual Studies
Quality appraisal and assessment of bias were performed with an appropriate checklist from the “Scottish Intercollegiate Guidelines Network” (SIGN), if available (available for RCT, non-RCT, cohort study, case-control study). Quality appraisal and risk of bias analysis were performed and discussed by two independent investigators (BvE and LW). Articles were appraised as low, acceptable, or high quality; those assessed as low quality were excluded from the review.
Data Analysis
We performed a meta-analysis of BMD to evaluate whether the mean BMD Z-score in the CG population differs from normative data (a mean BMD Z-score of zero in the general population). We used Review Manager 5.3 version 5.3.5 for the analysis. The inverse-variance method was used to pool study data, and the individual effect sizes were weighted according to the reciprocal of their variance (Deeks et al. 2010). I 2 was used as a measure of heterogeneity (The Cochrane Collaboration 2011). The p-value used to reject the null hypothesis of homogeneity was 0.1 (p-value of Q; Q = chi squared statistic). In case of low heterogeneity (0–30%) a fixed effects model was used to pool data. In case of moderate-to-high heterogeneity (30–100%), both fixed and random effects models were applied, resulting in a sensitivity analysis with description of differences between the fixed and random effects models and selection of the most appropriate model. Aggregate data and IPD were analyzed together using a two-stage approach: for IPD, a mean BMD Z-score and SD were calculated first, and were then included in the next step as aggregate data. BMD Z-scores were pooled based on the measurement site (lumbar spine, total hip, femoral neck, and total body). For the overall effect size, a p-value of 0.05 was considered statistically significant. Yet, in case of multiple testing, adjustment for Bonferroni correction was applied.
In order to assess clinical relevance of the mean BMD Z-score of the CG population, the normal distribution was used to find the percentiles of Z-scores, and thus the estimated proportion of patients with a BMD Z-score ≤−2 SD (low bone mass) using SPSS version 23. For this, two approaches were used, one with the SD of normative data (mean of zero, SD 1), another with the SD of the mean calculated in our meta-analysis.
The secondary outcome measures (BMC, minerals, vitamins, hormones, and BTM) were presented in a descriptive manner.
Results
Study Selection
The literature search identified 138 potential publications of which 94 remained after removing duplicates (n = 44). After screening the titles and abstracts of the unique publications, 71 were excluded because they did not relate to the research question. Detailed evaluation of the 23 selected publications led to the exclusion of 10 studies for various reasons (see Fig. 1: Flow diagram selection process).
Fig. 1.
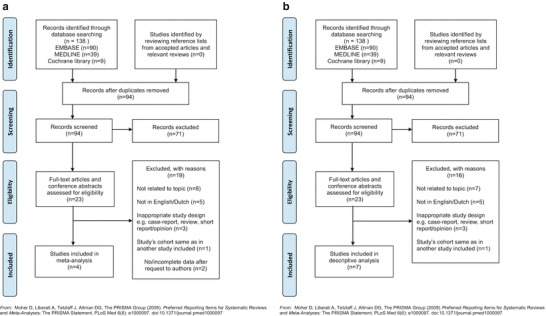
(a) Flow diagram selection process meta-analysis; (b) flow diagram selection process descriptive analysis
For the meta-analysis, eight studies were selected because they encompassed BMD measurements. Only one of these studies reported all data required for inclusion (Panis et al. 2004); the authors of the other seven studies were contacted for additional information. Three authors provided the required data at request (Waisbren et al. 2012; Tan et al. 2014; Doulgeraki et al. 2014) (Supplementary data S3, Data collection process). Accordingly, a total of four articles were included in our meta-analysis on BMD.
A total of seven studies reported on our secondary outcome measures and were therefore included in the descriptive analysis.
The complete study selection process is presented as a flow diagram, separately for the meta-analysis and the descriptive analysis (Fig. 1a, b).
Included Studies
Characteristics of the studies included in the meta-analysis or descriptive analysis are presented in Supplementary data S2a and S2b, Summary of evidence tables. Age of the patients in the included studies ranged from 2.5 to 59 years.
Individual Patient Data Integrity
No issues with IPD integrity were detected.
Assessment of Quality and Risk of Bias in Individual Studies
A quality appraisal with the standardized checklist was applicable for only one study, a randomized controlled trial (Panis et al. 2006). The paper was graded high quality as defined by the risk of bias checklist for randomized controlled trial of SIGN.
