Abstract
In the present study, six commonly used promoters, including cytomegalovirus major immediate-early (CMV), the CMV enhancer fused to the chicken beta-actin promoter (CAG), human elongation factor-1α (HEF-1α), mouse cytomegalovirus (mouse CMV), Chinese hamster elongation factor-1α (CHEF-1α), and phosphoglycerate kinase (PGK), a CMV promoter mutant and a CAG enhancer, were evaluated to determine their effects on transgene expression and stability in transfected CHO cells. The promoters and enhancer were cloned or synthesized, and mutation at C-404 in the CMV promoter was generated; then all elements were transfected into CHO cells. Stably transfected CHO cells were identified via screening under the selection pressure of G418. Flow cytometry, qPCR, and qRT-PCR were used to explore eGFP expression levels, gene copy number, and mRNA expression levels, respectively. Furthermore, the erythropoietin (EPO) gene was used to test the selected strong promoter. Of the six promoters, the CHEF-1α promoter yielded the highest transgene expression levels, whereas the CMV promoter maintained transgene expression more stably during long-term culture of cells. We conclude that CHEF-1α promoter conferred higher level of EPO expression in CHO cells, but the CMV promoter with its high levels of stability performs best in this vector system.
Subject terms: Expression systems, DNA recombination
Introduction
Currently, recombinant proteins are used in the treatment of different diseases1–3, and these therapeutic proteins are produced in a large number of cell types4. Chinese hamster ovary (CHO) cells are the preferred producers because of their capacity to perform proper protein folding, assembly, and post-translational modifications, similar to human cells5, 6. However, problems of instability and recombinant protein expression silencing need to be resolved urgently4–7 because they limit the application of recombinant proteins8, 9. To resolve these problems, many elements have been investigated, including promoters, matrix attachment regions (MARs), introns, and other cis-acting elements10–13.
A promoter is the region upstream of a specific gene that initiates its transcription. Promoters show different activities because of their composition or other cis-acting elements in the expression vector14. In addition, the promoter also shows cell compatibility in the episomal vector driven by human cytomegalovirus major (CMV) immediate early gene promoter4. Mouse CMV promoter is suggested to be stronger than human CMV promoter15, 16. The CMV promoter is a commonly used promoter for the production of high level recombinant protein in mammalian cells17. However, the expression level of the transgene driven by CMV promoter decreases with extended culture times because of transcriptional silencing, which is associated with DNA methylation18, 19. To achieve stable and increased expression of the transgene, other strong promoters of mammalian origin, combined with cis-acting elements and synthetic promoters, have been investigated. Human eukaryotic translation elongation factor 1 alpha (HEF-1α, gene symbol EEF1A1) promoter is constitutively active in a broad range of cell types and often active in cells in which viral promoters fail to express downstream genes and in cells in which the viral promoters are gradually silenced20, 21. The CHEF1-α promoter is more active in CHO cells compared to CMV and SV40 promoters alone20, 22. Simian virus 40 (SV40) is also a strong promoter for the production of therapeutic proteins in mammalian cells, providing a lower expression yield, but higher stability than the EF1a and CMV promoters14. Mouse phosphoglycerate kinase 1 (PGK) promoter was also used to drive transgene expression in different cell lines23, 24. Besides natural promoters, hybrid promoters and synthetic promoters were also developed to drive higher and long-term expression of a transgene24, 25. CAG promoter is a hybrid construct consisting of the cytomegalovirus (CMV) enhancer fused to the chicken beta-actin promoter, which is also used to drive transgene expression in different cell lines26, 27. Brown et al. designed 140 synthetic promoters to specifically regulate the expression of recombinant genes in CHO cells, which offer precise control of recombinant transcriptional activity in CHO cells spanning over two orders of magnitude12. Schlabach et al. took a synthetic biology approach for the generation and screening of transcription factor binding sites for activity in human cells, and yielded compound enhancers that were capable of a twofold greater enhancement activity than the CMV enhancer, with higher levels of activity than the original synthetic enhancer across multiple cell lines28.
Although previous reports attempted to identify strong promoters for transgene expression, none of the ideal promoters can significantly increase and maintain stable transgene expression. In this study, we report a systematic comparison of six commonly used promoters (CMV, mouse CMV, CHEF-1α, PGK, CAG, and HEF-1α), a CMV mutant, and a CAG enhancer in transfected CHO cell system. Our findings will benefit those choosing promoters during vector design to generate transfected CHO cell lines with both high expression level and long-term expression stability.
