Abstract
Cold exposure is necessary to activate human brown adipose tissue (BAT), resulting in heat production. Skin temperature is an indirect measure to monitor the body’s reaction to cold. The aim of this research was to study whether the most used equations to estimate parameters of skin temperature in BAT-human studies measure the same values of temperature in young lean men (n = 11: 23.4 ± 0.5 years, fat mass: 19.9 ± 1.2%). Skin temperature was measured with 26 ibuttons at 1-minute intervals in warm and cold room conditions. We used 12 equations to estimate parameters of mean, proximal, and distal skin temperature as well as skin temperature gradients. Data were analysed with Temperatus software. Significant differences were found across equations to measure the same parameters of skin temperature in warm and cold room conditions, hampering comparison across studies. Based on these findings, we suggest to use a set of 14 ibuttons at anatomical positions reported by ISO STANDARD 9886:2004 plus five ibuttons placed on the right supraclavicular fossa, right middle clavicular bone, right middle upper forearm, right top of forefinger, and right upper chest.
Introduction
In 1937, Hardy and Du Bois1 studied the effect of different environment exposures (cold and heat) over parameters of skin temperature. Thereafter, parameters of skin temperature and thermal responses were studied as thermal-physiological responses to different stimulus such as exercise2, anaesthesia-treatment3, circadian-rythms4, or heat5 and cold6 environment.
Since 2009, cold exposure is used in human studies as one of the main activators of brown adipose tissue (BAT) before performing a 18F-Fluorodeoxiglucose Positron emission tomography/computed tomography (18F-FDG-PET/CT)7–12. BAT is highly regulated by the sympathetic nervous system (SNS) increasing body temperature when mammals are exposed to cold13. Therefore, skin temperature measurements could play an important role as a possible indirect marker of BAT activity or volume14–17.
To date, consensus about which equations are better to estimate parameters of mean, proximal, or distal skin temperature and body-gradients is non-existent. Moreover, whereas some human BAT studies7, 14, 18–23 used different equations to estimate the same parameters of skin temperature, other studies did not report the equations used15, 24, 25. This fact hampers comparability between studies. The lack of knowledge about a set of validated equations or alternative instruments as “gold-standard” to measure skin temperature hinders comparisons between studies. Of note is that most of the human-studies used ibuttons (a valid26, 27 and reliable27 tool) to measure skin temperature.
Most of the equations used in the cooling protocols before BAT activation were not validated (i.e. mean, proximal, distal skin temperature, and body gradients) against any “gold-standard”, neither were most of the body-gradients used. To note is, however, that supraclavicular skin temperature14, 15, 28 and a supraclavicular gradient29 were validated against 18F-FDG-PET/CT and were postulated as an indirect measurement of BAT volume and activity. Sessler et al.3 validated a peripheral skin temperature gradient (see Table 1) as a proxy of peripheral vasoconstriction measured by laser Doppler monitor. Furthermore, there are some thermophysiological models used to predict local skin temperature25, 30, 31, yet these models need to be applied in the specific cooling protocols used in the BAT activation.
Table 1.
Equations used to estimate parameters of skin temperature.
| Outcome | Reference | ibuttons (n) | Anatomical positions. Fig. 1 | Participants (n) | Equations |
|---|---|---|---|---|---|
| Mean skin temperature | 14 ISO 9886–200441 (14-ISO) | 14 | From 1 to 14 | 9 | (Forehead*0.07) + (Neck*0.07) + (Right Scapula*0.07) + (Left Chest*0.07) + (Right Deltoid*0.07) + (Left Elbow*0.07) + (Right Abdomen*0.07) + (Left Hand*0.07) + (Left Lumbar *0.07) + (Right Thigh*0.07) + (Left Hamstring*0.07) + (Right Shinbone*0.07) + (Left Gastrocnemius*0.07) + (Right Instep*0.07) |
| 8 ISO 9886–200441 (8-ISO) | 8 | 1, 3, 4, 5, 6, 9, 10, 13 | 10 | (Forehead*0.07) + (Right Scapula*0.175) + (Left Chest*0.175) + (Right Deltoid*0.07) + (Left Elbow*0.07) + (Left Hand*0.05) + (Right Thigh*0.19) + (Left Gastrocnemius*0.2) | |
| 4 ISO 9886–200441 (4-ISO) | 4 | 2, 3, 9, 12 | 10 | (Neck*0.28) + (Right Scapula*0.28) + (Left Hand*0.16) + (Right Shinbone*0.28) | |
| Boon et al.15 (Boon) | 5 | 10, 16, 8, 9, 14 | 10 | (((Right Thigh*0.383) + (Right Clavicular *0.293) + (Right Abdomen*0.324)) + ((Left Hand + Right Instep)/2))/4) | |
| PROFITH | 26 | From 1 to 26 | 8 | (Forehead + Neck + Right Scapula + Left Chest + Right Deltoid + Left Elbow + Right Abdomen + Left Hand + Left Lumbar + Right Thigh + Left Hamstring + Right Shinbone + Left Gastrocnemius + Right Instep + Right Supraclavicular + Right Clavicular + Right Subclavicular + Left Forearm + Left top of forefinger + Right Forearm + Right top of forefinger + Left Shinbone + Right Gastrocnemius + Left Instep + Right Chest + Left Thigh)/26 | |
| Proximal skin temperature | Kräuchi et al.36 (Kräuchi) | 4 | 1, 10, 17, 8 | 10 | (Forehead*0.093) + (Right Thigh*0.347) + (Right Subclavicular*0.266) + (Right Abdomen*0.294)/4 |
| van Marken Lichtenbelt et al.26 (Van Marken) | 3 | 10, 17, 8 | 10 | (Right Thigh*0.383) + (Right Subclavicular*0.293) + (Right Abdomen*0.324) | |
| Schellen et al.32 (Schellen) | 4 | 3, 7, 4, 8 | 11 | (Right Scapula + Left Lumbar + Left Chest + Right Abdomen)/4 | |
| Boon et al.15 (Boon) | 3 | 10, 16, 8 | 10 | (Right Thigh*0.383) + (Right Clavicular*0.293) + (Right Abdomen*0.324) | |
| PROFITH | 5 | 8, 25, 4, 3, 2 | 11 | (Right Abdomen + Right Chest + Left Chest + Right Scapula + Neck)/5 | |
| Distal skin temperature | Kräuchi et al.36 (Krauchi) | 2 | 9, 14 | 11 | (Left Hand + Right Instep)/2 |
| PROFITH | 6 | 1, 19, 21, 14, 24, 9 | 11 | (Forehead + Left top of forefinger + Right top of forefinger + Right Instep + Left Instep + Left Hand)/6 | |
| Body temperature gradient | Boon et al.15 (Boon) | 5 | 9, 14, 10, 16, 8 | 10 | [(Left Hand + Right Instep)/2]- [(Right Thigh*0.383) + (Right Clavicular*0.293) + (Right Abdomen*0.324)] |
| PROFITH | 11 | 1, 19, 21, 14, 24, 9, 8, 25, 3, 2 | 11 | [(Forehead + Left top of forefinger + Right top of forefinger + Right Instep + Left Instep + Left Hand)/6]-[(Right Abdomen + Right Chest + Left Chest + Right Scapula + Neck)/5] | |
| Supraclavicular temperature gradient | PROFITH: (S-LC) | 2 | 15, 4 | 11 | (Right Supraclavicular(S)-Left Chest(LC)) |
| Lee et al.29 (Lee S-RC) | 2 | 15, 25 | 11 | (Right Supraclavicular(S)- Right Chest (RC)) | |
| PROFITH: (S-SB) | 2 | 15, 17 | 11 | (Right Supraclavicular(S)-Right Subclavicular(SB)) | |
| Peripheral temperature Gradient | Sessler et al.3 Right arm | 2 | 20, 21 | 11 | (Right Forearm-Right Top of forefinger) |
| PROFITH: Left arm | 2 | 18, 19 | 11 | (Left Forearm-Left Top of forefinger) | |
| PROFITH: Right Leg | 2 | 23, 14 | 11 | (Right Gastrocnemius- Right Instep) | |
| PROFITH: Left Leg | 2 | 13, 24 | 11 | (Left Gastrocnemius-Left Instep) |
Taking into account the lack of consensus on which equation to use and the high discrepancy that exists across studies that measured skin temperature in response to cold exposure, we studied if the most used equations to estimate parameters of skin temperature in human BAT studies measure the same in young lean men.
