Abstract
Noise stress is a common environmental pollutant whose adverse effect on offspring performance has been less studied. This study was novel in terms of using “noise” as a prenatal stress compared with physical stress to explore the effect of stress during gestation on HPA axis activation, cognitive performance, and motor coordination, as well as in investigating the effect of behavioral assessments on the corticosterone (CORT) levels. Three groups of C57BL/6 mice with a gestational history of either noise stress (NS), physical stress (PS), or no stress were examined in several behavioral tests. Plasma CORT level was significantly higher before starting the behavioral tests in NS group than the two other groups. It was significantly increased after the behavioral tests in both prenatal stressed groups relative to the controls. Stress caused anxiety-like behavior and reduced learning and memory performance in both stressed groups compared to the controls, as well as decreased motor coordination in the NS group relative to the other groups. The findings suggested that: prenatal NS severely changes the HPA axis; both prenatal stressors, and particularly NS, negatively impair the offspring’s cognitive and motor performance; and, they also cause a strong susceptibility to interpret environmental experiences as stressful conditions.
Introduction
The early life is one of the most important and sensitive periods during the development of an individual. A large body of evidence supports the conclusion that there are diverse effects of stress during gestation on the offspring’s brain and behavior in both human and non-human animals1–3. In humans, the relationship between prenatal stress and increased tendency for developing diverse psychosocial problems has been demonstrated in both childhood and adulthood. For example, the link between prenatal stress and cognitive, behavioral, physical, and emotional problems such as autism and attention-deficit hyperactivity disorder in children, as well as the relationship between prenatal stress and depression and schizophrenia in adulthood, has been well-documented in numerous studies1, 4, 5. Studies of laboratory rodents have shown negative neurodevelopmental, behavioral, neuroanatomical, neurochemical, and epigenetic outcomes subsequent to prenatal stress. The behavioral symptoms are broad including anxiety and depressive-like behavior, learning and memory deficits, distractibility and attention disorders, and non-directed locomotor behavior2, 4, 5.
Fetal development is regulated by hormones that are transported through the placenta6. Stress may also negatively affect the developing immune system leading to the higher incidence of respiratory and other infectious diseases5, 7, 8. In addition, maternal stress can influence fetal brain development by constricting the placental arteries and consequently reducing fetal blood flow and the supply of essential nutrients and oxygen9. Catecholamines, corticotropin releasing hormone (CRH), and adrenal steroids are stress hormones that penetrate the fetal brain from the maternal circulation. In addition to CRH, which is only shown in primates, other circulating stress hormones including cortisol, corticosterone (CORT), adrenocorticotropic hormone, and aldosterone are increased owing to maternal stress in both the mother and fetuses of rodents and non-human primates5. These hormones could then produce alterations in the brain structure and function depending on the hormone type, and amount and time of exposure.
The kind of stressor experienced is an important issue to address in considering the effect of prenatal stress on the offspring’s brain and behavior. Laboratory studies have used a wide range of stressors including crowding, social isolation, food deprivation, cage tilting, exposure to cold, intermittent electric shocks, forced swimming, and restraint alone or restraint with heat and bright light, and noise2, 4, 6. Among the diverse types of prenatal stressors, restraint is the most commonly used and noise is the least used stressor in rodent studies2. These two stressors differ in how much they are unpredictable and/or uncontrollable and thus likely differ in their induction of the hypothalamic–pituitary–adrenal (HPA) response in animals.
In a study on offspring of pregnant rats exposed to restraint, forced swimming, and crowding, although each stressor significantly increased the plasma CORT level compared with the controls (52 ng/mL), the increases varied by stressors including restraint (522 ng/mL), forced swimming (438 ng/mL), and crowding (247 ng/mL) respectively. The degree of control that an animal perceives it has over the stressful event is one of the important factors that differs among these stressors. Likely, the highest HPA axis response in restraint stress is the result of absolutely no control over the situation for the animal, whereas in both forced swimming or crowding the animal is able to move and therefore has a certain degree of control over the situation10. Studies in both humans and laboratory animals have shown that the sense of control over the stressor effects the prefrontal cortex regulation of stress hormones11.
Noise is an unwanted unpleasant auditory stimulus whose adverse effects on health have been less studied12. The most investigated non-auditory effects of noise on human health are perceived disturbance and annoyance, cognitive impairment (loss of learning and memory), sleep disturbance, and cardiovascular diseases13, 14. Noise interferes with daily activities, sleep, or rest, and can induce annoyance and negative responses, such as anger, displeasure, exhaustion, and stress-related symptoms15. Stressful noise causes the release of stress hormones, including catecholamines and glucocorticoids16. Although a growing body of evidence supports the conclusion that prenatal stressors produce adverse effects on brain development, neuroendocrine function, and behavior in offspring, only a few studies have examined the impact of noise stress on brain and behavior in rodents, particularly in the mouse. The present study was performed to compare the effect of a prenatal noise stress and physical stressors on cognitive and motor performance as well as the HPA axis activity in offspring. We also investigated how the HPA axis activity is modulated by training the mice on a set of behavioral tests.
Results
No significant differences were observed between the two control groups in any measures (p > 0.05). Therefore, the control groups pooled for results.
CORT levels
The CORT levels comparison before starting and after finishing the behavioral tests
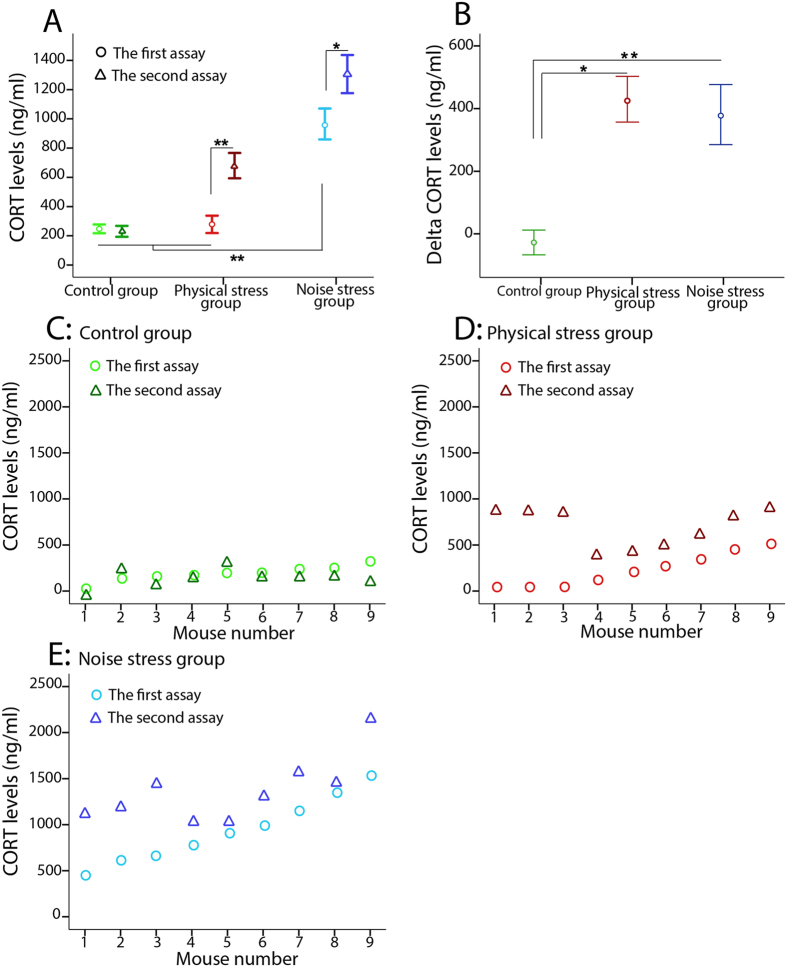
Blood collection was conducted twice one day before starting and one day after finishing all behavioral tests. Table 1 compares the two time periods of the CORT assay in each group. No significant difference was found between the two CORT assays in the control group, but in both stressed groups the plasma CORT levels were significantly higher in the second assay compared with the first assay (Fig. 1A). Figure 1B shows the delta CORT levels (the difference between the CORT levels in the first and the second measures) in the three groups. A significant difference was observed between the control group and both stressed groups in the delta CORT level (Table 1).
Table 1.