Results
Transfection efficiency and transient transgene expression
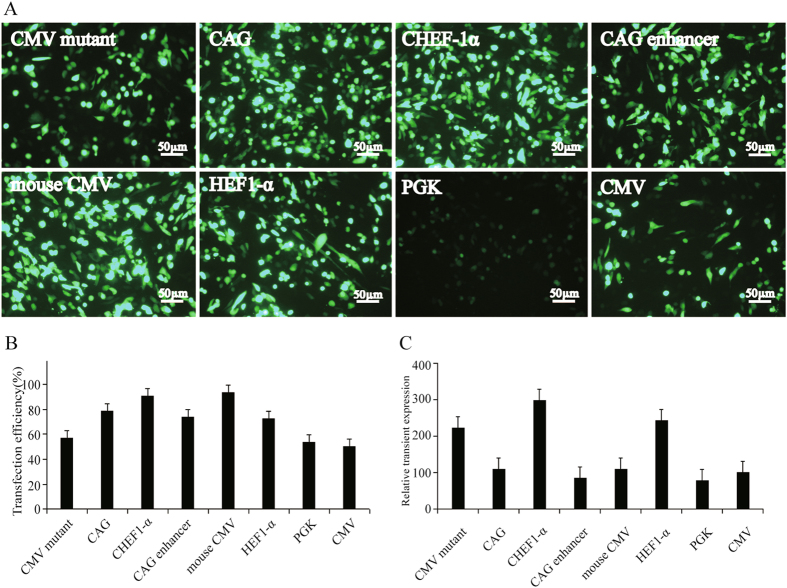
Transient gene expression is especially useful in early research when many potential therapeutic candidates are needed for evaluation or when a molecule is needed at short notice29, 30. At 48 h post-transfection, the fluorescence intensity of CHO cells transfected with different types of promoter was observed under a fluorescence microscope (Fig. 1A). CAG, HEF-1α, mouse CMV, and CHEF-1α showed enhanced transgene expression when compared with CMV. The transfection efficiency of CAG, HEF-1α, mouse CMV, and CHEF-1α was better than that of the CMV mutant and the CAG enhancer. We also observed that the mouse CMV presented a significantly higher transient efficiency (about 93.5%) than the control vector (about 57.6%), followed by CHEF1-α (90.7%), CAG (78.8%), and CAG enhancer (74.3%) (Fig. 1B). DNA length influences the transfection efficiency, but the PGK promoter was the sixth shortest in length, shorter than CAG, HEF1-α, CHEF-1α, the CMV mutant, and CMV, and showed the lowest activity. This demonstrated that the length of the promoter is not a major factor affecting transfection efficiency.
Figure 1.
Effect of different promoter on transfection efficiency and transient transgene expression. The pIRES-mediated vectors containing CMV, CAG, CHEF-1α, CMV mutant, HEF1-α, mouse CMV, CAG and PGK were transfected into CHO cells, and CHO cells were cultured in absence of G418 selection pressure for 48 h. (A) The eGFP of cells fluorescence profile was observed under fluorescence microscope; (B) The transfection efficiency were obtained using eGFP antibody analysis. (C) eGFP proteins transient expression levels. CMV promoter was regarded as 100, the MFI of other promoter were calculated. CMV, Cytomegalovirus major immediate-early; CAG, the CMV enhancer fused to the chicken beta-actin promoter; CHEF-1α, Chinese hamster elongation factor-1α; mouse CMV, mouse cytomegalovirus; HEF-1α, human elongation factor-1α; PGK, phosphoglycerate kinase; and CMV protein mutant, CAG enhancer. EPO, erythropoietin; SpA, simian virus 40 early polyadenylation signal; eGFP, enhanced green fluorescence protein.
Compared with the CMV promoter, the enhancement was the highest for CHEF1-α, which improved transgenic eGFP expression by 2.9-fold, followed by HEF1-α (2.4-fold). However, mouse CMV and CAG resulted in a slight increase in transient transgene expression (Fig. 1C).