Methods
A total of 11 men (23.4 ± 0.5 years) took part in the present study. All participants were healthy, lean (fat mass: 19.9 ± 1.2%) (see Table 2), non-smokers, and were not taking any medication that could have altered the cardiovascular or thermoregulatory responses to cold exposure. The study protocol and informed consent followed the Declaration of Helsinki (revision of 2013). This study was approved by The Human Research Ethics Committee of the University of Granada (n°924) and by the Servicio Andaluz de Salud (Centro de Granada, CEI-Granada).
Table 2.
Characteristics of the participants.
| Mean | SE | ||
|---|---|---|---|
| Age (years) | 23.4 | ± | 0.5 |
| Body mass index (kg/m2) | 23.2 | ± | 0.4 |
| Fat free mass (kg) | 58.2 | ± | 8.8 |
| Fat mass (kg) | 13.8 | ± | 0.9 |
| Fat mass (%) | 19.9 | ± | 1.2 |
| Bone mineral density (g/cm2) | 1.31 | ± | 0.03 |
SE: Standard error.
Previous conditions to the study day
The study was conducted between March and April 2016 in Granada (Southern Spain). Participants arrived at the research centre by bus or by car in fasted condition (at least 8 hours after their last meal), between 8 a.m. and 4 p.m. They were advised to refrain from any type of physical activity or exercise in the 48 hours prior to the study day. Additionally, participants were required not to drink alcoholic or caffeine-containing beverages in the 24 hours prior to the study day.
Skin temperature registration
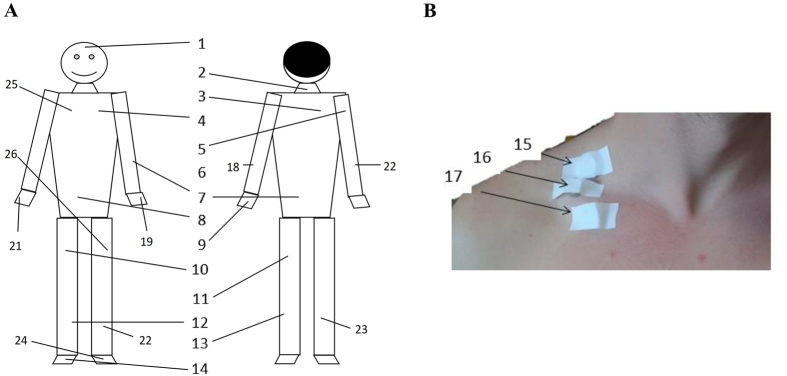
We measured skin temperature with 26 ibuttons32 (DS-1922 L, Thermochron; resolution: 0.0625 °C; Maxim, Dallas, USA). Ibuttons are valid and reliable devices to measure skin temperature in humans26, 27. We attached the ibuttons to the skin with adhesive tape (Fixomull, Beiersdorf AG, Hamburg, Germany) on different body sites (see Fig. 1)14, 26, 32–36. Skin temperature was recorded at 1-minute intervals. We reviewed which skin temperature equations were most used in human BAT studies7, 14, 18–23, and selected 12 equations to estimate parameters of skin temperature including mean, proximal, and distal skin temperature (Table 1). Moreover, we calculated the gradient over forearms minus top of forefingers as a measure of peripheral vasoconstriction in the arms37, as well as the heat loss capacity of the supraclavicular zone29 and of the whole body through different body gradients15. Taking into account the number of ibuttons used in the present study (n = 26), we computed 6 new equations using the highest number of ibuttons possible to estimate parameters of skin temperature (see PROFITH equations, Table 1). All data recorded by the devices and equations were analysed by the Temperatus software (http://profith.ugr.es/temperatus).
Figure 1.
Anatomical position of 26 ibuttons. (A) Distribution of the ibuttons over the body. (B) Distribution of the ibuttons on the right clavicular sites.
Cooling protocol
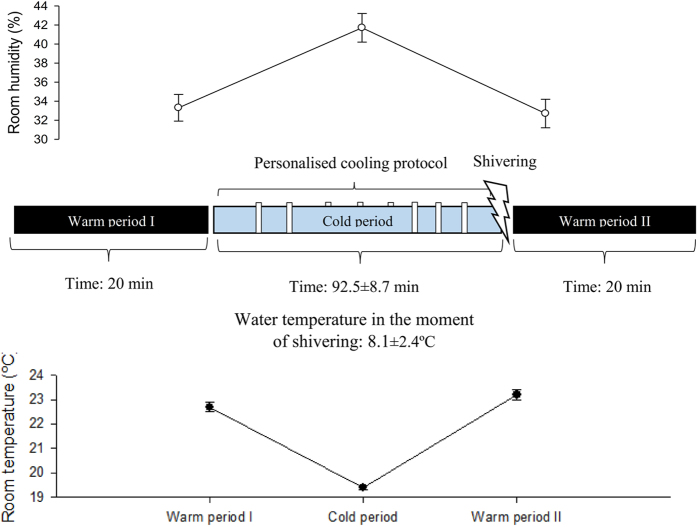
Participants were barefoot and wore a short standardized T-shirt [clo-value: 0.2038, 39]. We controlled the room temperature (see Fig. 2), and we avoided potential airflow in the room. The participants lay on a bed for 20 minutes in a warm room (warm period I, 22.7 ± 0.2 °C). Thereafter, participants were moved into a cold room (19.4 ± 0.1 °C) where we applied a cooling protocol until shivering occurred (Fig. 2). In the cold room, the participants lay on a bed for 15 minutes, after which they were equipped with a temperature-controlled water circulation-cooling vest (Polar Products Inc., Ohio, USA)40. The cooling vest covered the individuals’ clavicular region, as well as the chest, abdomen, and back. The water temperature started at 17 °C and decreased progressively (~1 °C) every 10 minutes until shivering occurred (cold period mean time: 92.5 ± 8.7 minutes; water mean temperature of shivering: 8.1 ± 2.41 °C, see Fig. 2). We determined shivering both visually and by asking the participants if they were experiencing shivering. Shivering was confirmed by EMG in 6 participants. Once shivering was determined, the participants returned to the warm room (23.2 ± 0.2 °C) and lay on the bed for another 20 minutes (warm period II) without the cooling vest. The participants were not allowed to move on the bed, read or watch a film, or to be covered by a blanket or a sheet. At the end of the study day, we measured body composition by Dual Energy X-ray Absorptiometry scan (HOLOGIC, QDR 4500 W).
Figure 2.
Cooling protocol. Each vertical white bars represents a decrease of the water temperature of the cooling vest. Upper graphic represents mean room humidity during warm period I, cold period, warm period II. Lower graphics represents mean room temperature during warm period I, cold period, warm period II.