(A) Comparison of CORT levels within each group to compare levels before starting (the first assay) and after finishing (the second assay) all behavioral tests. (B) Comparison of CORT levels among the three groups for the first assay, the second assay, and the delta CORT.
| a) In every group | Mean difference (delta CORT) | t | p | η2 | power | ||
|---|---|---|---|---|---|---|---|
| Control group | −28.017 | 0.505 | 0.497 | 0.059 | 0.096 | ||
| Physical stress group | 414.674 | −4.639 | 0.004 | 0.676 | 0.945 | ||
| Noise stress group | 396.218 | −3.146 | 0.021 | 0.602 | 0.761 | ||
| b) Among the groups | *Between groups’ p-values | **Significant main effects | |||||
| Control and PS | Control and NS | PS and NS | F | p | η 2 | power | |
| The first assay | 0.439 | <0.001 | <0.001 | 23.069 | <0.001 | 0.658 | 1.000 |
| The second assay | 0.021 | <0.001 | <0.001 | 22.226 | <0.001 | 0.649 | 1.000 |
| Delta CORT | 0.005 | 0.020 | 0.563 | 5.307 | 0.012 | 0.370 | 0.787 |
NS: noise stress, PS: physical stress, η2 = effect size, *the “between groups’ p-values” show p-values for the between group comparisons, **the “significant main effects” indicate the statistical results of a significant main effect for every measure.
Figure 1.
The CORT levels (ng/ml): (A) the CORT level in the first assay (○ = before starting behavioral tests) was significantly higher in the NS group compared with the two other groups. A significant increase in CORT levels were observed in the second assay (Δ = after finishing all behavioral tests) than the first assay in both stressed groups relative to the control group. (B) A significant difference was obtained in the delta CORT level between the control group and both stressed groups. Results reported as mean ± S.E.M. Asterisks indicate *p < 0.05 or **p < 0.01. (C) No significant difference was shown in CORT levels between the first assay and the second assay in the control group. (D and E) A significant difference was obtained in CORT levels between the first assay and the second assay in both PS and NS groups. N = 9 per group.
The CORT levels comparison among the three groups
As Table 1 shows, the CORT level was significantly higher in the noise stress (NS) group relative to the other groups in the first assay. After finishing the behavioral tests, the CORT levels significantly increased in both stressed groups compared to the control group. Figures 1C, 1D, and 1E illustrate the CORT levels of every animal twice (pre and post behavior measures) in each group: control group (n = 9), physical stress (PS) group (n = 9), and in the NS group (n = 9), respectively. In the Fig. 1D and E, animals were ordered from the lowest to the highest CORT levels measured before the behavioral tests.
Behavioral tests
Novel object recognition (NOR) test
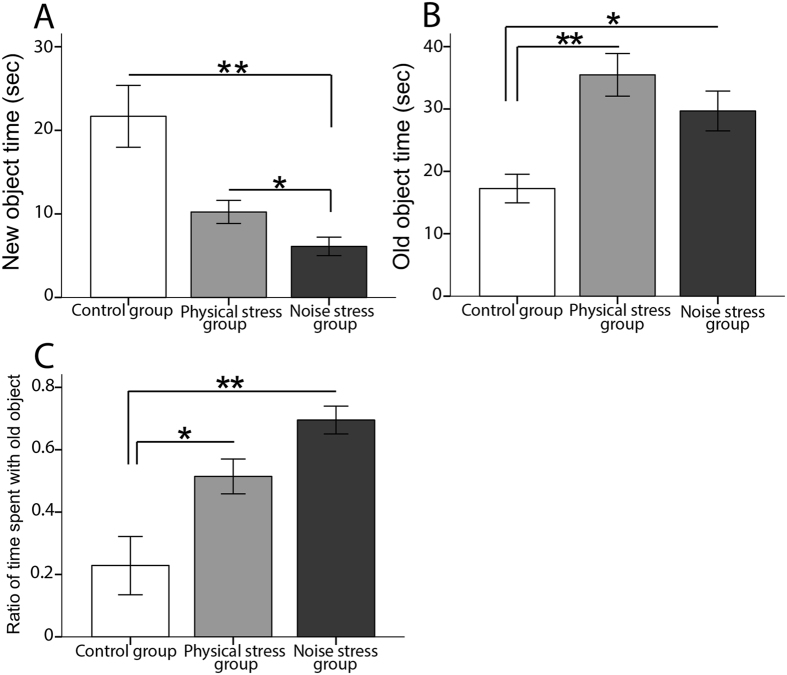
The NS group revealed a significantly shorter time spent with the new object compared with the two other groups (Table 2). Both stressed groups showed a significantly longer time spent with the old object than the control group. The ratio of time spent with old compared to new object was significantly higher in both stressed groups than the control group (Fig. 2). The difference among the groups was not significant in any measures of the locomotion behavior including movement time (F2,27 = 0.577, p = 0.571, η2 = 0.060, power = 0.131), movement number (F2,27 = 0.643, p = 0.537, η2 = 0.067, power = 0.141), total distance (F2,27 = 1.012, p = 0.385, η2 = 0.094, power = 0.203), or rest time (F2,27 = 0.913, p = 0.401, η2 = 0.101, power = 0.198).
Table 2.
Comparison among the three groups in different measures for all behavioral tests.
| NOR test | *Between groups’ p-values | **Significant main effects | |||||
|---|---|---|---|---|---|---|---|
| Control and PS | Control and NS | PS and NS | F | p | η2 | power | |
| New object time (sec) | 0.657 | 0.009 | 0.010 | 6.452 | 0.005 | 0.315 | 0.871 |
| Old object time (sec) | 0.001 | 0.038 | 0.151 | 5.233 | 0.012 | 0.275 | 0.789 |
| Ratio of old object time (%) | 0.022 | 0.001 | 0.098 | 6.903 | 0.004 | 0.330 | 0.893 |
| EPM test | |||||||
| Open arm time (sec) | <0.001 | <0.001 | 0.129 | 14.094 | <0.001 | 0.493 | 0.997 |
| Closed arm time (sec) | 0.025 | 0.032 | 0.896 | 3.225 | 0.048 | 0.202 | 0.597 |
| Number of entries to open arm | 0.001 | <0.001 | 0.102 | 13.686 | <0.001 | 0.486 | 0.996 |
| Number of entries to closed arm | 0.139 | <0.001 | 0.022 | 3.772 | 0.035 | 0.206 | 0.642 |
| BBT | |||||||
| Number of foot slips | 0.100 | 0.007 | 0.148 | 4.273 | 0.021 | 0.257 | 0.732 |
| Number of turns | 0.378 | 0.044 | 0.143 | 2.780 | 0.111 | 0.153 | 0.456 |
| MWT | |||||||
| Swim time (sec) | 0.004 | 0.014 | 0.784 | 5.481 | 0.004 | 0.011 | 0.850 |
| Swim speed (m/s) | <0.001 | 0.001 | 0.390 | 10.416 | <0.001 | 0.022 | 0.988 |
BBT: balance beam test; EPM: elevated plus maze; MWT: Morris water task, NOR: novel object recognition, NS: noise stress, PS: physical stress, η2 = Effect size, *the “between groups’ p-values” show p-values for the between group comparisons, **the “significant main effects” indicate the statistical results of a significant main effect for every measure.
Figure 2.
The NOR test: (A) The NS group significantly spent shorter time (sec) with the new object compared with the two other groups. (B) Both stressed groups significantly spent longer time (sec) with the old object relative to the control group. (C) The ratio of time spent with old versus new object was significantly higher in both stressed group than the control group. N = 10 per group. Results reported as mean ± S.E.M. Asterisks indicate *p < 0.05 or **p < 0.01.
Elevated plus maze (EPM) test
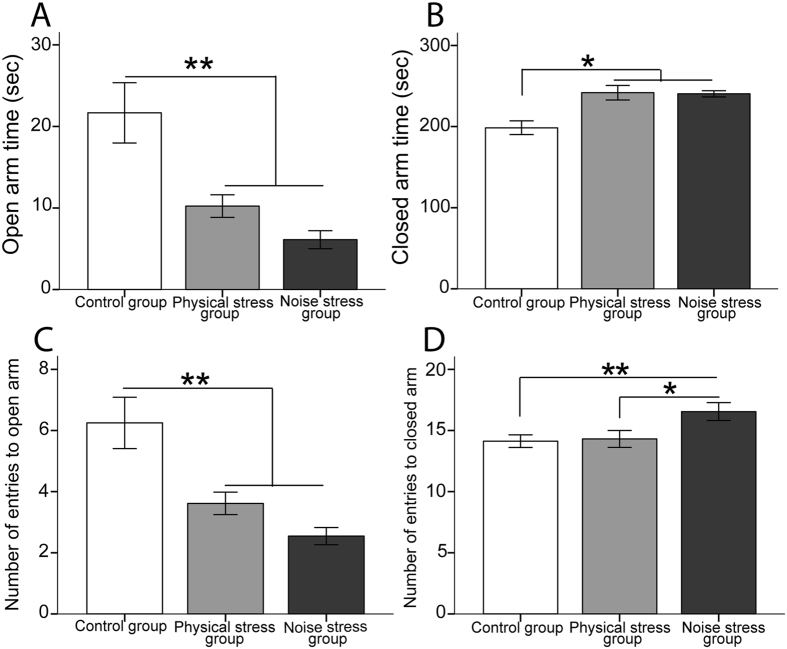
As Table 2 indicates, compared to the control group both stressed groups showed significantly less open arm time (time spent in the open arm; sec), greater closed arm time (time spent in the closed arm; sec), and a lower number of entries to open arms. The NS group had a significantly higher number of entries into closed arms compared with the other groups. Figure 3 compares the means of variables in all groups.