Recombinant protein expression of stably transfected cells
Transient expression cannot fully reveal the function of different promoters, and it is important that vectors can be stably expressed in cells. When stably transfected cell lines were screened out, the eGFP protein levels (MFI) were measured by using flow cytometry (Fig. 2A). The cells transfected with CHEF-1α promoter-containing vectors exhibited the highest expression levels, followed by those containing HEF-1α, CMV mutant, CMV, mouse CMV, CAG, CAG enhancer, and PGK. When the eGFP expression level under the CMV promoter was considered as 100, the expression levels under the CHEF-1α, HEF-1α, CMV mutant, mouse CMV, CAG, CAG enhancer, and PGK promoters were 218.13, 184.21, 164.33, 49.12, 47.95, 26.31, and 8.77, respectively (Fig. 2B). Therefore, expression under the HEF-1α, CHEF-1α, CMV mutant, and CMV promoters was higher than that under the mouse CMV, CAG, CAG enhancer, and PGK promoter. The highest activity was exhibited by the CHEF-1α promoter, with an MFI 2.18-fold that of the CMV promoter, 24.86-fold that of the PGK promoter, and 8.29-fold that of the CAG enhancer.
Figure 2.
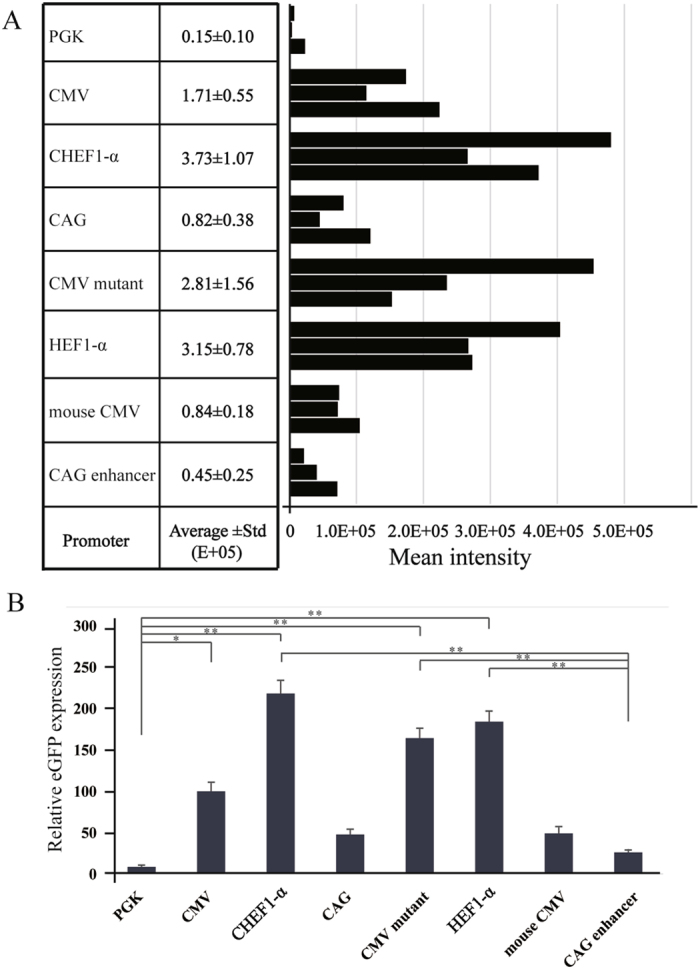
Cells were collected at 10 generation post-transfection and the eGFP MFI was measured by flow cytometry. (A) The stably transfected cells were screened in medium containing G418 (800 μg/mL). The eGFP MFI of stably transfected cell lines containing different promoters were detected. Black bar represent the results from after 10 generations analyzed by flow cytometry. (B) Fold statistical analysis results of expression level, and the eGFP MFI was normalized to CMV promoter. Three stably transfected pools were generated for each vector. Cells were collected and measured for the eGFP MFI with the FACS Calibur (*P < 0.05, **P < 0.01).
Recombinant mRNA expression
The mRNA expression level is closely related to that of recombinant protein31. We measured the mRNA expression levels in cells transfected with CMV, CAG, HEF-1α, and CHEF-1α promoters by qRT-PCR using eGFP as the target gene and GAPDH as the internal control. We found differences in recombinant mRNA expression levels among the cells transfected with CMV, CAG, HEF-1α, and CHEF-1α promoters; the highest level was found in CHEF-1α promoter-containing cells, followed by those containing the CMV, HEF-1α, and CAG promoters (Fig. 3). The mRNA expression level was consistent with the protein expression level, but the increasing fold was not directly proportional, suggesting that a promoter can increase transgene expression not only at the mRNA level, but also by affecting post-transcriptional regulation.