Meta-Analysis on BMD in Classic Galactosemia Patients
Four studies (three cross-sectional studies and one retrospective case series) were included in this meta-analysis of BMD Z-score (Panis et al. 2004; Waisbren et al. 2012; Tan et al. 2014; Doulgeraki et al. 2014). Panis et al. (2004) assessed 40 patients with a mean age of 8.9 years (range 3.0–17.3). Waisbren et al. (2012) evaluated BMD in 33 patients with a mean age of 32.6 years (range 18–59). Doulgeraki et al. (2014) reported BMD Z-scores of 14 patients with a mean age of 13.16 years (range 6.17–16.58 years). Tan et al. (2014) provided the individual patient data reported in their conference abstract as well as additional new data (n = 5). The mean age in these 25 patients was 13.5 years (range 5–41 years). Mean BMD Z-scores were calculated and are presented in Supplementary Data S2a.
Complete Cohort (Children and Adults)
A total of 112 CG patients assessed in four studies were included in this part of the meta-analysis. At the site of the lumbar spine, a mean BMD Z-score of −0.70 (95% CI: −0.88, −0.52) was found (Panis et al. 2004; Waisbren et al. 2012; Tan et al. 2014; Doulgeraki et al. 2014). Heterogeneity was zero (I 2 = 0%); a fixed effects model was used. The overall effect was statistically significant (p < 0.00001) when compared to the mean of normative data (BMD Z-score of zero). One of the studies crossed the line of no effect (Fig. 2).
Fig. 2.
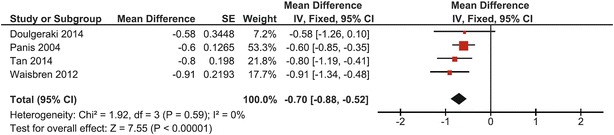
Forest plot of lumbar spine BMD Z-score in complete CG cohort
In 58 pooled patients included in two studies, mean total hip BMD Z-score was −0.89 (95% CI: −1.14, −0.64) (Waisbren et al. 2012; Tan et al. 2014). Heterogeneity was zero (I 2 = 0%); a fixed effects model was used. The overall effect was significantly different from the mean of normative data (p < 0.00001) and none of the studies crossed the line of no effect (Fig. 3).
Fig. 3.
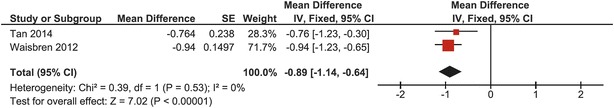
Forest plot of total hip BMD Z-score of the complete CG cohort
At the site of the femoral neck, 73 CG patients from 2 studies were pooled (Panis et al. 2004; Waisbren et al. 2012). The heterogeneity was high (I 2 = 91%), leading to determination of the mean BMD Z-score with both fixed and random effects models (Figs. 4 and 5, respectively). A mean BMD Z-score of −0.63 was found with both models, with a difference in 95% confidence interval (95% CI fixed effects model: −0.83, 0.44; 95% CI random effects model: −1.29, 0.02). As heterogeneity was very high and both studies are of adequate quality with comparable cohort sizes, it was chosen to base conclusions on the results of the random effects model. Even though both studies did not cross the line of no effect, the overall effect was not significantly different from the mean of normative data when using the random effects model (p = 0.06) (Fig. 5).
Fig. 4.
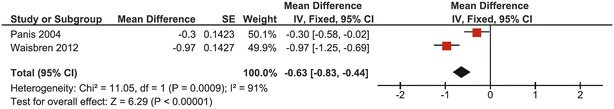
Forest plot of femoral neck BMD Z-score in complete CG cohort (fixed effects model)
Fig. 5.
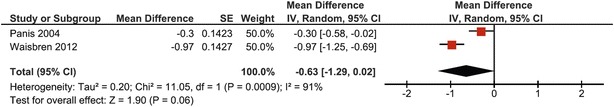
Forest plot of femoral neck BMD Z-score in complete CG cohort (random effects model)
Children with CG
In pooled data from 76 children from three studies, mean lumbar spine BMD Z-score was −0.64 (95% CI: −0.84, −0.43) (Panis et al. 2004; Tan et al. 2014; Doulgeraki et al. 2014). Heterogeneity was zero (I 2 = 0%); a fixed effects model was used. This was overall significantly different from the mean of normative data (p < 0.00001). One study crossed the line of no effect (same study as in lumbar spine BMD Z-score analysis of the entire patient group) (Fig. 6).