Figure 3.
The EPM test: Both stressed groups showed: (A) a shorter time in open arms (sec); (B) a longer time in closed arms (sec); and, (C) a lower number of entries to open arms compared with the control group. (D) The NS group revealed higher number of entries to closed arm than the two other groups. N = 10 per group. Results reported as mean ± S.E.M. Asterisks indicate *p < 0.05 or **p < 0.01.
Balance beam test (BBT)
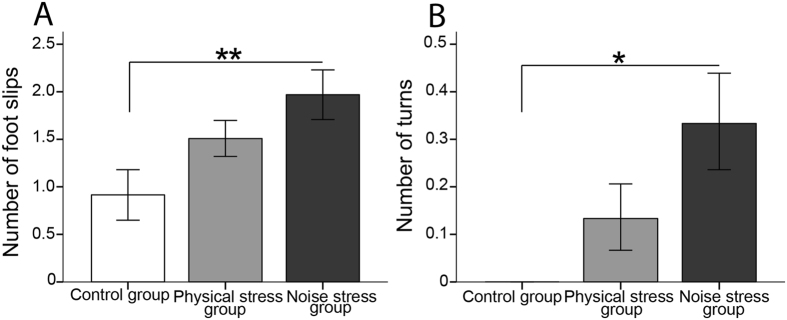
The latency to pass across the beam was higher in both stressed groups (PS group mean: 10.39 ± 3.90 sec, NS group: 9.82 ± 2.14 sec) relative to the control group (8.05 ± 2.50), but the difference was not significant (F2,27 = 1.612, p = 0.278, η2 = 0.091, power = 0.321). The NS group showed a significantly higher number of foot slips, and higher number of turns compared with the other groups (Table 2). Figure 4 shows mean scores of measured variables for the three groups.
Figure 4.
The BBT: The noise stress group revealed significantly (A) higher number of foot slips, and (B) higher number of turns compared with the two other groups. N = 10 per group. Results reported as mean ± S.E.M. Asterisk indicates *p < 0.05.
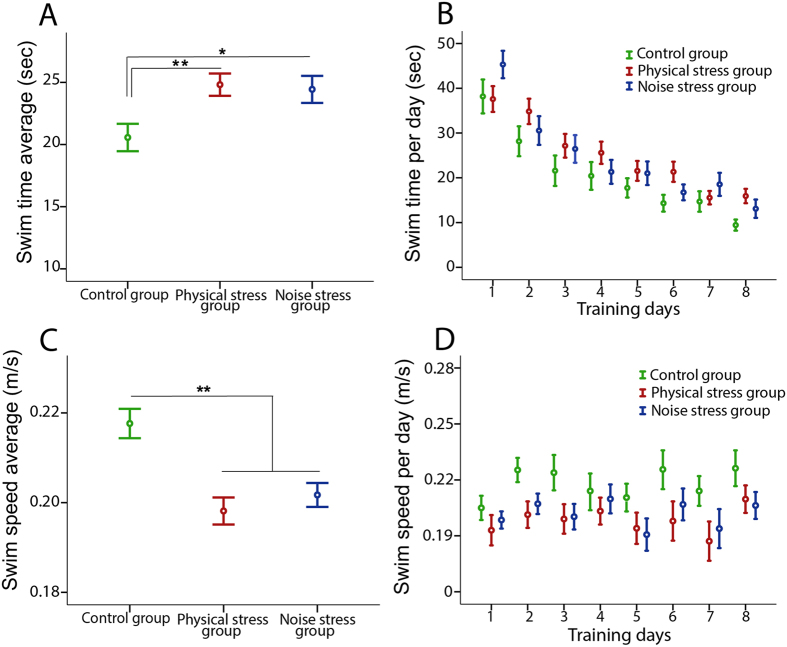
Morris water task (MWT)
Swim time
Both stressed groups showed significantly longer time to reach the platform relative to the control group. Figure 5A and B illustrates the learning process of the three groups during 8-day course of training in terms of latency to reach platform or swim time (sec). Table 2 compares the three groups in average of swim time during days of training on the MWT. Also in intra-group analysis, a significant difference was observed in swim time across days of training (Control group: F7,273 = 14.004, p < 0.001, partial η2 = 0.342, power = 1.0; PS group: F7,273 = 11.081, p < 0.001, partial η2 = 0.633, power = 1.0; NS group: F7,273 = 13.469, p = < 0.001, partial η2 = 0.771, power = 1.0) in each group.
Figure 5.
The MWT: (A) The swim time average (sec) and (B) the swim time during 8-days training in the three groups. A significantly longer swim time was observed in both stressed groups relative to the control group. (C) The swim speed average (m/s) and (D) the swim speed during 8-days training in the three groups. A significantly lower swim speed was obtained in both stressed groups versus the control group. N = 10 per group. Results reported as mean ± S.E.M. Asterisks indicate **p < 0.01.
Swim speed
Both stressed groups showed significantly slower swim speed to reach the platform compared with the control group. Figure 5C and D indicates the swim speed (m/s) of the three groups during days of training. Table 2 compares the three groups in average of swim speed (m/s) during course of training of the MWT. In an intra-group analysis, no significant difference was shown in swim speed (m/s) during eight days of training in the three groups (control group: F7,273 = 1.694, p = 0.071, partial η2 = 0.411, power = 0.765; PS group: F7,273 = 0.871, p = 0.537, partial η2 = 0.119, power = 0.331; NS group: F7,273 = 1.732, p = 0.142, partial η2 = 0.302, power = 0.591).
Swim distance
Although both stressed groups travelled a longer path (m) to reach the platform compared with the control group, this difference was not significant (F2,27 = 1.943, p = 0.143, partial η2 = 0.004, power = 0.405).
Probe test
Both stressed groups spent shorter time in the target quadrant than the control group, but this difference was not significant (F2,27 = 0.657, p = 0.473, partial η2 = 0.037, power = 0.136).
Discussion
The four main findings were: 1) the NS group had a higher baseline CORT level than the other groups; 2) behavioral testing increased the CORT levels in both stress groups but not in the controls; 3) both stress groups performed significantly differently from the control group on the elevated plus maze, novel recognition memory test, and the MWT; and, 4) the performance of the NS group in the motor task was impaired relative to the other groups. We consider these findings in turn.
Corticosterone is a major component of the stress system that along with the sympathetic nervous system plays a prominent role to mobilize energy stores during stressful conditions4, 17. In the current study although both stressors increased CORT levels, the NS procedure had almost a fourfold greater effect on offspring’s HPA axis response relative to the PS procedure. Interestingly, in the second measure, where we hypothesized that familiarization with the surrounding environment through the behavioral assessments could act as a positive factor leading to a decrease in the CORT levels, CORT levels were remarkably increased compared to the first measure in both prenatal stress groups. This finding suggests that encountering novel conditions during behavioral assessments was stressful for both prenatal stress groups. High levels of CORT concentrations before starting behavioral assessments in the NS animals could be the result of high levels of maternal CORT levels reaching the fetal brain through the placenta. Numerous studies suggest that prenatal stress is associated with higher baseline CORT release18–20. Furthermore, greater stress reactivity19, 21, 22 and slower recovery from the stressors18, 23 have been indicated in prenatally stressed rodents. The lipophilic structure of CORT naturally allows it to readily pass biological barriers such as the placenta. Although CORT is essential for fetal growth and the induction of certain enzymes to prepare the fetus for extrauterine life, fetal physiological CORT levels are much lower than maternal levels. The fetoplacental 11β-hydroxy steroid dehydrogenase type 2 (11β-HSD2) is an enzyme that catalyses the rapid metabolism of active CORT (cortisol) to physiologically inactive 11-keto forms (cortisone, 11-dehydro corticosterone) and makes a barrier to transport maternal CORTs. This barrier is not fully developed to protect the fetus against extra levels of CORT during stress exposure. Therefore, high proportions of maternal CORT reach to the fetal brain and elevate CORT levels24.
CORT is a systemic intercellular signal whose level dynamically increases with environmental and psychological stressors, and is associated with weight loss, adrenal hypertrophy, thymus involution, and reduced corticosteroid binding globulin (CBG), and each of these changes are other hallmark signs of general stress25. Given the extreme levels of CORT in the NS group prior to doing the behavioral tests in our study, further investigations on HPA axis-related parameters (i.e., thymus and adrenal weight, ACTH and CBG levels) would be helpful in providing a more comprehensive view regarding the harmful effects of prenatal stress on general health. Hypothalamic structural rearrangement owing to prenatal stress is also gender specific and differs between males and females26, that this aspect was not investigated in the present study.