Figure 3.
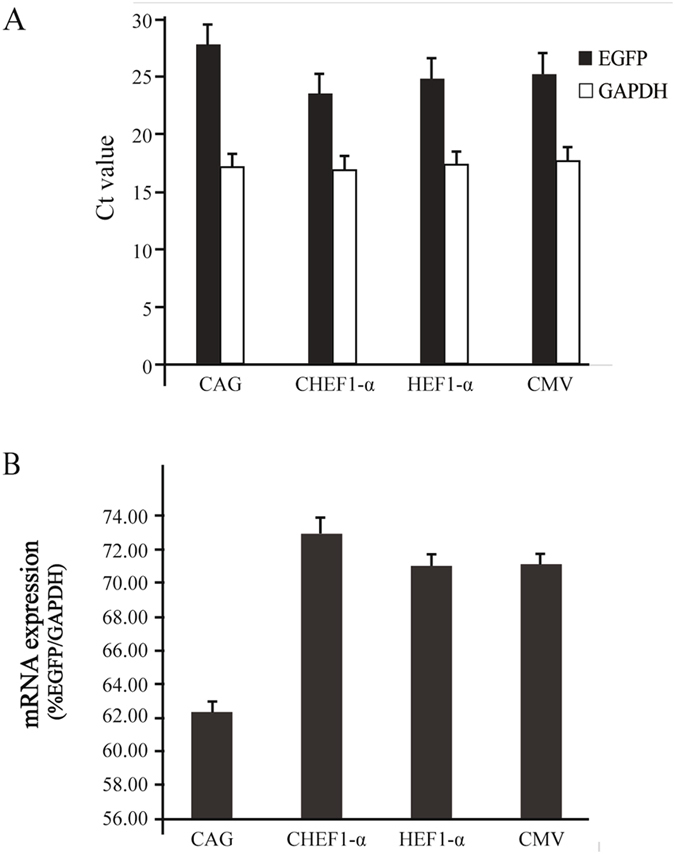
Recombinant expression at mRNA level in cells transfected with CMV, CAG, HEF-1α, and CHEF-1α promoters at 10 generations post-transfection. (A) Target gene (eGFP) and internal reference gene (GAPDH) were measured by qRT-PCR. (B) The mRNA expression levels of cells were calculated using percentage of eGFP/GAPDH qRT-PCR values. qRT-PCR results were obtained three independent measurements.
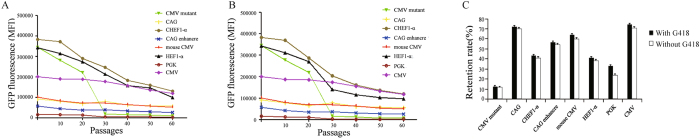
Analysis of the stability of recombinant protein expression. After un-transfected cells were killed, the stably transfected cells were screened out and divided into two groups: cultured in medium with G418 (500 μg/mL) to maintain the selection pressure, or without G418. MFI was detected to evaluate the intensity of the expressed eGFP until generation 60 post-transfection (Fig. 4A,B), and the relative retention of cells transfected with CMV, CMV mutant, CAG, HEF-1α, CAG enhancer, mouse CMV, PGK, and CHEF-1α vectors was calculated (Fig. 4C). Cells transfected with the vector containing the CMV promoter showed the most stable expression levels, the relative retention was 74.53% (with G418) and 70.59% (without G418) of the original expression level at 60 passages post-transfection, followed by CHEF1-α, 43.49% (with G418) and 40.97% (without G418), mouse CMV, 63.75% (with G418) and 59.78% (without G418), and CAG enhancer 56.58% (with G418) and 54.02% (without G418). Cells containing the CMV mutant, CHEF-1α, HEF-1α, and PGK promoters were unstable, with only 12.31% (with G418) and 11.37% (without G418), 43.49% (with G418) and 40.97% (without G418), 40.83% (with G418) and 38.10% (without G418) retention, and 32.85% (without G418), 23.84% (with G418), respectively. The transgene expression levels also differed and decreased gradually in stably transfected CHO cells.
Figure 4.