Fig. 6.
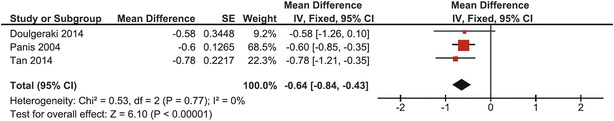
Forest plot of lumbar spine BMD Z-score in children with CG
Adults with CG
In pooled data from 36 adults with CG included in two studies, mean lumbar spine BMD Z-score was −0.94 (95% CI: −1.30, −0.57) (Waisbren et al. 2012; Tan et al. 2014). Heterogeneity was zero (I 2 = 0%); a fixed effects model was used. The overall effect was statistically significant from the mean of normative data (p < 0.00001), and none of the studies crossed the line of no effect (Fig. 7).
Fig. 7.
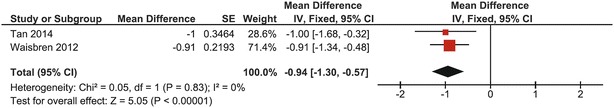
Forest plot of lumbar spine BMD Z-score in adults with CG
Mean BMD Z-score at the total hip in this group of adults was −0.91 (95% CI: −1.20, −0.63) (Waisbren et al. 2012; Tan et al. 2014). Heterogeneity was zero (I 2 = 0%); a fixed effects model was used. One study crossed the no-effect line, but the overall effect was significantly different from the mean of normative data (p < 0.00001) (Fig. 8).
Fig. 8.
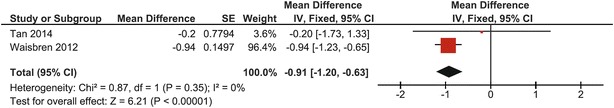
Forest plot of total hip BMD Z-score in adults with CG
We could not perform a subgroup analysis on BMD Z-scores for adult males and adult females separately since one study included data on only one or two patients.
Clinical Relevance
The normal distribution was used to find the percentiles of Z-scores, and thus the estimated proportion of patients with a BMD Z-score ≤−2 SD (low bone mass). Two approaches were used: one with an SD of 1.9 (resulting from the 95% CI of the calculated mean lumbar spine BMD Z-score of −0.7 in the complete CG group) and another with an SD of 1.0 (according to the normal distribution curve of BMD in the general population). Accordingly, 10–25% of CG patients are estimated to be at risk for a BMD Z-score ≤−2 SD, and thus a low bone mass, whereas this is only 2.3% in the general population (normally distributed parameter).
Descriptive Analysis
Bone Mineral Content
Another indicator of bone health is BMC, which reflects other aspects of bone mass acquirement than BMD and might therefore be of additional value in children (Mølgaard et al. 1997). BMC of femoral neck, lumbar spine, and total body was assessed in a single study by Panis et al. (2006) (40 patients, age range 3–17 years) (Panis et al. 2006). In this randomized controlled trial, in which the effect of supplementation of calcium, vitamin K1, and vitamin D3 on bone health was studied, baseline measurements of BMC Z-scores in 40 children with CG (age 3–17 years) were conducted. Mean BMC Z-scores (varying between −0.3 and −1.1 for different subgroups) were lower than in controls.
Vitamins, Minerals, and Hormones
Six studies evaluated vitamin D status in CG patients (n = 197) (Rubio-Gozalbo et al. 2002; Panis et al. 2004; Gajewska et al. 2006; Gajewska et al. 2008; Sirrs et al. 2010; Waisbren et al. 2012). One study assessed 1,25-OH-D concentrations only (Panis et al. 2004), four measured 25-OH-D only, and one evaluated both (Rubio-Gozalbo et al. 2002). The studies varied with regard to vitamin D reference ranges and units, and in some the specified range of desired vitamin D concentrations was not stated. Two studies in adults with CG reported that most patients had levels in the low reference range (Sirrs et al. 2010; Waisbren et al. 2012). Compliance to vitamin D supplements differed between the two cohorts. The remaining four studies, performed in children with CG, found vitamin D levels to be within reference range.