The open field tests, such as EPM, have been used to detect potential anxiety-like behavior resulting from prenatal stress in rodents2 and the EPM is likely the most employed animal model of anxiety in past studies27. In our study, both prenatal stress groups revealed significantly less time in open arms, longer time in closed arms, and a lower number of entries in open arms than the control group. The NS group also revealed a higher number of entries into closed arms compared the other groups. The number of entries into open arms and time spent in open arms has been suggested as a primary anxiety index28. The results also suggest that the noise stress can be stressful enough to produce anxiety-like behavior in offspring similar to that seen after physical stressors. The findings are a replication of numerous publications in offspring of pregnant rodents with the EPM16, 29–35.
There was also an effect of prenatal stressors in the NOR test. The tendency to explore a new object more than an old familiar one has been taken as a sensitive test of stimulus recognition memory in rodents36, 37. In our study, the NS group spent significantly less time with the new object than the other groups. Both prenatal stress groups also spent significantly longer time with the old object, and their ratio of time spent with old object was significantly higher relative to the control animals. Previous studies suggest maternal stress during a critical period of fetal brain development could highly increase the likelihood of anxiety and depressive disorders. Factors such as the type, timing, and intensity of the maternal stress, as well as gender, age, and test condition also can affect the results4, 5. Furthermore, no significant difference was observed among the three groups in any measure of the locomotion behavior, i.e., movement time, movement number, total distance, and rest time. This finding indicates that the increase in the time spent in the closed arms in the EPM is not a result of a decrease in total locomotor activity27, 29.
Whereas all groups indicated a significant improvement in learning behavior during eight days-training of the MWT, both prenatal stress groups showed markedly reduced performance in spatial learning compared with the controls. They showed a significantly longer latency and lower speed to reach the platform than the control group, whereas no significant difference was revealed in memory retention. The results thus show an adverse impact of both stress paradigms in spatial learning and allocentric navigation compared with the control mice. Similarly in recent studies, reduced spatial learning, but not memory retention, has been suggested in pre-pubertal and adult male C57/BL mice owing to the prenatal stress38, 39. In this regard, many studies have shown retardation and/or alternation of neuronal development in the fetal brain, as well as smaller hippocampal size and reduced number of hippocampal granule cells resulting from high levels of maternal CORT40–45. As almost 85% of hippocampal granule cells are created postnatally in rodents, the negative effects of maternal high plasma CORT levels appear to extend long after birth. Several studies also suggested diverse rates of reduction in dendritic arborization and synaptic loss in the CA1 and CA3 areas due to prenatal stress as measured on different postnatal days41, 46, 47. Allocentric navigation involves the hippocampus, entorhinal cortex, and surrounding structures48, 49, and the interaction of these structures with the prefrontal, anterior cingulate, parietal, and retrosplenial cortices are required for memory consolidation and retrieval50. Our results are in agreement with past studies51–53 that suggested that prenatal stress negatively affects the neural networks involved in allocentric navigation. It has also been shown to reduce adult hippocampal neurogenesis54, 55.
The swim speed of the prenatal stressed groups was also significantly slower than the control group. Previous studies have indicated that regardless of possible confounding factors such as appetite or differences in body weight, rodents typically swim at similar speeds in the MWT, and changes in swim speed owing to a treatment are typically small. Therefore, a difference in swim speed might not account for a difference in learning the platform location49. A reduced swim speed could also indicate an increased passive swimming behavior, which can be taken as evidence of increased depressive-like behavior. Using tests sensitive to depressive-like behavior such as the forced swim test (FST) might support such an assumption. The FST, which is one of the most commonly used animal models for assessing depressant-like behavior, has two major behaviors in rodents including active (swimming and struggling) and passive (immobility) behaviors, and the latter one is typically described as depressive-like behavior56. In our study, however, a significant slower swim speed, might be related to the impact of the stress procedures on offspring mental state, attentional skills, or mood56–59. This needs to be explored further in the future.
Besides glucocorticoids, there are other neuronal regulators that have an important role in the development and survival of neurons in the central nervous system. Brain-derived neurotrophic factor (BDNF) is a growth factor is expressed in the hippocampus60, and has a critical role for learning and memory formation61. Recent studies in rodents suggest that prenatal stress reduces the levels of BDNF mRNA, presumably under modulation of epigenetic mechanisms29, 62, 63. Like BDNF, noradrenaline contributes on learning and memory through activating B-adrenergic receptors and regulating hippocampal and neocortical functions64, 65. Noradrenaline and glucocorticoids each has an ‘inverted U-shaped influence on learning and memory, and even a slight change in these neural regulators impairs prefrontal cortex (PFC) and hippocampal functions11.
While numerous human and animal studies have demonstrated that prenatal stress affects behavioral, mental, and cognitive development in offspring, few studies have explored the outcomes of prenatal stress on motor development. Recent human studies suggest that prenatal stress or anxiety during gestation negatively modifies infant neuromotor development66, 67. A longitudinal study of motor development from the Western Australian Pregnancy (Raine) Study cohort (n = 2,900), which used the McCarron Assessment of Neuromuscular Development, showed a negative relation between the number of stressful events, especially in late pregnancy, and the mean neuromuscular development index measured at 10, 14, and 17 years67. In rodents, the BBT has been used as a sensitive test of early detecting balance coordination deficits68. The NS group in our study showed a significantly higher number of foot slips and a higher number of turns compared with the control group in the BBT. In a study on pregnant rats exposed to an acute or a repeated stress (presence of a cat) either at the gestational day (GD) 10 or 14, the development of the vibrissae placing response, the righting reflex, and the negative geotaxis behavior was delayed, particularly with a repeated stressor, in the offspring of dams stressed at the GD 10 when offspring were assessed through the first two weeks of life. The delay in motor development was interpreted as an alteration in maturation of nervous structures due to prenatal stress69. Another study revealed that hindlimb unloading, a ground-based model widely used to plan the absence of weight support on hindlimbs, severely impaired motor activity and skilled walking in rats. Prenatal stress (restraint for 45 min three times daily from GD11 until delivery) exacerbated the effects of unloading, owing to immaturity of sensorimotor systems in response to environmental challenges during adulthood70. The authors concluded that a steady prenatal environment is necessary for normal development of the sensorimotor systems. In contrast, in a study on Swiss mice, Pallares et al.71 found that prenatal stress (restraint under bright light for 45 min, three times per day from the GD15 until birth), led to improvement in motor performance of the offspring in the BBT and this effect was sex and age dependent. The authors proposed that the prenatal stress had some type of adaptive or protective effect on motor functions. These two inconsistent studies used different durations of prenatal stress and used different species suggesting that further studies are needed to clarify effects of prenatal stress on motor development. We believe functional assessment of brain dynamics using optogenetic and neuroimaging tools72–75 combined with animal behaviour is ideally suited to monitor in real time the process by which prenatal stress changes the structure and function of different brain circuits.
Conclusion
This study compared the effects of prenatal physical stressors and “noise” stress on cognitive and motor performance as well as CORT levels. There was a higher level of CORT before starting behavioral assessments in the NS group compared to the other groups. Both stressed groups had increased CORT effects after behavioral testing. The results of behavioral assessments revealed signs of anxiety-like behavior and reduced learning and memory performance in the prenatal stress groups relative to the controls. Decreased motor coordination was only observed in the NS group. Overall, the results imply loud noise exposure during gestation negatively affects the HPA axis as well as cognitive and motor performance as large as or stronger than the physical stressors in the adult offspring.
Methods
Animals
All experiments were carried out in accordance with the Canadian Council of Animal Care and approved by the University of Lethbridge Animal Care Committee. All animals were given access to food and water ad libitum and were maintained on a 12:12-h light:dark cycle in a temperature-controlled breeding room (21 °C) with less than 66 ± 2 dBC room noise level. Thirty female C57BL/6 mice between 8 to 11 weeks of age were individually mated with thirty male C57BL/6 mice in standard shoe-box cages at 4:00 pm. For recording of gestational length, the female mice were assessed three hours later at 7:00 pm and the next morning for breeding signs such as sperm plug and red/swollen vaginal opening 16. If a plug or sperm was present, the female was considered possibly pregnant and removed from the male. Once a female was left with a male overnight, she was not paired with a male again until the lack of pregnancy was confirmed. The weight gain of the female mice was followed every day to confirm pregnancy. On the gestational day (GD) 11, a weight gain of at least 3.5 g usually signifies that conception has occurred. This method allows a determination of the length of gestation with a 0.5-day precision6. When the pups were born, the dams were kept individually with the litters. At the age of 21–23 days, the pups were weaned from their mothers, and only one male pup was randomly selected from each litter (n = 10 in each group).
Experimental design
Stress Procedure
Pregnant mice were randomly assigned to three groups consisting of two stress groups and one control group. We exposed animals to stress on GDs 12–16 because the corticogenesis process occurs from embryonic day 10 to 17 in mice, and the layer II/III, IV, and V mainly develop during GDs 12–1676, 77. This timeframe also corresponds to the second trimester in human pregnancy when substantial neural development occurs78.