Statistical Analysis
Data are presented as mean and standard error. The skin temperature data were taken as average in the last five minutes of the warm period I (before initializing cooling protocol), in the last five minutes of the warm period II, and in the five minutes prior to shivering (cold period). We excluded the data from the equations in the analysis when at least one ibutton failed during the measurements (see Table 1). We analysed differences across the study equations using analysis of variance (ANOVA) with Bonferroni adjustments for post-hoc comparisons, by periods (warm period I, cold period, and warm period II). We compared mean differences of skin temperature across temperature conditions, using ANOVA for repeated measurements. All the analyses were conducted using the Statistical Package for Social Sciences (SPSS, v. 22.0, IBM SPSS Statistics, IBM Corporation), and the level of significance was set to < 0.05.
Results
Warm period I
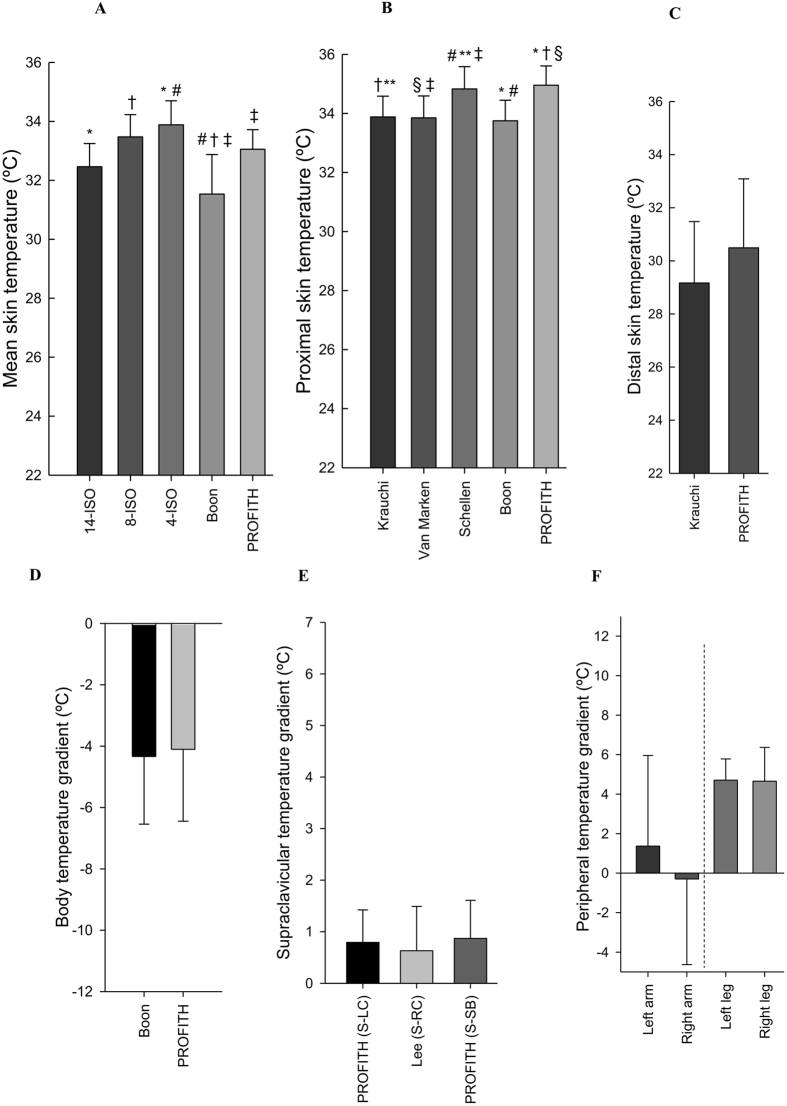
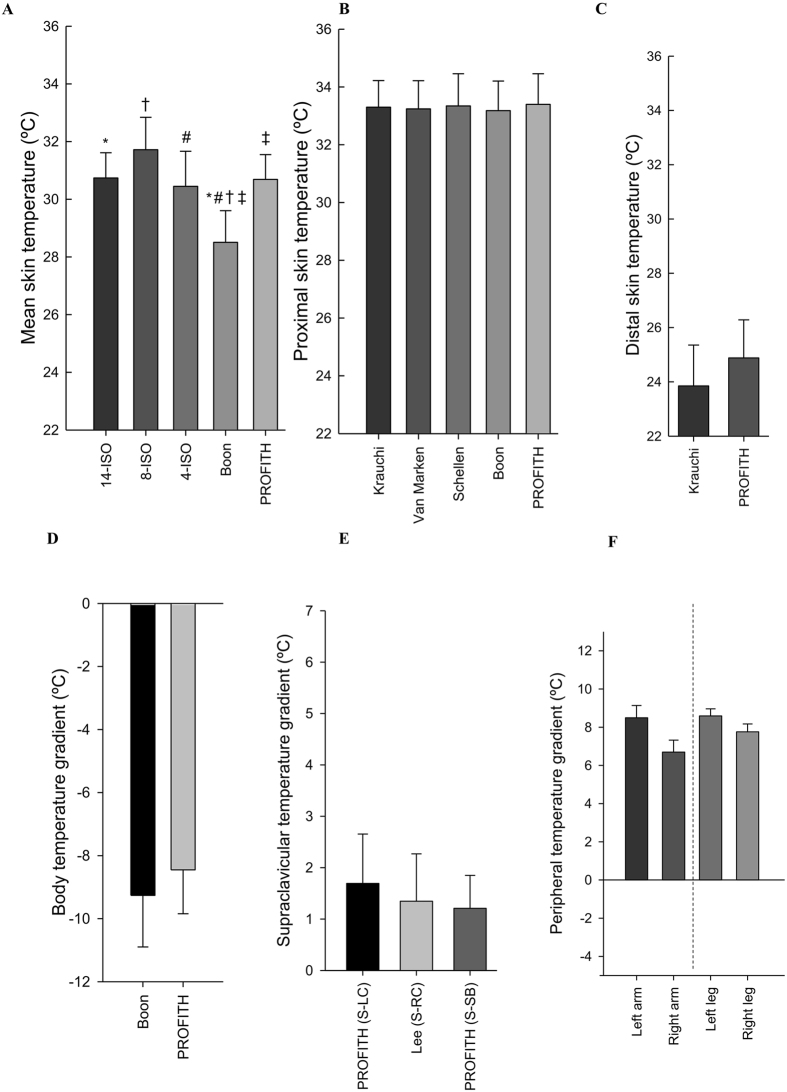
Figure 3 shows the mean skin temperature (A), proximal skin temperature (B), distal skin temperature (C), body temperature gradient (D), supraclavicular temperature gradient (E), and temperature gradients as a proxy for upper (left and right arm) and lower (left and right leg) peripheral vasoconstrictions (F) in the last five minutes of warm period I as estimated with the equations used in literature (Table 1). ANOVA showed differences across mean skin temperature equations (overall P < 0.001, Fig. 3A). The post-hoc analysis showed significant differences in mean skin temperature using the equation reported by 4-ISO41 compared with 14-ISO41 (mean difference 1.35 °C; 95% confidence interval: 0.12 °C–2.57 °C; P = 0.022) and between the equation reported by Boon et al.15 and 8-ISO41 (−1.83 °C; −3.02 °C–0.64 °C; P < 0.001), 4-ISO (−2.15 °C; −3.35 °C–0.96 °C; P = 0.001), and PROFITH (−1.40 °C; −2.67 °C–0.14 °C; P = 0.021).
Figure 3.