Four studies with a total of 148 patients assessed serum calcium levels (Rubio-Gozalbo et al. 2002; Panis et al. 2004; Gajewska et al. 2006; Gajewska et al. 2008). In all studies calcium levels were found to be within reference range. Rubio-Gozalbo et al. (2002) found no correlation between BMD results and calcium intake in their cohort of children with CG (Rubio-Gozalbo et al. 2002).
Parathormone was also measured in the studies by Rubio-Gozalbo et al. (2002) and Panis et al. (2004) and revealed values within reference range. Only one study measured 17-beta-estradiol levels in a cohort of 40 children aged 3–17.3 years (mean age 8.9 years) (Panis et al. 2004). Mean levels did not differ from reference values.
Bone Turnover Markers
Four cross-sectional studies examined BTM in CG patients (Rubio-Gozalbo et al. 2002; Panis et al. 2004; Gajewska et al. 2006; Gajewska et al. 2008): three studies included children only (<18 years) and one study included patients up to 20 years. A total of 148 patients were included in these studies (range 11–62 patients/study). There were no studies reporting on markers of bone status in adult patients.
Carboxy-terminal telopeptide of type 1 collagen, measured in all four studies, was found to be significantly reduced in children but not in adolescents when compared to controls. Panis et al. (2004) found it to be inversely correlated with BMD Z-score of the femoral neck and lumbar spine (Panis et al. 2004). Amino-terminal telopeptide of type I collagen levels were measured in two study cohorts and levels were found to be significantly low in both cohorts of CG children (Rubio-Gozalbo et al. 2002; Panis et al. 2004). Gajewska et al. (2008) reported about 30% higher values of bone-specific alkaline phosphatase in adolescents than in controls (Gajewska et al. 2008), whereas normal values were found in children. In addition, Gajewska et al. found increased osteocalcin (OC; sum of carboxylated [cOC] and under-carboxylated osteocalcin [ucOC]) levels in adolescents with CG (Gajewska et al. 2008), whereas Panis et al. (2004) reported significantly decreased cOC levels with normal ucOC levels in children (Panis et al. 2004). Normal values of OC (Gajewska et al. 2006; Gajewska et al. 2008) and cOC and ucOC (Rubio-Gozalbo et al. 2002) in children were reported in three studies. Furthermore, Panis et al. (2004), the only study measuring IGF-1 levels, reported reduced IGF-1 Z-scores in children (Panis et al. 2004) and found that IGF-1 Z-score was a strong positive predictor of femoral neck and lumbar spine BMD.
Fracture History
Waisbren et al. (2012) reported that 45% of 33 patients (mean age 32.6 years, range 18–59) had broken a bone, 6 during childhood and the others at ages 20–46 years (Waisbren et al. 2012).
Discussion
In this systematic review we evaluated bone mass in patients with CG through a meta-analysis of BMD. The results of our meta-analysis indicate that mean bone mass in the CG population, reflected by mean BMD Z-score, is more than a half-standard deviation lower than in the general population. While this is still within two SD of the normative mean, this result indicates that an increased proportion of individuals with CG will have a BMD Z-score ≤−2 SD, and thus a low bone mass for age, as compared to individuals in the general population. Based on the meta-analysis, estimated prevalence of BMD Z-score ≤−2 SD in patients with CG is 10–25%, which is higher than in the general population (2.3%). Mean BMD Z-scores in pooled data of adults and children with CG were reduced at all sites (lumbar spine −0.70, total hip −0.89, femoral neck −0.63), of which only the latter was not statistically significant, probably due to heterogeneity between included study cohorts. Findings from our descriptive analysis support the need for improved evaluation and optimization of vitamin D concentrations. Though serum calcium levels were within reference range in all studies that addressed calcium status, ensuring sufficient intake of calcium remains a point of attention in this population. Data on the role of BMC and BTM as other indicators of bone status are very limited and, in the case of BTM, highly ambiguous. Therefore, routine screening of these indicators in CG patients does not seem of additional value at present. Literature on bone mass in CG is scarce and study cohorts are small as a result of the rarity of the disease. This limits the number of studies and patients included in our meta-analysis, which hampers extensive subgroup analyses and solid conclusions. However, this systematic review is currently the most comprehensive study evaluating bone health in CG patients, thereby providing results that are more representative for the whole population of CG patients.