Physical stress group
Two stressors, restraint and elevated platform (EP), were applied daily from GDs 12 through 1679. For restraint, mice (n = 10) were maintained in a transparent Plexiglas container (5 cm inner diameter), 20 minutes per day at 10:00 am. The container maintained the mice in a standing position without compression of the body80, 81. For the EP stressor, each mouse was placed on an elevated platform (1 m height, 21 × 21 cm), 30 minutes twice a day at 9:00 am and 3:00 pm47, 82.
Noise stress group
On GDs 12, 14, and 16, a female pregnant mouse was transferred into a standard cage and moved to a sound chamber. A speaker, which emitted an intermittent 3000 Hz frequency sound of 90 dB7, 83, 84 for 1 sec duration and 15 sec inter-stimulus interval (ISI)85, was placed in the cage. The sound pressure level was measured daily inside the cage without an animal (Tektronix RM3000, Digital Phosphor Oscilloscope). The mice (n = 10) were exposed to the NS for 24 hrs starting at 8:00 am6, 79. A similar protocol was previously used in rats, including a low-frequency sound of 300 Hz for 1 sec in the intervals of 15 sec during 24 hrs.85. Here we used a 3000 Hz frequency tone, since (a) is audible by mice86, 87 and (b) is relatively similar to environmental and traffic noises which are largely made up of low to mid frequency tones88, 89. The intensity of NS exposure used in previous studies was also between 95 to 130 dB7, 16, 83, 84. We applied an intermittent stimulus intensity (90 dB) to prevent noise-induced hearing loss. In addition, 24 hrs rest after every stress exposure provided enough time for to recover from possible temporary threshold shifts90.
Control group
There were two sets of control animals: one served as a control for NS dams and another was a control for PS dams. In NS control group, pregnant mice (n = 5) on GDs 12, 14, and 16 were individually transferred into a standard cage and moved to a sound chamber. A silent speaker was placed in the cage. The mice were left undisturbed for 24 hrs starting at 8:00 am. In PS control group, pregnant mice (n = 5) were removed daily from the home cage for 20 or 30 minutes (depending on the type of stressor) during GDs 12–16, transferred to the same testing room for the PS, left undisturbed, and then returned to their home cages. In the control group, no stress was given.
Plasma CORT assay
The blood sampling procedure was performed at 7:30 to 8:30 am91 one day before starting behavioral tests and a day after finishing all behavioral tests. Blood was taken from the submandibular vein92 for all animals by one of the authors (ZJ) in order to control for any possible effects of experience and/or inter-tester difference on the results. The submandibular bleeding of mice is a single-use lancet method to quickly draw blood without the use of the anesthesia92. No obvious difference in the response of the different animals to the procedure was observed. Approximately 0.1 ml of submandibular blood was collected in heparin-coated tubes. These tubes were centrifuged at 6000 rpm at 4 °C for 15 min to collect the plasma93. Collected plasma samples then were stored at −80 °C until further analysis. A commercially available ELISA kit was used to quantify the levels of CORT in the plasma. The assay was carried out as per the manufacturer’s instructions79. The optical density of CORT was read at 450 nm wavelength using a microplate reader (Synergy HT BioTek®). The concentration of CORT in samples was calculated using KC4 Bio-Tek® Microplate Data Collection and Analysis software. To reduce intra-plate variability, the coefficient of variation (CV) for all samples was determined using the same standards and controls across all plates, and only samples with a CV less than 10 percent were included in the analysis. The CORT concentration in plasma samples was expressed in ng/ml16, 91, 94–97.
Behavioral assessments
Several behavioral tests were done when pups reached 8 weeks old to measure the effect of prenatal stress on cognitive and motor performance of the offspring. Tests of NOR, EPM, BBT, and MWT, including a probe trial, were conducted respectively in separate days, only one test per day, with an alternating order of animals, by the same examiner in the mornings at 9–11am (Fig. 6).
Figure 6.
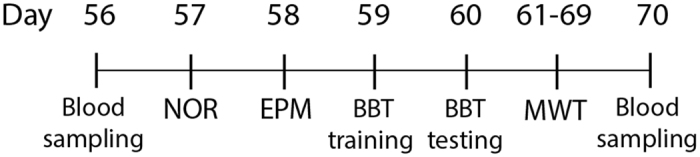
Time course of blood samplings and behavioral tests: The first blood sampling was performed when pups reached 8 weeks old (56 days). After doing a set of behavioral tests including NOR (novel object recognition), EPM (elevated plus maze), BBT (balance beam test), MWT (Morris water task), and probe test, the second blood sampling was conducted a day after the last behavioral test (70 days).
Novel object recognition (NOR) test
Each mouse was placed in an open-field arena (47 cm width × 50 cm length × 30 cm height) made of white Plexiglas. In the first trial, the mouse was placed in the arena with two identical objects and was allowed to explore the field for 5 min. The animal was removed and placed in a transport box for 3 min, and one of the objects was randomly replaced with a new object. The mouse was then returned to the arena, and the animal’s exploration was filmed (30 frames/second) for 3 minutes. The time spent with each object was only calculated during the second session. If the nose of the mouse was within 1 cm of the object, it was considered to be in contact with an object6, 82, 98. The ratio of time spent with the old compared to the new object was calculated by subtracting times spent with old from the new object divided by the total time spent for exploration99. In addition, behavioural measures including movement time (the time in seconds spent moving in the arena), movement number (the number of movements after the animal remained immobile for more than one second), total distance (the total length of paths traveled by animals, in centimeters), and rest time (the time in seconds spent immobile) during the second session were calculated to determine the locomotor behavior of the animals.
Elevated plus maze (EPM) test
The EPM has been constructed from black Plexiglas®, with a base measuring 48 cm high. The two open arms were 5 cm wide and 27 cm long. The two closed arms were 5 cm wide, 27 cm long, and had walls measuring 21 cm high. The maze was housed in an empty room and a dim light was used during filming. The camera for filming was placed at the end of an open arm slightly above the maze. Mice were placed with their front paws in the center of the square maze facing a closed arm. Each mouse was filmed for 5 min and scored for time spent in the open arms, time spent in the closed arms, number of entries to open arms, and number of entries into closed arms100–102. Animals were considered to be in an arm when the first half of the body was within the arm3, 103. The center zone connecting all arms was excluded6, 104.
Balance beam test (BBT)
The mice were required to traverse an elevated, narrow aluminium beam (1 cm diameter, 100 cm long and 50 cm above a foam pad to cushion falling mice) to reach an enclosed escape box. Mice were first trained (4–5 trials) and were tested (3 trials) on the next day. We calculated the mean latency (sec), distance travelled (cm), number of foot slips, number of turns, and number of falls across the 3 testing trials 6, 105.
Morris water task (MWT)
The water task consisted of a pool (153 cm diameter) filled with water (23–25 ± 1 °C) up to a level of ~15 cm from the top edge of the tank. The water was made opaque by non-toxic white tempura paint. The pool was located in a room rich with distal cues, which remained unobstructed throughout the duration of the experiment106. During all hidden platform trials, the platform was submerged ~1.0 cm under the surface of the water. The tank was divided into four quadrants, 1, 2, 3 and 4 using software, with starting points at north, west, east and south. The starting positions of the mice were located at the intersection of the quadrants, 4–5 cm away from the edge of the tank. Animals were trained with 4 trials per day for 8 consecutive days under regular room light (Water2100 Software vs.7, 2008). Each trial began with the mouse being placed in the pool in a pseudo-random sequence at one of the four cardinal compass positions around the perimeter of the pool. The acquisition trial was started by placing a mouse facing the wall of the tank at one of the 4 starting locations. Testing was stopped after the mouse reached the platform or, if the mouse did not find the platform, at the 60 second trial time limit. If a mouse found the platform within this 60 sec period, it was allowed to remain on the platform for five additional seconds. If it did not find the platform during the selected time, it was placed onto the platform for 15 sec by the experimenter before being returned to his home cage. Data were recorded using an automated tracking system (HVS Image Hampton, U.K.). Following each swim trial, the animal was dried with a soft fabric cloth and placed back into the home cage where it was allowed to rest for at least 5 min before the start of the next swim trial. The swim time (sec), swim speed (m/s), and swim distance (m) were calculated for analysis31, 79.
The probe trial was carried out on the ninth day, in which the platform was removed and each mouse was allowed to swim freely for 60 sec. In order to preclude the possible impact of working memory on retention 107 this trial was performed 24 hours after the last acquisition trial. The time spent in the quadrant where the platform had been located was measured.