Measures of skin temperature at the last five minutes of the first warm period as estimated with the equations used in the respective references. (A) Mean skin temperature: *P = 0.022: 14-ISO vs. 4-ISO; †P < 0.001: 8-ISO vs. Boon; #P = 0.001: 4-ISO vs. Boon; ‡P = 0.021: Boon vs. PROFITH. (B) Proximal skin temperature: †P = 0.015: Krauchi vs. PROFITH; **P = 0.048: Krauchi vs. Schellen; §P = 0.011: Van Marken vs. PROFITH; ‡P = 0.036: Van Marken vs. Schellen; #P = 0.014 Schellen vs. Boon; *P = 0.004: Boon vs. PROFITH. (C) Distal skin temperature: P = 0.222: Krauchi vs PROFITH. (D) Body temperature gradients: P = 0.931: Boon vs PROFITH. (E) Supraclavicular temperature gradients: (S: Supraclavicular; LC: Left Chest; RC: Right Chest; SB: Subclavicular); All P = 1.000. (F) Peripheral temperature gradient: All P = 1.000. Data are mean and standard error.
Similarly, significant differences were observed across proximal skin temperature equations (overall P < 0.001, Fig. 3B). The post-hoc analysis revealed significant differences between the equation reported by Schellen et al.32 compared to Boon et al.15 (mean difference 1.03 °C; 95% confident interval: 0.13 °C-1.93 °C; P = 0.014), Kräuchi et al.36 (0.90 °C; 0.01 °C–1.81 °C; P = 0.048), and van Marken Lichtenbelt et al.26 (0.93 °C; −0.82 °C–1.84 °C; P = 0.036). We also observed significant differences between PROFITH and Boon et al.15 (1.16 °C; 0.26 °C–2.06 °C; P = 0.004), Kraüchi et al.36 (1.03 °C; 0.13 °C–1.93 °C; P = 0.015), and van Marken Lichtenbelt et al.26 (1.07 °C; 0.16 °C–1.96 °C; P = 0.011).
There were no significant differences between the equations used to estimate distal skin temperature (Fig. 3C), body (Fig. 3D), and supraclavicular (Fig. 3E) and peripheral (Fig. 3F) temperature gradients (all P ≥ 0.1).
Cold period
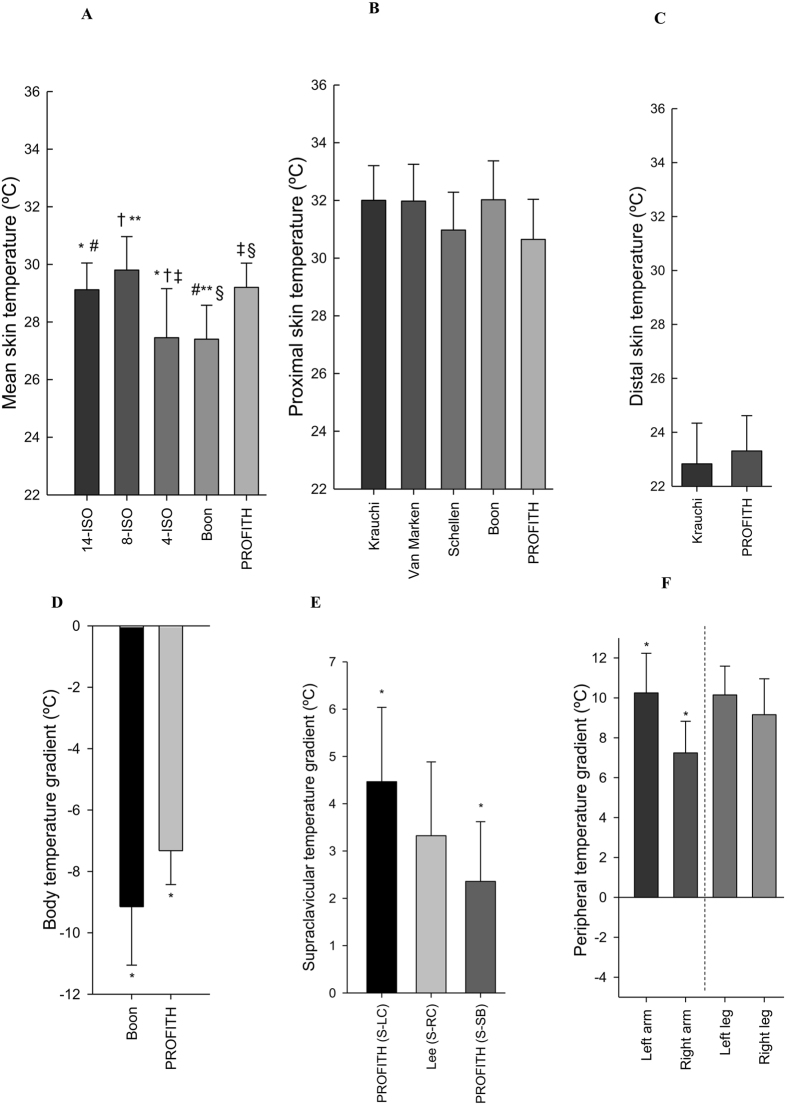
Figure 4 shows the mean skin temperature (A), proximal skin temperature (B), distal skin temperature (C), body temperature gradient (D), supraclavicular temperature gradient (E), and temperature gradients as a proxy for upper (left and right arm) and lower (left and right leg) peripheral vasoconstrictions (F) in the last five minutes of the cold period as estimated with the various equations used in literature (Table 1). Differences across mean skin temperature equations were found in warm room conditions (overall P < 0.001, Figure 4A). The post-hoc analysis showed significant differences between the equation reported by Boon et al.15 and 14-ISO41 (mean difference −1.71 °C; 95% confident interval: −3.30 °C–0.12 °C; P = 0.027), 8-ISO (−2.36 °C; −3.90 °C–0.80 °C; P = 0.001), and PROFITH (−1.79 °C; −3.44 °C–0.15 °C; P = 0.024). Similarly, there were significant differences between 4-ISO41 and 14-ISO41 (−1.73 °C; −3.32 °C–0.14 °C; P = 0.025), 8-ISO41 (−2.37 °C; 0.83 °C–3.93 °C; P ≤ 0.001), and PROFITH (−1.81 °C; −3.45 °C–0.16 °C; P = 0.022).
Figure 4.
Measures of skin temperature at the last five minutes of the cold period as estimated with the equations used in the respective references. (A) Mean skin temperature: *P = 0.025: 14-ISO vs. 4-ISO; #P = 0.027: 14-ISO vs. Boon; †P ≤ 0.001: 8-ISO vs. 4-ISO; **P = 0.001: 8-ISO vs. Boon; ‡P = 0.022: 4-ISO vs. PROFITH; §P = 0.024 Boon vs. PROFITH. (B) Proximal skin temperature: All P ≥ 0.123. (C) Distal skin temperature: P = 0.438. (D) Body temperature gradients: *P = 0.010 Boon vs. PROFITH. (E) Supraclavicular temperature gradients: (S: Supraclavicular; LC: Left Chest; RC: Right Chest; SB: Subclavicular) *P = 0.006; PROFITH (S-LC) vs. PROFITH (S-SB). (F) Peripheral temperature gradient: *P = 0.001; Left Arm vs. Right Arm. Data are mean and standard error.
There were no significant differences across equations used to estimate proximal (P = 0.123, Fig. 4B) and distal skin temperature (P = 0.438, Fig. 4C). There were, however, significant differences between the equations used to estimate the body temperature gradient (−1.82 °C; 95% confident interval: −0.82 °C− −2.83 °C; P = 0.01, Fig. 4D). Similarly, there were differences between the method to estimate supraclavicular temperature gradient zone when the skin temperature of the left chest zone was used instead of the right subclavicular zone (2.10 °C; 0.51 °C–3.70 °C; P = 0.006, Fig. 4E), as well as between the left and right gradient of the arms to estimate peripheral temperature gradients (3.01 °C; −0.97 °C −5.04 °C; P = 0.001, Figure 4 F).