The stability of eGFP expression in transfected CHO cells grown in the presence G418 selection pressure or absence of G418. (A) The stably transfected cells were passaged until 60 generation in the presence of G418 selection pressure (B) or in the absence of G418 selection pressure. The intensity of eGFP of cells were detected by flow cytometry at the passage 0, 10, 20, 30,40, 50, 60, respectively. The experiments were performed in triplicates. (C) The eGFP expression retention was calculated as the ratio of the MFI at the 60 generations stability testing to the MFI at the transient expression MFI testing. Retention of eGFP expression levels in cells transfected with CMV, CAG, CHEF-1α, CMV mutant, HEF1-α, mouse CMV, and CAG enhancer promoter-containing vectors (n = 3).
Gene copy number analysis
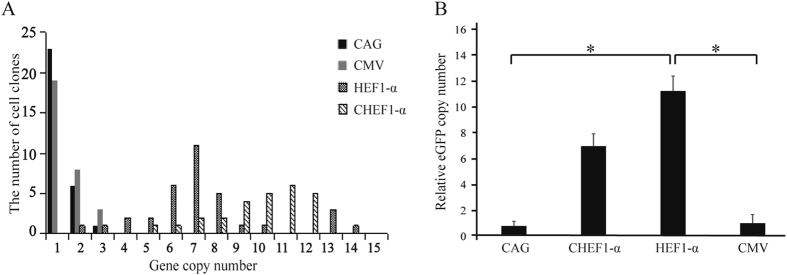
To further study the mechanism of the promoters, 30 single cell clones transfected with different vectors, HEF-1α, CHEF-1α, CMV, and CAG, were selected and the gene copy number was further analyzed by using qPCR. Some differences in the number of plasmids per genome were detected among the cells, indicating that the ratios of transgene copy number per genome were disparate for all plasmids in this experiment. The copy numbers are presented in Fig. 5A. The relative mean gene copy numbers were 0.73 ± 0.09 (CAG), 6.89 ± 1.43 (CHEF-1α), 10.98 ± 0.94 (HEF-1α), 1 ± 0.17 (CMV) (Fig. 5B). This finding indicated that transgene expression level was not related with gene copy number, suggesting that the promoter enhancing activity did not involve an increase in gene copy numbers.
Figure 5.
Gene copies per genome as determined by qPCR analysis. The gene copy number of CHO cells that stably transfected with CMV, CAG, CHEF-1α, HEF1-α element-containing vector was detected using qPCR at 10 generations post-transfection. (A) Thirty single stably clones was picked out and the gene copy number were analyzed by qPCR to detected the relationship between gene copy number. (B) The cells transfected with CMV promoter-containing vector was considered as 1.0. The relative copy number of the CAG, CHEF-1α, HEF1-α gene was calculated (*P < 0.05).
EPO expression levels
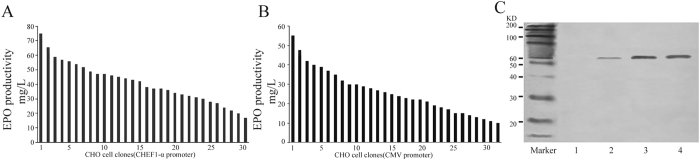
To evaluate the effect of promoter on the secreted protein expression, we selected a therapeutic protein, erythropoietin (EPO), and analyzed its expression in CHO cells under serum-free medium culture conditions. We transfected the EPO-expressing vectors driven by the CHEF1-α or CMV promoter into CHO cells and analyzed the expression levels of EPO by using ELISA and Western blot. The mean EPO expression level of cells transfected with the CHEF1-α driven vector yielded 49.07 ± 2.4 mg/L (Fig. 6A), and those transfected with the CMV driven vector yielded 23.56 ± 0.9 mg/L (Fig. 6B). The results showed that EPO expression driven by the CHEF1-α promoter was about 2.08-fold higher than that driven by the CMV promoter. The highest production of EPO in the single cell clone driven by CHEF1-α and CMV was 75.3 mg/L, 54.9 mg/L, respectively. In addition, Western blot results were further demonstrated that EPO expression driven by the CHEF1-α promoter was higher than that of CMV promoter (Fig. 6C).
Figure 6.
Analysis of EPO protein. The vectors containing EPO were transfected into CHO cells, and the stably transfected cells were screened under G418 selective pressure and thirty single cell clones were picked out. Cells supernatant were collected for analysis by ELISA to determine volumetric EPO production (mg/L). (A) CHEF-1α-EPO-containinng vector; (B) CMV-EPO-containing vector; (C) Western blot analysis. Lane 1, Un-transfected cells; Lane 2, CMV promoter; 3, CHEF1-α promoter, 4, Positive control.