Statistical analysis
All statistical analyses were performed using SPSS Statistics 24.0 at a significance level of 0.05 or better. Normally distributed data were analyzed using the Kolmogorov–Smirnov test. Multivariate analysis of variance (MANOVA) was conducted to compare the three groups in terms of different parameters of the behavioral tests. A two-way MANOVA was performed to compare the three groups in CORT levels one day before starting and one day after finishing all behavioral tests, and the delta CORT level (difference between the two CORT levels measured) as well. To determine the possible effect of swim speed on the swim time, a multivariate analysis of covariance (MANCOVA) was done to compare the three groups in swim time where swim speed was considered as a covariate. A repeated measures ANOVA was conducted to compare the eight-days of training in the MWT, as well as the CORT levels one day before starting and one day after finishing all behavioral tests in every group. The F values, p values, estimations of the effect size (partial η2), and observed power have been reported for the statistical analyses. For multiple comparisons of group means in each measurement, the Tukey post-hoc test was performed.
Acknowledgements
We would like to acknowledge Gerlinde A.S. Metz for using her lab equipment for the CORT assay. This work was supported by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery Grant #40352 (MHM), Campus Alberta for Innovation Program Chair, Alberta Alzheimer Research Program (MHM), Alzheimer Society of Canada (MHM) and a Canadian Institute for Advanced Research grant (BK). This study was part of a postdoctoral fellowship to ZJ in Canadian Center for Behavioural Neuroscience (CCBN) at the University of Lethbridge. ZJ would like to thank the Iran University of Medical Sciences (IUMS) sabbatical leave committee for their approval of her study leave.
Author Contributions
Z.J., B.E.K. and M.H.M. conceived and designed the method, and prepared and reviewed the manuscript, Z.J. performed experimental work and data analysis, Jogender Mehla assisted for the CORT assay, and M.H.M. and B.E.K. provided project leadership.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bryan E. Kolb, Email: kolb@uleth.ca
Majid H. Mohajerani, Email: mohajerani@uleth.ca
References
- 1.Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. 2014;43:137–162. doi: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Weinstock M. Prenatal stressors in rodents: Effects on behavior. Neurobiol Stress. 2017 doi: 10.1016/j.ynstr.2016.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muhammad A, et al. Training on motor and visual spatial learning tasks in early adulthood produces large changes in dendritic organization of prefrontal cortex and nucleus accumbens in rats given nicotine prenatally. Neuroscience. 2013;252:178–189. doi: 10.1016/j.neuroscience.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 4.Charil A, Laplante DP, Vaillancourt C, King S. Prenatal stress and brain development. Brain Res Rev. 2010;65:56–79. doi: 10.1016/j.brainresrev.2010.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Jafari Z, et al. The Adverse Effects of Auditory Stress on Mouse Uterus Receptivity and Behaviour. Sci Rep. 2017;7 doi: 10.1038/s41598-017-04943-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arck PC, Merali FS, Manuel J, Chaouat G, Clark DA. Stress-triggered abortion: inhibition of protective suppression and promotion of tumor necrosis factor-alpha (TNF-alpha) release as a mechanism triggering resorptions in mice. Am J Reprod Immunol. 1995;33:74–80. doi: 10.1111/j.1600-0897.1995.tb01141.x. [DOI] [PubMed] [Google Scholar]
- 8.Trump S, et al. Prenatal maternal stress and wheeze in children: novel insights into epigenetic regulation. Sci Rep. 2016;6 doi: 10.1038/srep28616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers RE. Maternal psychological stress and fetal asphyxia: a study in the monkey. Am J Obstet Gynecol. 1975;122:47–59. doi: 10.1016/0002-9378(75)90614-6. [DOI] [PubMed] [Google Scholar]
- 10.Murmu MS, et al. Changes of spine density and dendritic complexity in the prefrontal cortex in offspring of mothers exposed to stress during pregnancy. Eur J Neurosci. 2006;24:1477–1487. doi: 10.1111/j.1460-9568.2006.05024.x. [DOI] [PubMed] [Google Scholar]
- 11.Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Basner M, et al. Auditory and non-auditory effects of noise on health. Lancet. 2014;383:1325–1332. doi: 10.1016/S0140-6736(13)61613-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritschi, L. B. A., Kim, R., Schwela, D. H. & Kephalopoulos, S. Burden of disease from environmental noise. (World Health Organization 2011).
- 14.Jafari Z, Kolb BE, Mohajerani MH. Effect of acute stress on auditory processing: a systematic review of human studies. Rev Neurosci. 2017;28:1–13. doi: 10.1515/revneuro-2016-0043. [DOI] [PubMed] [Google Scholar]
- 15.Ohrstrom E, Svensson SA, Gidlof-Gunnarsson H. A. Effects of road traffic noise and the benefit of access to quietness. J Sound Vibrat. 2006;295:40–59. doi: 10.1016/j.jsv.2005.11.034. [DOI] [Google Scholar]
- 16.Barzegar M, Sajjadi FS, Talaei SA, Hamidi G. & Salami, M. Prenatal exposure to noise stress: anxiety, impaired spatial memory, and deteriorated hippocampal plasticity in postnatal life. Hippocampus. 2015;25:187–196. doi: 10.1002/hipo.22363. [DOI] [PubMed] [Google Scholar]
- 17.Handa RJ, Weiser MJ. Gonadal steroid hormones and the hypothalamo-pituitary-adrenal axis. Frontiers Neuroendocrinol. 2014;35:197–220. doi: 10.1016/j.yfrne.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maccari S, et al. Adoption reverses the long-term impairment in glucocorticoid feedback induced by prenatal stress. J Neurosci. 1995;15:110–116. doi: 10.1523/JNEUROSCI.15-01-00110.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinstock M, Matlina E, Maor GI, Rosen H, McEwen BS. Prenatal stress selectively alters the reactivity of the hypothalamic-pituitary adrenal system in the female rat. Brain Res. 1992;595:195–200. doi: 10.1016/0006-8993(92)91049-K. [DOI] [PubMed] [Google Scholar]
- 20.Ward HE, Johnson EA, Salm AK, Birkle DL. Effects of prenatal stress on defensive withdrawal behavior and corticotropin releasing factor systems in rat brain. Physiol Behav. 2000;70:359–366. doi: 10.1016/S0031-9384(00)00270-5. [DOI] [PubMed] [Google Scholar]
- 21.Koehl M, et al. Prenatal stress alters circadian activity of hypothalamo-pituitary-adrenal axis and hippocampal corticosteroid receptors in adult rats of both gender. J Neurobiol. 1999;40:302–315. doi: 10.1002/(SICI)1097-4695(19990905)40:3<302::AID-NEU3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 22.Takahashi LK, Kalin NH. Early developmental and temporal characteristics of stress-induced secretion of pituitary-adrenal hormones in prenatally stressed rat pups. Brain Res. 1991;558:75–78. doi: 10.1016/0006-8993(91)90715-8. [DOI] [PubMed] [Google Scholar]
- 23.Fride E, Dan Y, Feldon J, Halevy G, Weinstock M. Effects of prenatal stress on vulnerability to stress in prepubertal and adult rats. Physiol Behav. 1986;37:681–687. doi: 10.1016/0031-9384(86)90172-1. [DOI] [PubMed] [Google Scholar]
- 24.Seckl JR, Holmes MC. Mechanisms of disease: glucocorticoids, their placental metabolism and fetal ‘programming’ of adult pathophysiology. Nat Clin Pract Endocrinol Metab. 2007;3:479–488. doi: 10.1038/ncpendmet0515. [DOI] [PubMed] [Google Scholar]
- 25.Spencer RL, Deak T. A users guide to HPA axis research. Physiol Behav. 2016 doi: 10.1016/j.physbeh.2016.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Caceres, C. et al. Gender differences in the long-term effects of chronic prenatal stress on the HPA axis and hypothalamic structure in rats. Psychoneuroendocrinology35. [DOI] [PubMed]
- 27.Campos AC, Fogaca MV, Aguiar DC, Guimaraes FS. Animal models of anxiety disorders and stress. Rev Bras Psiquiatr. 2013;35(Suppl 2):S101–111. doi: 10.1590/1516-4446-2013-1139. [DOI] [PubMed] [Google Scholar]
- 28.Calabrese EJ. An assessment of anxiolytic drug screening tests: hormetic dose responses predominate. Crit Rev Toxicol. 2008;38:489–542. doi: 10.1080/10408440802014238. [DOI] [PubMed] [Google Scholar]
- 29.Jia N, et al. Alterations of Group I mGluRs and BDNF Associated with Behavioral Abnormity in Prenatally Stressed Offspring Rats. Neurochem Res. 2015;40:1074–1082. doi: 10.1007/s11064-015-1565-6. [DOI] [PubMed] [Google Scholar]