This study was limited in that not all original patient data could be obtained. In addition, data used in this study were all cross-sectional, and conclusions about progression over time must therefore be interpreted with caution. Longitudinal studies following patients in time are needed to enable firm conclusions on this. Moreover, ISCD recommendations for pediatric DXA (Crabtree et al. 2014) were not unanimously followed by the researchers, as they used different sites of measurement and did not take into account the presence of short stature or growth delay, with the exception of only one study (Doulgeraki et al. 2014). Furthermore, data on fracture history were obtained in only one study (Waisbren et al. 2012), though this is required to establish a diagnosis of osteoporosis. Future studies should consider the ISCD recommendations to further improve insights into bone health in CG.
Conclusions
BMD Z-scores in individual CG patients are within two SD of the normative mean in the majority of patients with CG. However, results from our meta-analysis demonstrate that the mean BMD Z-score in the CG population is lower than the mean BMD Z-score in the general population. These results suggest that bone health in general is mildly affected, and that an estimated proportion of 10–25% of patients with CG could be at risk for a low bone mass (BMD Z-score ≤−2) as compared to the general population. With the currently available literature, which lacks data on fracture prevalence, it is impossible to draw conclusions about osteoporosis risk. Vitamin D levels are low in many patients, emphasizing the need for monitoring of 25(OH) D levels and vitamin D supplementation. Optimization of calcium intake remains important. Evaluation of the importance of other parameters of bone health (BCM, BTM, hormones) was inconclusive due to a limited number of studies with inconsistent results. Concluding, it is important that treating physicians are aware that patients with CG are at risk for having or developing low bone mass, so that patients will be screened and treated appropriately.
Electronic Supplementary Material
Acknowledgements
The authors would like to thank the clinical librarian A.G.E. Leenders (Academic Medical Center, Amsterdam, the Netherlands) for developing the search strategies for this review, as well as Dr. M.J. van Kroonenburgh (Maastricht University Medical Center, Department of Nuclear Medicine) for answering queries on the technical aspects of bone mass evaluation.
Take-Home Message
It is important that treating physicians are aware that patients with CG are at risk for having or developing low bone mass, warranting appropriate screening and treatment.
Contributions of Individual Authors
Britt van Erven: conception and design, acquisition of data, analysis and interpretation of data, drafting the chapter, revising the chapter critically for important intellectual content.
Lindsey Welling: conception and design, acquisition of data, analysis and interpretation of data, drafting the chapter, revising the chapter critically for important intellectual content.
Sandra C. van Calcar: analysis and interpretation of data, revising the chapter critically for important intellectual content.
Artemis Doulgeraki: acquisition of data, revising the chapter critically for important intellectual content.
François Eyskens: analysis and interpretation of data, revising the chapter critically for important intellectual content.
Joanna Gribben: acquisition of data, revising the chapter critically for important intellectual content.
Eileen P. Treacy: acquisition of data, revising the chapter critically for important intellectual content.
Rein Vos: statistical design, revising the chapter critically for important intellectual content.
Susan E. Waisbren: acquisition of data, revising the chapter critically for important intellectual content.
M. Estela Rubio-Gozalbo: conception and design, analysis and interpretation of data, drafting the chapter, revising the chapter critically for important intellectual content.
Annet M. Bosch: conception and design, analysis and interpretation of data, drafting the chapter, revising the chapter critically for important intellectual content.
All authors read and approved the manuscript before submission.
Guarantor
M. Estela Rubio-Gozalbo.
Conflict of Interest Statement
Annet M. Bosch is in receipt of research grants from Nutricia and was a member of an advisory board for Nutricia.
Sandra C. van Calcar declares that she has no conflict of interest.
Artemis Doulgeraki declares that she has no conflict of interest.
Britt van Erven declares that she has no conflict of interest.
François Eyskens declares that he has no conflict of interest.
Joanna Gribben declares that she has no conflict of interest.
M. Estela Rubio-Gozalbo declares that she has no conflict of interest.
Eileen P. Treacy declares that she has no conflict of interest.
Rein Vos declares that he has no conflict of interest.
Susan E. Waisbren declares that she has no conflict of interest.
Lindsey Welling declares that she has no conflict of interest.
Details of Funding
No financial support from sponsors was received for the conduct of this research.
Ethics Approval
Not required. This chapter does not contain any interventional studies with human or animal subjects performed by any of the authors.