Warm period II
Figure 5 shows the mean skin temperature (A), proximal skin temperature (B), distal skin temperature (C), body temperature gradient (D), supraclavicular temperature gradient (E), and temperature gradients as a proxy for upper (left and right arm) and lower (left and right leg) peripheral vasoconstriction (F) in the last five minutes of warm period II as estimated with the various equations used in literature (Table 1). Just as in warm period I, differences were found across mean skin temperature equations (overall P < 0.001, Figure 5A). The post-hoc analysis showed significant differences between the equation reported by Boon et al.15 and 14-ISO41 (mean difference −2.18 °C; 95% confident interval: −3.56 °C–0.79 °C; P ≤ 0.001), 8-ISO (−3.10 °C; −4.45 °C–1.76 °C; P ≤ 0.001), 4-ISO (−1.87 °C; −3.21 °C–0.52 °C; P = 0.002), and PROFITH (−2.12 °C; −3.55 °C–0.70 °C; P = 0.001). There were no significant differences (all P ≥ 0.1) between the equations used to estimate proximal (Fig. 5B) and distal skin temperature (Fig. 5C), as well as body (Fig. 5D), supraclavicular (Fig. 5E), and peripheral (Fig. 5F) temperature gradients.
Figure 5.
Measures of skin temperature at the last five minutes of the second warm period as estimated with the equations used in the respective references. (A) Mean skin temperature: *P ≤ 0.001: 14-ISO vs. Boon; †P ≤ 0.001: 8-ISO vs. Boon; #P = 0.002: 4-ISO vs. Boon; ‡P = 0.001: Boon vs. PROFITH. (B) Proximal skin temperature: All P = 1.000. (C) Distal skin temperature: P = 0.113. (D) Body temperature gradients: P = 0.252. (E) Supraclavicular temperature gradients: All P ≥ 0.575. (F) Peripheral temperature gradient: All P = 1.000. Data are mean and standard error.
Discussion
Cold activates human BAT, which produces heat. Skin temperature is an indirect measure to monitor how the body reacts to cold. The present study analysed the impact of the most used equation in BAT-human studies to estimate parameters of skin temperature in warm and cold room conditions in young lean men. We observed differences across equations to measure the same parameters of skin temperature in warm and cold room conditions, which hamper comparisons across studies.
Mean skin temperature
The equation reported by ISO STANDARD 9886:2004 using 14 ibuttons (14-ISO)41 is the most commonly used equation in BAT-related studies7, 14, 18–23, yet it has been used with substantial modifications15, 24, 25. Furthermore, studies report values of mean skin temperature without providing information on how the calculations were made12, 17, 29, which hampers between-study comparisons. Mean skin temperature estimated with 14-ISO was similar to that estimated using 26 ibuttons (PROFITH equation, see Table 1), which suggests that the 14-ISO equation covers the most important body sites. The ISO STANDARD 9886:2004 suggested another set of 8 ibuttons (8-ISO)41 to measure mean skin temperature. However, the 8-ISO slightly overestimates temperature in both warm and cold conditions, at least when compared with the other equations used in this study.
Similarly, the equation based on 4 ibuttons (4-ISO)41 overestimates mean temperature in warm room conditions and underestimates mean temperature in cold conditions compared to 14-ISO. The anatomical sites used in 4-ISO may partially explain the observed differences. For instance, the temperatures of the shin bone and the hand decreased after cold exposure14 probably due to peripheral vasoconstriction, while these two anatomical zones contribute 50% of the estimated mean temperature in the equation 4-ISO. Based on these findings, we suggest using the 14-ISO equation to measure mean skin temperature because (i) it is the most used equation in BAT-human studies, (ii) it is supported by the International Standard Organization, and (iii) the outcome of temperature is practically the same when it is compared with an equation with a higher number of ibuttons.
Proximal skin temperature
We did not observe differences between the equations used to estimate proximal skin temperature during cold exposure or during the warm period II. On the other hand, there were differences between the study equations during the warm period I. The equations reported by Kräuchi et al.36 and Schellen et al.30 used four ibuttons while the equations reported by van Marken Lichtenbelt et al.26 and Boon et al.15 used three ibuttons. The equations reported by both van Marken Lichtenbelt et al.26 and Boon et al.15 are based on the same anatomical points. However, Boon et al.15 used the clavicular zone whereas van Marken Lichtenbelt et al.26 used the subclavicular zone (Table 1). These equations showed a similar decrease of the mean temperature after cold exposure: −1.72 ± 0.85 °C15 and −1.87 ± 0.76 °C26, respectively. To assess proximal skin temperature, we suggest the equation reported by Boon et al.15 as it includes a button at the clavicular site which is close to BAT deposits. In addition, the outcome of proximal temperature could be more representative from a body reaction to cold than other equations.
Distal skin temperature
To measure distal skin temperature, the majority of BAT-related studies7, 14, 18–23 used the equation reported by Kraüchi et al.36 (2 ibuttons placed on the left hand and right instep). Indeed, this equation is one of the easiest to estimate distal skin temperature. We did not observe differences between distal skin temperature measured by this and other equations with a higher number of distal anatomical points (6 ibuttons, see PROFITH, Table 1) in warm or cold conditions (all P ≥ 0.113). In addition, the 14 anatomical points recommended by ISO STANDARD 9886:200441 include the anatomical positions used by the equation reported by Kraüchi et al.36. Thus, under these study conditions, the equation reported by Kraüchi et al.36 is a valid choice to estimate distal skin temperature. Moreover, two distal ibuttons (hand and feet) can measure the same as other equations with a higher number of ibuttons (i.e. 6 devices in PROFITH equation, see Table 1).
Whole body temperature gradients
The heat loss capacity of the body can be estimated as the gradient between distal and proximal skin temperature. Such gradients have been used in various fields including circadian rhythm34, 36, 42, anaesthesia24, exercise27, 43, 44, and following BAT activation7, 14, 18–23. We observed no differences between the gradients calculated by the equation reported by Boon et al.15 (5 ibuttons) and other equations with a higher number of anatomical points (11 ibuttons, see PROFITH, Table 1). Therefore, we suggest to use the equation reported by Boon et al.15 as it obtains the same outcome of temperature with a lower number of ibuttons than other equations.
Another interesting gradient that has recently been used in the field of BAT research is the supraclavicular temperature gradient proposed by Lee et al.16. This gradient is based on the difference between the temperature of the right supraclavicular fossa and right upper chest and aims to estimate BAT heat loss capacity during or after a cold exposure. Yoneshiro et al.12 and Chondronikola et al.17 used the same gradient albeit at the left side of the body. The importance of the side of the body is unknown and further studies are warranted. Nevertheless, we observed differences when the supraclavicular temperature gradient is calculated on the right or the left side, as well as on the chest or the subclavicular zone. We propose to use the supraclavicular temperature gradient reported by Lee et al.16, 29. This gradient was validated against 18F-FDG-PET/CT and infrared thermography in 87 lean individuals29 while the validity of other gradients has not yet been proved12, 17.