- 30.Xu J, et al. Effects of duration and timing of prenatal stress on hippocampal myelination and synaptophysin expression. Brain Res. 2013;1527:57–66. doi: 10.1016/j.brainres.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 31.Akatsu S, Ishikawa C, Takemura K, Ohtani A, Shiga T. Effects of prenatal stress and neonatal handling on anxiety, spatial learning and serotonergic system of male offspring mice. Neurosci Res. 2015;101:15–23. doi: 10.1016/j.neures.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 32.Zohar I, Shoham S, Weinstock M. Perinatal citalopram does not prevent the effect of prenatal stress on anxiety, depressive-like behaviour and serotonergic transmission in adult rat offspring. Eur J Neurosci. 2016;43:590–600. doi: 10.1111/ejn.13150. [DOI] [PubMed] [Google Scholar]
- 33.de Souza MA, et al. Prenatal stress produces social behavior deficits and alters the number of oxytocin and vasopressin neurons in adult rats. Neurochem Res. 2013;38:1479–1489. doi: 10.1007/s11064-013-1049-5. [DOI] [PubMed] [Google Scholar]
- 34.Miyagawa K, Tsuji M, Fujimori K, Saito Y, Takeda H. Prenatal stress induces anxiety-like behavior together with the disruption of central serotonin neurons in mice. Neurosci Res. 2011;70:111–117. doi: 10.1016/j.neures.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 35.Sun H, et al. Involvement of NR1, NR2A different expression in brain regions in anxiety-like behavior of prenatally stressed offspring. Behav Brain Res. 2013;257:1–7. doi: 10.1016/j.bbr.2013.08.044. [DOI] [PubMed] [Google Scholar]
- 36.Grayson B, et al. Assessment of disease-related cognitive impairments using the novel object recognition (NOR) task in rodents. Behav Brain Res. 2015;285:176–193. doi: 10.1016/j.bbr.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 37.Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behav Brain Res. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- 38.Zhao D, et al. Prenatal stress disturbs hippocampal KIF17 and NR2B in spatial cognition in male offspring. J Neurosci Res. 2013;91:535–544. doi: 10.1002/jnr.23172. [DOI] [PubMed] [Google Scholar]
- 39.Benoit JD, Rakic P, Frick KM. Prenatal stress induces spatial memory deficits and epigenetic changes in the hippocampus indicative of heterochromatin formation and reduced gene expression. Behav Brain Res. 2015;281:1–8. doi: 10.1016/j.bbr.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Avishai-Eliner S, Brunson KL, Sandman CA, Baram TZ. Stressed-out, or in (utero)? Trends Neurosci. 2002;25:518–524. doi: 10.1016/S0166-2236(02)02241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hayashi A, et al. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/S0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 42.Schmitz C, et al. Depression: reduced number of granule cells in the hippocampus of female, but not male, rats due to prenatal restraint stress. Mol Psychiatry. 2002;7:810–813. doi: 10.1038/sj.mp.4001118. [DOI] [PubMed] [Google Scholar]
- 43.Szuran T, Zimmermann E, Welzl H. Water maze performance and hippocampal weight of prenatally stressed rats. Behav Brain Res. 1994;65:153–155. doi: 10.1016/0166-4328(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 44.Lemaire V, Koehl M, Le Moal M, Abrous DN. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lemaire V, Lamarque S, Le Moal M, Piazza PV, Abrous DN. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Barros VG, Duhalde-Vega M, Caltana L, Brusco A, Antonelli MC. Astrocyte-neuron vulnerability to prenatal stress in the adult rat brain. J Neurosci Res. 2006;83:787–800. doi: 10.1002/jnr.20758. [DOI] [PubMed] [Google Scholar]
- 47.Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66:308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- 48.Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. Ilar j. 2014;55:310–332. doi: 10.1093/ilar/ilu013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vorhees CV, Williams MT. Value of water mazes for assessing spatial and egocentric learning and memory in rodent basic research and regulatory studies. Neurotoxicol Teratol. 2014;45:75–90. doi: 10.1016/j.ntt.2014.07.003. [DOI] [PubMed] [Google Scholar]
- 50.Maviel T, Durkin TP, Menzaghi F, Bontempi B. Sites of neocortical reorganization critical for remote spatial memory. Science (New York, N.Y.) 2004;305:96–99. doi: 10.1126/science.1098180. [DOI] [PubMed] [Google Scholar]
- 51.Manikandan S, et al. Effects of chronic noise stress on spatial memory of rats in relation to neuronal dendritic alteration and free radical-imbalance in hippocampus and medial prefrontal cortex. Neurosci Lett. 2006;399:17–22. doi: 10.1016/j.neulet.2006.01.037. [DOI] [PubMed] [Google Scholar]
- 52.Wu J, et al. Prenatal restraint stress impairs learning and memory and hippocampal PKCbeta1 expression and translocation in offspring rats. Brain Res. 2007;1141:205–213. doi: 10.1016/j.brainres.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 53.Negron-Oyarzo I, Neira D, Espinosa N, Fuentealba P, Aboitiz F. Prenatal Stress Produces Persistence of Remote Memory and Disrupts Functional Connectivity in the Hippocampal-Prefrontal Cortex Axis. Cereb Cortex. 2015;25:3132–3143. doi: 10.1093/cercor/bhu108. [DOI] [PubMed] [Google Scholar]
- 54.Ortega-Martinez S. Influences of prenatal and postnatal stress on adult hippocampal neurogenesis: the double neurogenic niche hypothesis. Behav Brain Res. 2015;281:309–317. doi: 10.1016/j.bbr.2014.12.036. [DOI] [PubMed] [Google Scholar]
- 55.Kraus KS, et al. Noise trauma impairs neurogenesis in the rat hippocampus. Neuroscience. 2010;167:1216–1226. doi: 10.1016/j.neuroscience.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Slattery DA, Cryan JF. Using the rat forced swim test to assess antidepressant-like activity in rodents. Nat Protoc. 2012;7:1009–1014. doi: 10.1038/nprot.2012.044. [DOI] [PubMed] [Google Scholar]
- 57.Reid KM, Taylor MG. Social support, stress, and maternal postpartum depression: A comparison of supportive relationships. Soc Sci Res. 2015;54:246–262. doi: 10.1016/j.ssresearch.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 58.Plamondon A, et al. Spatial working memory and attention skills are predicted by maternal stress during pregnancy. Early Hum Dev. 2015;91:23–29. doi: 10.1016/j.earlhumdev.2014.11.004. [DOI] [PubMed] [Google Scholar]
- 59.Pawluski JL, et al. Effects of stress early in gestation on hippocampal neurogenesis and glucocorticoid receptor density in pregnant rats. Neuroscience. 2015;290:379–388. doi: 10.1016/j.neuroscience.2015.01.048. [DOI] [PubMed] [Google Scholar]
- 60.Zhang Q, et al. BDNF promotes EGF-induced proliferation and migration of human fetal neural stem/progenitor cells via the PI3K/Akt pathway. Molecules. 2011;16:10146–10156. doi: 10.3390/molecules161210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012;64:238–258. doi: 10.1124/pr.111.005108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ratajczak P, et al. Biochemical and cognitive impairments observed in animal models of schizophrenia induced by prenatal stress paradigm or methylazoxymethanol acetate administration. Acta Neurobiol Exp (Wars) 2015;75:314–325. [PubMed] [Google Scholar]
- 63.Yeh CM, Huang CC, Hsu KS. Prenatal stress alters hippocampal synaptic plasticity in young rat offspring through preventing the proteolytic conversion of pro-brain-derived neurotrophic factor (BDNF) to mature BDNF. J Physiol. 2012;590:991–1010. doi: 10.1113/jphysiol.2011.222042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 65.Bramham CR, Bacher-Svendsen K, Sarvey JM. LTP in the lateral perforant path is beta-adrenergic receptor-dependent. Neuroreport. 1997;8:719–724. doi: 10.1097/00001756-199702100-00028. [DOI] [PubMed] [Google Scholar]
- 66.Grace T, Bulsara M, Robinson M, Hands B. The Impact of Maternal Gestational Stress on Motor Development in Late Childhood and Adolescence: A Longitudinal Study. Child Dev. 2016;87:211–220. doi: 10.1111/cdev.12449. [DOI] [PubMed] [Google Scholar]
- 67.van Batenburg-Eddes T, et al. Maternal symptoms of anxiety during pregnancy affect infant neuromotor development: the generation R study. Dev Neuropsychol. 2009;34:476–493. doi: 10.1080/87565640902964508. [DOI] [PubMed] [Google Scholar]
- 68.Brooks SP, Dunnett SB. Tests to assess motor phenotype in mice: a user’s guide. Nat Rev Neurosci. 2009;10:519–529. doi: 10.1038/nrn2652. [DOI] [PubMed] [Google Scholar]
- 69.Patin V, Vincent A, Lordi B, Caston J. Does prenatal stress affect the motoric development of rat pups? Brain Res Dev Brain Res. 2004;149:85–92. doi: 10.1016/j.devbrainres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- 70.Canu MH, Darnaudery M, Falempin M, Maccari S, Viltart O. Effect of hindlimb unloading on motor activity in adult rats: impact of prenatal stress. Behav Neurosci. 2007;121:177–185. doi: 10.1037/0735-7044.121.1.177. [DOI] [PubMed] [Google Scholar]