Footnotes
The authors “Britt van Erven” and “Lindsey Welling” are equal first authors.
The authors “M. Estela Rubio-Gozalbo” and “Annet M. Bosch” are equal first authors.
Electronic supplementary material: The online version of this chapter (doi:10.1007/8904_2016_28) contains supplementary material, which is available to authorized users.
Contributor Information
M. Estela Rubio-Gozalbo, Email: estela.rubio@mumc.nl.
Collaborators: Matthias R. Baumgartner, Marc Patterson, Shamima Rahman, Verena Peters, Eva Morava, and Johannes Zschocke
References
- Batey LA, Welt CK, Rohr F, et al. Skeletal health in adult patients with classic galactosemia. Osteoporos Int. 2013;24:501–509. doi: 10.1007/s00198-012-1983-0. [DOI] [PubMed] [Google Scholar]
- Bons JA, Michielsen EC, de Boer D, et al. A specific immunoprecipitation method for isolating isoforms of insulin-like growth factor binding protein-3 from serum. Clin Chim Acta. 2008;387:59–65. doi: 10.1016/j.cca.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Coss KP, Doran PP, Owoeye C, et al. Classical Galactosaemia in Ireland: incidence, complications and outcomes of treatment. J Inherit Metab Dis. 2013;36:21–27. doi: 10.1007/s10545-012-9507-9. [DOI] [PubMed] [Google Scholar]
- Crabtree NJ, Arabi A, Bachrach LK, et al. Dual-energy X-ray absorptiometry interpretation and reporting in children and adolescents: the revised 2013 ISCD Pediatric Official Positions. J Clin Densitom. 2014;17:225–242. doi: 10.1016/j.jocd.2014.01.003. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JPT, on behalf of the Statistical Methods Group of The Cochrane Collaboration (2010) Statistical algorithms in Review Manager:5 Retrieved from: http://ims.cochrane.org/revman/documentation/Statistical-methods-in-RevMan-5.pdf. Accessed 24 Oct 2016
- Doulgeraki A, Monopolis I, Deligianni D, et al. Body composition in young patients with galactose metabolic disorders: a preliminary report. J Pediatr Endocrinol Metab. 2014;27:81–86. doi: 10.1515/jpem-2013-0161. [DOI] [PubMed] [Google Scholar]
- El-Bassyouni HT, Ashour AM, Ezzat A, et al. The effect of diet on antioxidant status in patients with galactosemia. J Med Sci. 2006;6:452–457. doi: 10.3923/jms.2006.452.457. [DOI] [Google Scholar]
- Fridovich-Keil JL, Gubbels CS, Spencer JB, et al. Ovarian function in girls and women with GALT-deficiency galactosemia. J Inherit Metab Dis. 2011;34:357–366. doi: 10.1007/s10545-010-9221-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewska J, Ambroszkiewicz J, Radomyska B, et al. Serum markers of bone turnover in children and adolescents with classic galactosemia. Adv Med Sci. 2008;53:214–220. doi: 10.2478/v10039-008-0026-8. [DOI] [PubMed] [Google Scholar]
- Gajewska J, Ambroszkiewicz J, Radomyska B, Laskowska-Klita T. Bone turnover markers in prepubertal children with classical galactosemia. Indian J Gastroenterol. 2006;25:221–222. [PubMed] [Google Scholar]
- Gordon C, Leonard MB, Zemel BS, International Society for Clinical Densitometry 2013 Pediatric Position Development Conference: executive summary and reflections. J Clin Densitom. 2014;17:219–224. doi: 10.1016/j.jocd.2014.01.007. [DOI] [PubMed] [Google Scholar]
- Haidich AB. Meta-analysis in medical research. Hippokratia. 2010;14:29–37. [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B, Wendel U, Schweitzer-Krantz S. Cross-sectional analysis of speech and cognitive performance in 32 patients with classic galactosemia. J Inherit Metab Dis. 2011;34:421–427. doi: 10.1007/s10545-011-9297-5. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV, Johansson H, et al. European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2013;24:23–57. doi: 10.1007/s00198-012-2074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman FR, Loro ML, Azen C, et al. Effect of hypogonadism and deficient calcium intake on bone density in patients with galactosemia. J Pediatr. 1993;123:365–370. doi: 10.1016/S0022-3476(05)81733-0. [DOI] [PubMed] [Google Scholar]