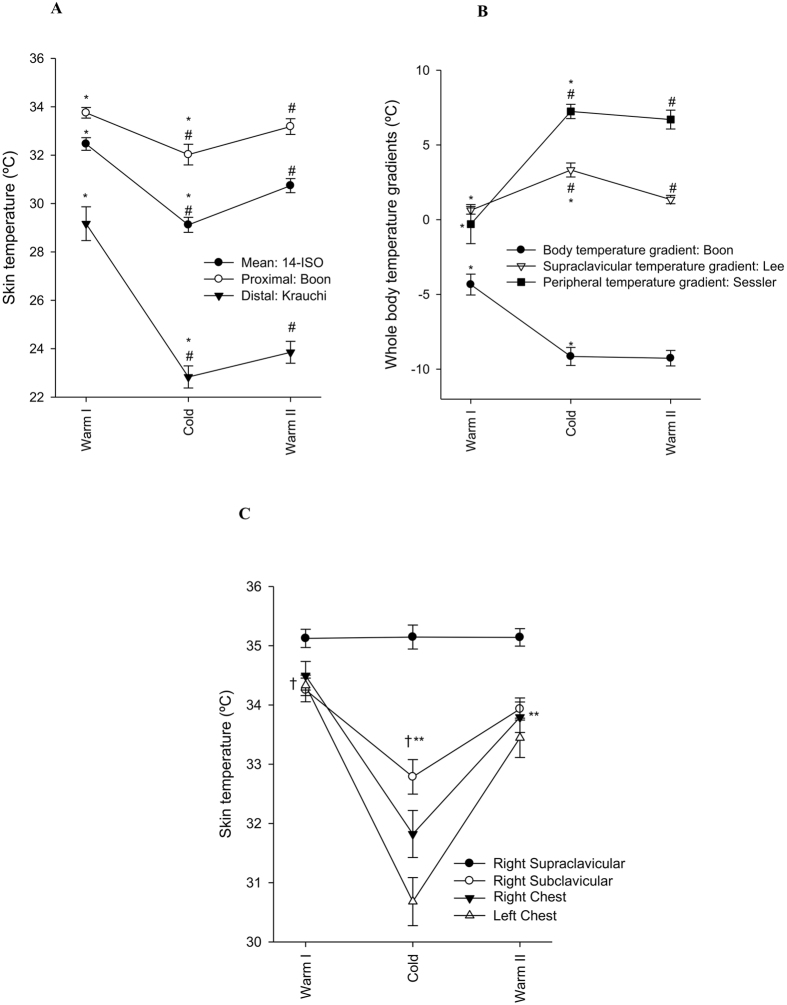
Interestingly, we identified the right supraclavicular skin temperature as the only marker that did not decrease during cold exposure (Fig. 6), which is in line with the hypothesis that cold exposure activates BAT, and that BAT generates heat. Besides, this region was properly covered by the cooling vest. Although we have no data on BAT activity and volume of the participants, this finding concurs with other studies that showed that supraclavicular skin temperature was positively associated with BAT activity and volume in the supraclavicular zone in healthy young men12, 14, 15, 17, 45. However, we cannot ignore that the absence of supraclavicular skin temperature decrease upon cold exposure is due to the presence of large blood vessels (i.e. aorta) close to the skin in this area. More studies are required to confirm this finding, as well as to elucidate the role of body mass index and subcutaneous adipose tissue in the measurement of all parameters of skin temperature45–48.
Figure 6.
Suggested equations to measure different parameters of skin temperature during warm and cold exposures. (A) Mean skin temperature (14-ISO): *P ≤ 0.001: Warm I vs. Cold; #P ≤ 0.001: Cold vs. Warm II; #P = 0.002. Proximal skin temperature (Boon): *P ≤ 0.001: Warm I vs. Cold; #P ≤ 0.001: Cold vs. Warm II. Distal skin temperature (Krauchi): *P ≤ 0.001: Warm I vs. Cold; #P ≤ 0.001: Cold vs. Warm II. (B) Body temperature gradient (Boon): *P ≤ 0.001: Warm I vs. Cold. Supraclavicular temperature gradient (Lee): *P ≤ 0.001: Warm I vs. Cold; #P ≤ 0.001: Cold vs. Warm II. Peripheral temperature gradient (Sessler): *P ≤ 0.001: Warm I vs. Cold. (C) Raw data of all anatomic points integrated in the equations to estimate supraclavicular temperature gradients: all †P≤0.001: Warm I vs. Cold; all **P ≤ 0.001: Cold vs. Warm II. No significant differences were found in the supraclavicular skin temperature across the exposures. Data are mean and standard error.
Peripheral temperature gradient is a proxy of peripheral vasoconstriction. This gradient has been used as a marker for changes in the blood flow in peripheral zones. The peripheral vasoconstriction is a strategy of the body to keep the organs warm during cold exposure5 carrying some of the peripheral blood to the central part of the body. House & Tipton37 proposed a gradient between the temperature of the right top of the forefinger and the temperature of the middle part of the right forearm. They validated this gradient against laser Doppler flowmetry and reported that a difference of 2 °C may indicate peripheral vasoconstriction. In contrast, a gradient lower than 0 °C suggests peripheral vasodilatation. Sessler et al.24, 49 reported vasoconstriction by a difference of ≥4 °C, while a difference lower than 4 °C points to peripheral vasodilatation. Of note is that both thresholds are reached in our experimental conditions. However, other studies used the same peripheral temperature gradient as a proxy of peripheral vasoconstriction but in different anatomical positions14 or calculated the gradient in a different way25. The best way to estimate peripheral vasoconstriction is currently not known and further studies are warranted. We calculated the same gradient but in the lower part of the body (instep-gastrocnemius) and observed an increase of this gradient after cold exposure. Therefore, a peripheral vasoconstriction occurs in the lower part of the body, as it happens in the upper part of the body, as previously reported14, 50.
Limitations
Results of this study should be considered with caution. Data are based on a single cooling protocol, using a cooling vest (that covers only chest and back zones), and we do not know whether the results apply to different cooling protocols or instruments (e.g. cooling blankets or ice blocks) or to longer cold exposure after shivering occurs. Additionally, the study was conducted on young lean men and we do not know whether these results apply to older people, to women, or to persons with higher (or lower) levels of body fat or in narrower ranges of hours. This study was performed in the south of Spain, and the equations were used under a personalised cooling protocol. Therefore, we are unaware if the results match those of other countries or environments and whether they can differ under warming or exercise protocols. We cannot ignore that the time of the day when our study was conducted may have influenced the results. Due to the methodological nature of this study, our findings are not comparable with other studies because they have used other cooling or warming protocols7, 14, 18–23.
Conclusion and Recommendations
We detected differences in skin temperature across the studied equations in both warm and cold room conditions. Based on these findings, we suggest a set of 19 ibuttons to estimate mean, proximal, and distal skin temperatures as well as body temperature gradients. We recommend to measure mean skin temperature with the 14-ISO equation41; proximal and body gradient of skin temperature with the Boon et al. equation15; distal skin temperature with the Krauchi et al. equation36; supraclavicular temperature gradient with Lee et al. equation16, 29 and peripheral temperature with Sessler et al. equation3 (Table 3). These equations are based on the 14 anatomical positions reported by ISO STANDARD 9886:200441 plus five more ibuttons placed on the right supraclavicular fossa, right middle clavicular bone, right middle upper forearm, right top of forefinger, and right upper chest (Fig. 1: ibuttons 1–16, 20, 21, and 25, respectively). Moreover, we have seen that all selected equations are sensitive to the cooling protocol study (see Fig. 6), except the supraclavicular skin temperature which was similar across temperature conditions.
Table 3.