- 71.Pallares ME, Scacchi Bernasconi PA, Feleder C, Cutrera RA. Effects of prenatal stress on motor performance and anxiety behavior in Swiss mice. Physiol Behav. 2007;92:951–956. doi: 10.1016/j.physbeh.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 72.Mohajerani MH, et al. Spontaneous cortical activity alternates between motifs defined by regional axonal projections. Nature Neurosci. 2013;16:1426–1435. doi: 10.1038/nn.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mohajerani MH, Aminoltejari K, Murphy TH. Targeted mini-strokes produce changes in interhemispheric sensory signal processing that are indicative of disinhibition within minutes. Proc Natl Acad Sci USA. 2011;108:E183–191. doi: 10.1073/pnas.1101914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kyweriga M, Mohajerani MH. Optogenetic Approaches for Mesoscopic Brain Mapping. Methods Mol Biol. 2016;1408:251–265. doi: 10.1007/978-1-4939-3512-3_17. [DOI] [PubMed] [Google Scholar]
- 75.Lim DH, et al. In vivo Large-Scale Cortical Mapping Using Channelrhodopsin-2 Stimulation in Transgenic Mice Reveals Asymmetric and Reciprocal Relationships between Cortical Areas. Front Neural Circuits. 2012;6 doi: 10.3389/fncir.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kolb B, Mychasiuk R, Muhammad A, Gibb R. Brain plasticity in the developing brain. Prog Brain Res. 2013;207:35–64. doi: 10.1016/B978-0-444-63327-9.00005-9. [DOI] [PubMed] [Google Scholar]
- 77.Kolb B, et al. Experience and the developing prefrontal cortex. Proc Natl Acad Sci U S A. 2012;109(Suppl 2):17186–17193. doi: 10.1073/pnas.1121251109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Clancy B, Finlay BL, Darlington RB, Anand KJ. Extrapolating brain development from experimental species to humans. Neurotoxicology. 2007;28:931–937. doi: 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jafari Z, Mehla J, Afrashteh N, Kolb BE, Mohajerani MH. Corticosterone response to gestational stress and postpartum memory function in mice. PloS One. 2017;12 doi: 10.1371/journal.pone.0180306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ward ID, et al. Transgenerational programming of maternal behaviour by prenatal stress. BMC Pregnancy Childbirth. 2013;13(Suppl 1) doi: 10.1186/1471-2393-13-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Erickson ZT, Falkenberg EA, Metz GA. Lifespan psychomotor behaviour profiles of multigenerational prenatal stress and artificial food dye effects in rats. PloS one. 2014;9 doi: 10.1371/journal.pone.0092132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 83.Haque SF, et al. Anesthesia and acoustic stress-induced intra-uterine growth retardation in mice. J Reprod Dev. 2004;50:185–190. doi: 10.1262/jrd.50.185. [DOI] [PubMed] [Google Scholar]
- 84.Kondoh E, et al. Stress affects uterine receptivity through an ovarian-independent pathway. Hum Reprod. 2009;24:945–953. doi: 10.1093/humrep/den461. [DOI] [PubMed] [Google Scholar]
- 85.Mazurek B, et al. Stress induces transient auditory hypersensitivity in rats. Hear Res. 2010;259:55–63. doi: 10.1016/j.heares.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 86.Heffner HE, Heffner RS. Hearing ranges of laboratory animals. J Am Assoc Lab Anim Sci. 2007;46:20–22. [PubMed] [Google Scholar]
- 87.Heffner HE. Hearing in Glires: Domestic rabbit, cotton rat, feral house mouse, and kangaroo rat. J Acoust Soc Am. 1980;68:1584–1599. doi: 10.1121/1.385213. [DOI] [Google Scholar]
- 88.Chang TY, et al. Road traffic noise frequency and prevalent hypertension in Taichung, Taiwan: a cross-sectional study. Environ Health. 2014;13 doi: 10.1186/1476-069X-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Berglund B, Hassmen P, Job RF. Sources and effects of low-frequency noise. J Acoust Soc Am. 1996;99:2985–3002. doi: 10.1121/1.414863. [DOI] [PubMed] [Google Scholar]
- 90.White DR, Boettcher FA, Miles LR, Gratton MA. Effectiveness of intermittent and continuous acoustic stimulation in preventing noise-induced hearing and hair cell loss. J Acoust Soc Am. 1998;103:1566–1572. doi: 10.1121/1.421303. [DOI] [PubMed] [Google Scholar]
- 91.Barriga C, Martin MI, Tabla R, Ortega E, Rodriguez AB. Circadian rhythm of melatonin, corticosterone and phagocytosis: effect of stress. J Pineal Res. 2001;30:180–187. doi: 10.1034/j.1600-079X.2001.300307.x. [DOI] [PubMed] [Google Scholar]
- 92.Golde WT, Gollobin P, Rodriguez LL. A rapid, simple, and humane method for submandibular bleeding of mice using a lancet. Lab Anim (NY) 2005;34:39–43. doi: 10.1038/laban1005-39. [DOI] [PubMed] [Google Scholar]
- 93.Browne CA, et al. Effect of acute swim stress on plasma corticosterone and brain monoamine levels in bidirectionally selected DxH recombinant inbred mouse strains differing in fear recall and extinction. Stress (Amsterdam, Netherlands) 2014;17:471–483. doi: 10.3109/10253890.2014.954104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Malisch JL, et al. Baseline and stress-induced plasma corticosterone concentrations of mice selectively bred for high voluntary wheel running. Physiol Biochem Zool. 2007;80:146–156. doi: 10.1086/508828. [DOI] [PubMed] [Google Scholar]
- 95.Malisch JL, Breuner CW, Gomes FR, Chappell MA, Garland T., Jr. Circadian pattern of total and free corticosterone concentrations, corticosteroid-binding globulin, and physical activity in mice selectively bred for high voluntary wheel-running behavior. Gen Comp Endocrinol. 2008;156:210–217. doi: 10.1016/j.ygcen.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 96.Hare BD, Beierle JA, Toufexis DJ, Hammack SE, Falls WA. Exercise-associated changes in the corticosterone response to acute restraint stress: evidence for increased adrenal sensitivity and reduced corticosterone response duration. Neuropsychopharmacology. 2014;39:1262–1269. doi: 10.1038/npp.2013.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gong S, et al. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PloS one. 2015;10 doi: 10.1371/journal.pone.0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richards S, Mychasiuk R, Kolb B, Gibb R. Tactile stimulation during development alters behaviour and neuroanatomical organization of normal rats. Behav Brain Res. 2012;231:86–91. doi: 10.1016/j.bbr.2012.02.043. [DOI] [PubMed] [Google Scholar]
- 99.Hannesson DK, Howland JG, Phillips AG. Interaction between perirhinal and medial prefrontal cortex is required for temporal order but not recognition memory for objects in rats. J Neurosci. 2004;24:4596–4604. doi: 10.1523/JNEUROSCI.5517-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Muhammad A, Kolb B. Prenatal tactile stimulation attenuates drug-induced behavioral sensitization, modifies behavior, and alters brain architecture. Brain Res. 2011;1400:53–65. doi: 10.1016/j.brainres.2011.05.038. [DOI] [PubMed] [Google Scholar]
- 101.Solati J, Kleehaupt E, Kratz O, Moll GH, Golub Y. Inverse effects of lipopolysaccharides on anxiety in pregnant mice and their offspring. Physiol Behav. 2015;139:369–374. doi: 10.1016/j.physbeh.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 102.Kim MH, Leem YH. Chronic exercise improves repeated restraint stress-induced anxiety and depression through 5HT1A receptor and cAMP signaling in hippocampus. J Exerc Nutrition Biochem. 2014;18:97–104. doi: 10.5717/jenb.2014.18.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Raza S, Harker A, Richards S, Kolb B, Gibb R. Tactile stimulation improves neuroanatomical pathology but not behavior in rats prenatally exposed to valproic acid. Behav Brain Res. 2015;282:25–36. doi: 10.1016/j.bbr.2014.12.055. [DOI] [PubMed] [Google Scholar]
- 104.McBrayer ZL, et al. Forebrain-Specific Loss of BMPRII in Mice Reduces Anxiety and Increases Object Exploration. PloS one. 2015;10 doi: 10.1371/journal.pone.0139860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Stover KR, Campbell MA, Van Winssen CM, Brown RE. Analysis of motor function in 6-month-old male and female 3xTg-AD mice. Behav Brain Res. 2015;281:16–23. doi: 10.1016/j.bbr.2014.11.046. [DOI] [PubMed] [Google Scholar]
- 106.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Baldi E, Efoudebe M, Lorenzini CA, Bucherelli C. Spatial navigation in the Morris water maze: working and long lasting reference memories. Neurosci Lett. 2005;378:176–180. doi: 10.1016/j.neulet.2004.12.029. [DOI] [PubMed] [Google Scholar]