- Marchall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;18:1254–1259. doi: 10.1136/bmj.312.7041.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mølgaard C, Thomsen BL, Prentice A, et al. Whole body bone mineral content in healthy children and adolescents. Arch Dis Child. 1997;76:9–15. doi: 10.1136/adc.76.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panis B, Forget PP, Van Kroonenburgh MJPG, et al. Bone metabolism in galactosemia. Bone. 2004;35:982–987. doi: 10.1016/j.bone.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Panis B, Gerver W-JM, Rubio-Gozalbo ME. Growth in treated classical galactosemia patients. Eur J Pediatr. 2007;166:443–446. doi: 10.1007/s00431-006-0255-4. [DOI] [PubMed] [Google Scholar]
- Panis B, Vermeer C, van Kroonenburgh MJPG, et al. Effect of calcium, vitamins K1 and D3 on bone in galactosemia. Bone. 2006;39:1123–1129. doi: 10.1016/j.bone.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Rasmussen RK, Andreassen AB, Stromme P, Hansen TW. Learning disabilities and language pathology in patients with galactosemia. Logoped Phoniatr Vocol. 1996;21:157–162. doi: 10.3109/14015439609098884. [DOI] [PubMed] [Google Scholar]
- Renner C, Razeghi S, Uberall MA, et al. Hormone replacement therapy in galactosaemic twins with ovarian failure and severe osteoporosis. J Inherit Metab Dis. 1999;22:194–195. doi: 10.1023/A:1005482826929. [DOI] [PubMed] [Google Scholar]
- Rubio-Agusti I, Carecchio M, Bhatia KP, et al. Movement disorders in adult patients with classical galactosemia. Mov Disord. 2013;28:804–810. doi: 10.1002/mds.25348. [DOI] [PubMed] [Google Scholar]
- Rubio-Gozalbo ME, Hamming S, van Kroonenburgh MJ, et al. Bone mineral density in patients with classic galactosaemia. Arch Dis Child. 2002;87:57–60. doi: 10.1136/adc.87.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford PJ, Davidson DC, Matthai SM. Dietary calcium in galactosaemia. J Hum Nutr Diet. 2002;15:39–42. doi: 10.1046/j.1365-277X.2002.00330.x. [DOI] [PubMed] [Google Scholar]
- Schousboe J, Shepherd JA, Bilezikian JP, Baim S. Executive summary of the 2013 International Society for Clinical Densitometry Position Development Conference on bone densitometry. J Clin Densitom. 2013;16:455–466. doi: 10.1016/j.jocd.2013.08.004. [DOI] [PubMed] [Google Scholar]
- Schweitzer S, Shin Y, Jakobs C, Brodehl J. Long-term outcome in 134 patients with galactosaemia. Eur J Pediatr. 1993;152:36–43. doi: 10.1007/BF02072514. [DOI] [PubMed] [Google Scholar]
- Shield JP, Wadsworth EJ, MacDonald A, et al. The relationship of genotype to cognitive outcome in galactosaemia. Arch Dis Child. 2000;83:248–250. doi: 10.1136/adc.83.3.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirrs S, Bosdet T, Branov J, et al. (2010) High prevalence of vitamin D insufficiency in galactosemic adults despite compliance with supplementation. J Inherit Metab Dis 33:S64
- Tan C, Stonestreet K, Winstone R, et al. (2014) Management and long-term outcomes of classical galactosaemia-a single centre experience in the UK. Conf Annu Symp Soc Study Inborn Errors Metab SSIEM 2014 Innsbruck Austria Conf Start 20140902 Conf End 20140905 Conf Publ (var.pagings) 37(Suppl 1):S57
- The Cochrane Collaboration (2011) Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]
- Van Erven B, Römers MM, Rubio-Gozalbo ME. Revised proposal for the prevention of low bone mass in patients with classic galactosemia. JIMD Rep. 2014;17:41–46. doi: 10.1007/8904_2014_331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waisbren SE, Potter NL, Gordon CM, et al. The adult galactosemic phenotype. J Inherit Metab Dis. 2012;35:279–286. doi: 10.1007/s10545-011-9372-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesmann UN, Rosé-Beutler B, Schlüchter R. Leguminosae in the diet: the raffinose-stachyose question. Eur J Pediatr. 1995;154:93–96. doi: 10.1007/BF02143812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