Recommended equations to measure skin temperature.
| Outcome | Reference | ibuttons (n) | Anatomical positions (Fig. 1) | Equation | Rationale to select the equation |
|---|---|---|---|---|---|
| Mean skin temperature | 14 ISO 9886–200441 (14-ISO) | 14 | From 1 to 14 | (Forehead*0.07) + (Neck*0.07) + (Right Scapula*0.07) + (Left Chest*0.07) + (Right Deltoid*0.07) + (Left Elbow*0.07) + (Right Abdomen*0.07) + (Left Hand*0.07) + (Left Lumbar *0.07) + (Right Thigh*0.07) + (Left Hamstring*0.07) + Right Shinbone*0.07) + (Left Gastrocnemius*0.07) + (Right Instep*0.07) | Mean skin temperature estimated with 14-ISO was similar to that estimated using 26 ibuttons (PROFITH equation), which suggests that the 14-ISO equation covers the most important body sites with less ibuttons. |
| Proximal skin temperature | Boon et al.15 (Boon) | 3 | 10, 16, 8 | (Right Thigh*0.383) + (Right Clavicular*0.293) + (Right Abdomen*0.324) | This equation includes less ibuttons than other equations, and it also includes an ibutton at the clavicular site which is close to BAT deposits. |
| Distal skin temperature | Kräuchi et al.36 (Krauchi) | 2 | 9, 14 | (Left Hand + Right Instep)/2 | Distal skin temperature estimated with this equation was similar to that estimated using 6 ibuttons (PROFITH equation), which suggests that this equation covers the most important body sites with less ibuttons. |
| Body temperature gradient | Boon et al.15 (Boon) | 5 | 9, 14, 10, 16, 8 | [(Left Hand + Right Instep)/2] − [(Right Thigh*0.383) + (Right Clavicular*0.293) + (Right Abdomen*0.324)] | Body temperature gradient estimated with this equation was similar to that estimated using 11 ibuttons (PROFITH equation), which suggests that this equation covers the most important body sites with less ibuttons. |
| Supraclavicular temperature gradient | Lee et al.29 (Lee S-RC) | 2 | 15, 25 | (Right Supraclavicular(S) - Right Chest (RC)) | This equation has been validated against 18F-FDG-PET/CT and infrared thermography. |
| Peripheral temperature Gradient | Sessler et al.3 Right arm | 2 | 20, 21 | (Right Forearm-Right Top of forefinger) | This equation has been validated against laser Doppler flowmetry. |
Acknowledgements
We are grateful to Alberto Quesada-Aranda for helping with the development of the Temperatus software (Free trial in http://profith.ugr.es/temperatus). We are grateful to Ms. Carmen Sainz-Quinn for assistance with the English language. This study is part of a PhD thesis conducted in the Biomedicine Doctoral Studies of the University of Granada, Spain. The study was supported by the Spanish Ministry of Economy and Competitiveness, Fondo de Investigación Sanitaria del Instituto de Salud Carlos III (PI13/01393), Fondos Estructurales de la Unión Europea (FEDER), by the Spanish Ministry of Science and Innovation (RYC-2010–05957), by the Spanish Ministry of Education (FPU 13/04365 and 15/04059), by the Fundación Iberoamericana de Nutrición (FINUT), by the Redes temáticas de investigación cooperativa RETIC (Red SAMID RD12/0026/0015 and RD16/0022), by AstraZeneca HealthCare Foundation and by the University of Granada, Plan Propio de Investigación 2016, Excellence actions: Units of Excellence; Unit of Excellence on Exercise and Health (UCEES). Mariëtte R. Boon is supported by the Dutch Diabetes Foundation (grant 2015.81.1808). Patrick C.N. Rensen is an Established Investigator of the Netherlands Heart Foundation (grant 2009T038).
Author Contributions
Conception and design of research: B.M.T. and J.R.R.; B.M.T., G.S.D., F.M.A., and J.M.A.A. performed the experiments; B.M.T., G.S.D., and J.R.R. analysed the data; B.M.T., G.S.D., M.R.B., P.C.R., and J.R.R. interpreted the results; B.M.T. and J.R.R. prepared the figures and drafted manuscript; B.M.T., G.S.D., F.M.A., J.M.A.A., M.R.B., P.C.R., and J.R.R. critically revised the manuscript and approved the final version.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hardy JD, Bois EF. Du. The Technic of Measuring Radiation and Convection. J. Nutr. 1938;15:461–475. [Google Scholar]
- 2.Priego Quesada JI, et al. Effects of graduated compression stockings on skin temperature after running. J. Therm. Biol. 2015;52:130–136. doi: 10.1016/j.jtherbio.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Sessler DI, Olofsson CI, Rubinstein EH, Beebe JJ. The thermoregulatory threshold in humans during halothane anesthesia. Anesthesiology. 1988;68:836–42. doi: 10.1097/00000542-198806000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Mendt S, et al. Circadian rhythms in bed rest: Monitoring core body temperature via heat-flux approach is superior to skin surface temperature. Chronobiol. Int. 2016;0:1–11. doi: 10.1080/07420528.2016.1224241. [DOI] [PubMed] [Google Scholar]
- 5.Benzinger TH. Heat regulation: homeostasis of central temperature in man. Physiol. Rev. 1969;49:671–759. doi: 10.1152/physrev.1969.49.4.671. [DOI] [PubMed] [Google Scholar]
- 6.Brychta, R. J. & Chen, K. Y. Cold-induced thermogenesis in humans. Eur. J. Clin. Nutr. 1–8, doi:10.1038/ejcn.2016.223 (2016). [DOI] [PMC free article] [PubMed]
- 7.Hanssen, M. J. et al. Short-term cold acclimation recruits brown adipose tissue in obese humans. Diabetes31 (2015). [DOI] [PubMed]
- 8.Vosselman MJ, Vijgen GHEJ, Kingma BRM, Brans B, van Marken Lichtenbelt WD. Frequent extreme cold exposure and brown fat and cold-induced thermogenesis: a study in a monozygotic twin. PLoS One. 2014;9 doi: 10.1371/journal.pone.0101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 10.Admiraal WM, et al. Cold-induced activity of brown adipose tissue in young lean men of South-Asian and European origin. Diabetologia. 2013;56:2231–2237. doi: 10.1007/s00125-013-2938-5. [DOI] [PubMed] [Google Scholar]
- 11.Blondin DP, et al. Contributions of white and brown adipose tissues and skeletal muscles to acute cold-induced metabolic responses in healthy men. J. Physiol. 2015;593:701–14. doi: 10.1113/jphysiol.2014.283598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yoneshiro T, et al. Brown adipose tissue is involved in the seasonal variation of cold-induced thermogenesis in humans. Am. J. Physiol. Regul. Integr. Comp. Physiol. ajpregu. 2016;00057 doi: 10.1152/ajpregu.00057.2015. [DOI] [PubMed] [Google Scholar]
- 13.Boon, M. R. & van M Lichtenbelt, W. D. In Handbook of experimental pharmacology233, 301–319 (2015). [DOI] [PubMed]
- 14.van der Lans AaJJ, Vosselman MJ, Hanssen MJW, Brans B, van M Lichtenbelt WD. Supraclavicular skin temperature and BAT activity in lean healthy adults. J. Physiol. Sci. 2016;66:77–83. doi: 10.1007/s12576-015-0398-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boon MR, et al. Supraclavicular Skin Temperature as a Measure of 18F-FDG Uptake by BAT in Human Subjects. PLoS One. 2014;9 doi: 10.1371/journal.pone.0098822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee P, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chondronikola M, et al. Brown Adipose Tissue Is Linked to a Distinct Thermoregulatory Response to Mild Cold in People. Front. Physiol. 2016;7 doi: 10.3389/fphys.2016.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vijgen GHEJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2012;97:E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 19.Vijgen GHEJ, et al. Increase in brown adipose tissue activity after weight loss in morbidly obese subjects. J. Clin. Endocrinol. Metab. 2012;97:E1229–33. doi: 10.1210/jc.2012-1289. [DOI] [PubMed] [Google Scholar]