Discussion
An ideal vector should ensure persistent transgene expression without epigenetic effects32. The landscape of epigenetic silencing is complex, and many factors could influence transgene silencing33, 34. The promoter is a major element in the expression cassette of gene therapy vectors, and optimal promoter selection can increase target specificity and gene expression14. Several reports investigated the effect of promoters on transgene expression35, 36. At present, CMV is the most commonly used promoter for the production of recombinant protein37. However, the CMV promoter cannot maintain production stability over time and it has many potential methylation sites; mutations and methylation will lead to lower productivity of recombinant proteins38. Nonetheless, we demonstrated that the CMV promoter is more stable than the mouse CMV, CHEF-1α, PGK, CAG, HEF-1α, and CMV mutant promoters, and the CAG enhancer. Previous studies revealed that EF-1α can enhance transgene expression in a lentiviral vector-mediated system and produce high-level, stable transgene expression39. We also found that CHEF-1α can significantly improve exogenous gene expression, but with reduced stability in CHO cells. One report demonstrated that the CAG promoter could enhance exogenous gene expression when it was cloned into the pCAGGS-GFP vector and transfected into Balb/c mice40. However, we found that the CAG promoter cannot enhance transgene expression in long-term culture. This phenomenon may result from methylation of the CAG promoter41. Mammalian DNA is frequently methylated at cytosine bases that are part of CpG dinucleotides42. In this study, the CMV mutant was unable to improve exogenous gene expression when the cytosine at position 404 was point-mutated to guanine; hence, we speculate that C-404-G point mutations may inhibit transgene expression.
In the present study, the relative activities of six commonly used promoters (CMV, CAG, HEF-1α, mouse CMV, CHEF-1α, and PGK), the CAG enhancer, and a CMV mutant were compared to explore the effects on transgene expression in CHO cells. During the long-term passage of cells, non-viral-mediated vectors that contain large DNA fragments can cause deficient transgene expression. Among the six promoters, CMV mutant, and CAG enhancer tested here, the CAG enhancer was the shortest in length, followed by the mouse CMV and PGK promoters. The highest positive eGFP gene expression rate was achieved with the HEF1-α promoter-containing vector, indicating that transgene expression is not only affected by the length of the fragment, but also by multiple factors, including cell growth conditions, cell type, and cell state. Our results show that, among the six promoters, CAG enhancer and CMV mutant, CMV and CHEF-1α, are better promoters than the CMV mutant, CAG, CAG enhancer, mouse CMV, HEF-1α, and PGK in mammalian cells for long-term cell culture. Results obtained by flow cytometry and qPCR experiments revealed that the vector containing HEF-1α was integrated into the host chromosome with the highest copy number, followed by CHEF-1α, CMV, and CAG. These results demonstrated that there was no direct relationship between exogenous gene expression levels and gene copy number. qRT-PCR was used to analyze the expression levels of mRNA and showed that the cells transfected with CHEF-1α had the highest expression levels, followed by HEF-1α, CMV, and CAG. These results are consistent with the results of eGFP expression.
To further investigate the effect of strong promoter on the expression of a gene of interest, we evaluated the expression of a therapeutic secreted protein, erythropoietin (EPO), driven by the CHEF-1α and CMV promoters under serum-free medium culture conditions. The results showed that EPO expression level in CHO cells transfected with the vector containing CHEF-1α was significantly higher than of that of cells transfected with the vector containing the CMV promoter, which was consistent with eGFP gene expression.
Many studies investigated synthetic promoters43. Human CMV and HEF-1α constructs increased transgene expression by up to ten-fold44. However, human CMV usually peaks 1–2 days after transfection and the activity is rapidly lost43. Considering the effects of CMV on the relative maintenance of transgenes and the activity of CHEF-1α on transgene expression, we can use a CMV core and CHEF-1α to construct a synthetic promoter. The synthetic promoter would shorten the vector length and potentially contribute to the improvement of transfection efficiency.
In conclusion, we found that CHEF-1α showed high transgene expression activity and CMV derived from an IRES-mediated vector presented the highest retention rate. However, this experiment was performed only in CHO cells and the results cannot be extrapolated to other cell lines. The development of such expression systems is a major strategic task continually required for the expression of target proteins for research and is necessary for any future long-term clinical application. Our study makes a significant contribution to such research and applications.