- 20.Vijgen GHEJ, et al. Brown adipose tissue in morbidly obese subjects. PLoS One. 2011;6 doi: 10.1371/journal.pone.0017247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vosselman MJ, et al. Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans. Diabetes. 2012;61:3106–3113. doi: 10.2337/db12-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanssen MJW, et al. Glucose uptake in human brown adipose tissue is impaired upon fasting-induced insulin resistance. Diabetologia. 2015;58:586–595. doi: 10.1007/s00125-014-3465-8. [DOI] [PubMed] [Google Scholar]
- 23.van der Lans AAJJ, et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 2013;123:3395–3403. doi: 10.1172/JCI68993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puhakka K, Anttonen H, Niskanen J, Ryhänen P. Calculation of mean skin temperature and changes in body heat content during paediatric anaesthesia. Br. J. Anaesth. 1994;72:548–53. doi: 10.1093/bja/72.5.548. [DOI] [PubMed] [Google Scholar]
- 25.Martínez N, et al. Validation of the thermophysiological model by Fiala for prediction of local skin temperatures. Int. J. Biometeorol. 2016;60:1969–1982. doi: 10.1007/s00484-016-1184-1. [DOI] [PubMed] [Google Scholar]
- 26.van Marken Lichtenbelt WD, et al. Evaluation of wireless determination of skin temperature using iButtons. Physiol. Behav. 2006;88:489–97. doi: 10.1016/j.physbeh.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Smith aDH, Crabtree DR, Bilzon JLJ, Walsh NP. The validity of wireless iButtons and thermistors for human skin temperature measurement. Physiol. Meas. 2010;31:95–114. doi: 10.1088/0967-3334/31/1/007. [DOI] [PubMed] [Google Scholar]
- 28.Lee P, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–309. doi: 10.1016/j.cmet.2013.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee P, et al. Hot fat in a cool man: Infrared thermography and brown adipose tissue. Diabetes, Obes. Metab. 2011;13:92–93. doi: 10.1111/j.1463-1326.2010.01318.x. [DOI] [PubMed] [Google Scholar]
- 30.Kingma BRM, Schellen L, Frijns aJH, van Marken Lichtenbelt WD. Thermal sensation: a mathematical model based on neurophysiology. Indoor Air. 2012;22:253–262. doi: 10.1111/j.1600-0668.2011.00758.x. [DOI] [PubMed] [Google Scholar]
- 31.Kingma BRM, Vosselman MJ, Frijns aJH, van Steenhoven aa, van Marken Lichtenbelt WD. Incorporating neurophysiological concepts in mathematical thermoregulation models. Int. J. Biometeorol. 2014;58:87–99. doi: 10.1007/s00484-012-0628-5. [DOI] [PubMed] [Google Scholar]
- 32.Schellen L, Loomans MGLC, de Wit MH, Olesen BW, van Marken Lichtenbelt WD. The influence of local effects on thermal sensation under non-uniform environmental conditions–gender differences in thermophysiology, thermal comfort and productivity during convective and radiant cooling. Physiol. Behav. 2012;107:252–61. doi: 10.1016/j.physbeh.2012.07.008. [DOI] [PubMed] [Google Scholar]
- 33.Kolodyazhniy V, et al. Estimation of human circadian phase via a multi-channel ambulatory monitoring system and a multiple regression model. J. Biol. Rhythms. 2011;26:55–67. doi: 10.1177/0748730410391619. [DOI] [PubMed] [Google Scholar]
- 34.Kräuchi K, et al. Diurnal blood pressure variations are associated with changes in distal-proximal skin temperature gradient. Chronobiol. Int. 2012;29:1273–83. doi: 10.3109/07420528.2012.719961. [DOI] [PubMed] [Google Scholar]
- 35.Schellen L, Loomans MGLC, De Wit MH, Olesen BW, Lichtenbelt WDVM. Effects of different cooling principles on thermal sensation and physiological responses. Energy Build. 2013;62:116–125. doi: 10.1016/j.enbuild.2013.01.007. [DOI] [Google Scholar]
- 36.Kräuchi K, Cajochen C, Möri D, Graw P, Wirz-Justice A. Early evening melatonin and S-20098 advance circadian phase and nocturnal regulation of core body temperature. Am. J. Physiol. 1997;272:R1178–88. doi: 10.1152/ajpregu.1997.272.4.R1178. [DOI] [PubMed] [Google Scholar]
- 37.House JR, Tipton MJ. Using skin temperature gradients or skin heat flux measurements to determine thresholds of vasoconstriction and vasodilatation. Eur. J. Appl. Physiol. 2002;88:141–145. doi: 10.1007/s00421-002-0692-3. [DOI] [PubMed] [Google Scholar]
- 38.Iso, 9920. Ergonomics of the thermal environment – estimation of the thermal insulation and evaporative resistance of a clothing ensemble. Int. Stand. Organ. 3 (1992).
- 39.American Society of Heating Refrigerating and Air-Conditioning Engineers. in (2005).
- 40.Sanchez-Delgado G, et al. Activating brown adipose tissue through exercise (ACTIBATE) in young adults: Rationale, design and methodology. Contemp. Clin. Trials. 2015;45:416–25. doi: 10.1016/j.cct.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 41.ISO-standard 9886:2004 Ergonomics – Evaluation of thermal strain by physio- logical measurements, International Standards Organization, Geneva, S. in (2004).
- 42.Kräuchi K, Cajochen C, Danilenko KV, Wirz-Justice A. The hypothermic effect of late evening melatonin does not block the phase delay induced by concurrent bright light in human subjects. Neurosci. Lett. 1997;232:57–61. doi: 10.1016/S0304-3940(97)00553-3. [DOI] [PubMed] [Google Scholar]
- 43.James CA, Richardson AJ, Watt PW, Maxwell NS. Reliability and validity of skin temperature measurement by telemetry thermistors and a thermal camera during exercise in the heat. J. Therm. Biol. 2014;45:141–149. doi: 10.1016/j.jtherbio.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 44.Stern JS, Glick Z, Horwitz BA, Wickler S. J. exposed to a treadmill. 1987;36:76–81. doi: 10.1016/0026-0495(87)90067-9. [DOI] [PubMed] [Google Scholar]
- 45.Gatidis S, et al. Is It Possible to Detect Activated Brown Adipose Tissue in Humans Using Single-Time-Point Infrared Thermography under Thermoneutral Conditions? Impact of BMI and Subcutaneous Adipose Tissue Thickness. PLoS One. 2016;11 doi: 10.1371/journal.pone.0151152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Giangreco A, Qin M, Pintar JE, Watt FM. Epidermal stem cells are retained in vivo throughout skin aging. Aging Cell. 2008;7:250–259. doi: 10.1111/j.1474-9726.2008.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wu P, et al. Evo-Devo of amniote integuments and appendages. Int. J. Dev. Biol. 2004;48:249–270. doi: 10.1387/ijdb.15272390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Alexander CM, et al. Dermal white adipose tissue: a new component of the thermogenic response. J. Lipid Res. 2015;56:2061–2069. doi: 10.1194/jlr.R062893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sessler DI. Skin-temperature gradients are a validated measure of fingertip perfusion. Eur. J. Appl. Physiol. 2003;89:401–402. doi: 10.1007/s00421-003-0812-8. [DOI] [PubMed] [Google Scholar]
- 50.Vosselman MJ, et al. Low brown adipose tissue activity in endurance-trained compared with lean sedentary men. Int. J. Obes. 2015;39:1696–1702. doi: 10.1038/ijo.2015.130. [DOI] [PubMed] [Google Scholar]