Materials and Methods
Vector construction
The vector containing the CMV promoter was obtained by cloning the enhanced green fluorescent protein (eGFP) from pEGFP-C1 (Clontech, Mountain View, USA) into the pIRES-neo vector (Clontech, Mountain View, USA). Vectors containing the CMV mutant, CAG enhancer (GenBank no: AJ575208.1, position 100-386), CAG (GenBank no: GU299216.1, position 3-1664), HEF-1α (GenBank no: AY188393.1, position 10964-12623), mouse CMV (GenBank no: KT343252.1, position 1103-1625), CHEF-1α (GenBank no: KY447299.1, position 12-1346), and PGK (GenBank no: KJ175229.1, position 641-1195) were constructed by using the vector containing the CMV promoter, which acted as the original vector based on previously described IRES-mediated vectors45 (Fig. 7A, supplement 1). The CMV mutant, CAG enhancer, and CAG, CHEF-1α, mouse CMV, HEF1-α, PGK, and CMV promoters were 589 bp, 287 bp, 1662 bp, 1335 bp, 523 bp, 1659 bp, 555 bp, and 589 bp in length, respectively (Fig. 7B); eGFP was inserted into the vectors as a reporter gene. The sequences of elements were synthesized by General Biosystems (Chuzhou, China). Meanwhile, to detect the effect of promoter on secreted protein, we selected two promoters, CMV and CHEF1-α, to construct vectors containing the EPO cDNA (Fig. 7A). The EPO gene was synthesized according to the codon optimization sequence and further cloned into the vector using standard methods46.
Figure 7.
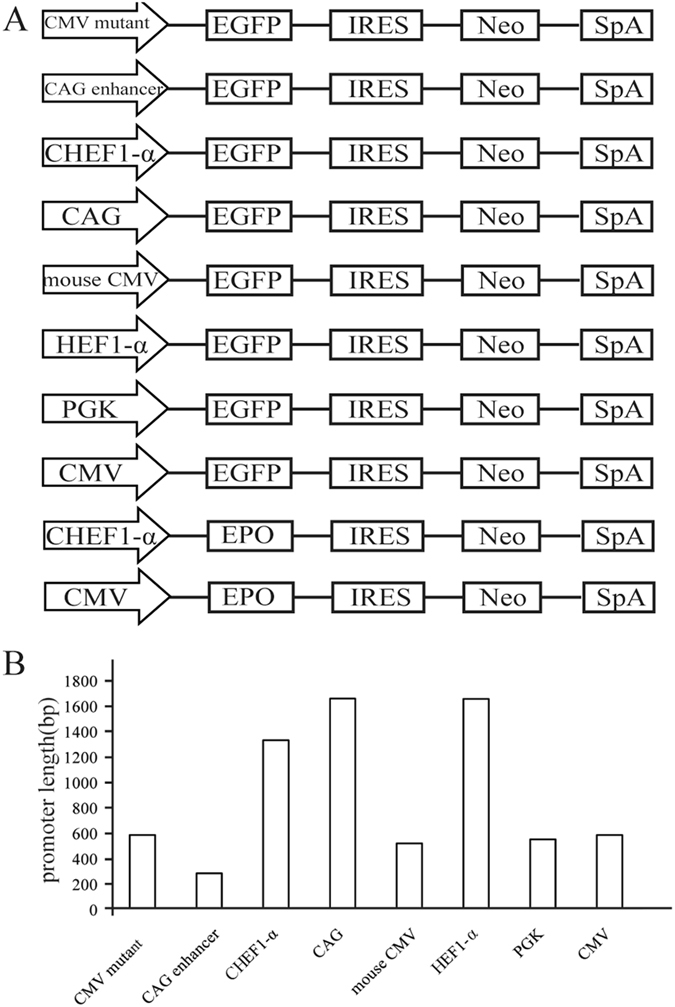
Schematic representation of vectors containing different promters and the length of different elements. (A) The vectors construction that containing different element. (B) The lengths of the six different promoters, and CMV mutant, CAG enhancer.
Cell culture and transfection
CHO-S cells (Life Technologies # A11557-01) were maintained in Dulbecco’s modified Eagle’s medium + F12 (Gibco, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum (Gibco, arlsbad, CA, USA), 1% penicillin and streptomycin (Beyotime, Shanghai, China). The cells were cultured in a humidified atmosphere at 37 °C and 5% CO2. The cells were plated at a concentration of 2 × 105 cells/well in 24-well plates and allowed to attach overnight. On the second day, after reaching 80% confluence, the cells in each well were transfected with the vectors containing CMV, CMV mutant, CAG enhancer, CAG, CHEF-1α, mouse CMV, HEF-1α, or PGK using 1 μL Lipofectamine 3000 Transfection Reagent (Invitrogen, Carlsbad, CA, USA) per 1 μg vector according to the manufacturer’s instructions. At 48 h post-transfection, G418 (800 μg/mL) was used to kill the un-transfected cell lines. Meanwhile, cells transfected with the different vectors were digested and reseeded at a concentration of 5 × 104 cells/well in 12-well plates. Each cell type was plated into three wells.
Transient expression
At 48 h post-transfection, transfection efficiency and transient eGFP expression levels were analyzed by assessing the fluorescence intensity of transfected cells using fluorescence microscopy (Nikon ECLIPSE Ti, Nikon, Japan). In order to more accurately analyze the results, the cells were assessed at 200 × magnification with an emission wavelength of 530 nm using a 530/15 bandpass filter for green fluorescence. Briefly, cells were harvested after digestion with trypsin at 48 h post transfection. The MFI and transfection efficiency were detected. Cells were resuspended in 100 µL of mouse anti-GFP antibody (ZSGB-Bio, Beijing, China) and analyzed by using a flow cytometer. The numbers of eGFP-positive and eGFP-negative cells were calculated according to the flow cytometry results, and the transfection efficiency was represented by the ratio of the number of eGFP-positive cells to the total cell number.
Stable expression of transfected cells and flow cytometry analysis
Long-term stable expression of the target gene is required for industrial production of recombinant proteins. Thus, cells transfected with plasmid vectors were passaged in pools and further cultured under G418 (800 μg/mL) selection for 16 days. When the non-transfected cells were dead, the stably transfected cell colonies were screened out and the concentration of G418 was reduced to 500 μg/mL to maintain the selection pressure. When cell colonies reached 90% confluence, the cells were harvested by using 0.25% trypsin/EDTA. Analyses were performed with a Guava EasyCyte™ 8HT flow cytometer (MilliporeSigma, Darmstadt, Germany) using FlowJo software 7.6 (Tree Star, Ashland, OR, USA). This software provides values for the media fluorescence intensity (MFI) based on the fluorochromes in each cell captured by flow cytometry. Therefore, the eGFP MFI of each sample can be used as a reporter of transgene expression level. For EPO gene expression, the cells transfected with EPO gene were screened under G418 (800 μg/mL) selection for about 15 days and the stably transfected cells appeared. When cell colonies reached 90% confluence, the cells were harvested and then were seeded into 96-well plate with a limited dilution method to produce the one cell/well. The cell colonies took up about 15% of the surface of the well when cells grew about one week, and further transferred into 24- well, 6-well, gradualy. When the total cell number achieved 1 × 107, the cells were cultured in protein-free, serum-free, chemically-defined CD CHO medium (Life Technologies # 10743-029) supplemented with 8 mM L-glutamine (Life Technologies # 25030-024) in 125 mL Corning shake flasks (Sigma # 431255) with 20 mL medium for about 5 days and the supernatant were collected for EPO expression level analysis.
Analysis of long-term transgene expression stability
CHO cells stably transfected with the vectors containing human CMV, CMV mutant, CAG, CHEF-1α, CAG enhancer, mouse CMV, and HEF-1α were passaged in pools and further cultured. The MFI for each vector type was measured by using the Guava EasyCyte™ 8HT flow cytometer, and the relative retention of eGFP expression for each vector was calculated as the ratio of the MFI after generation 60 to that at the start of stability testing. Each ten passages, the expression level of eGFP was determined by fluorescence intensity.
qPCR analysis of gene copy number
Genomic DNA was isolated from 4 × 106 transfected CHO-S cells using a genomic DNA Mini Preparation kit with spin column (Beyotime). The gene copy numbers were determined through qPCR technique using the eGFP and the GAPDH reference gene. Oligonucleotide primer sequences are provided in Table 1. Copy numbers were determined in triplicate and are presented as ratios of individual copy numbers relative to the control. Double-stranded DNA fragments were prepared from the oligonucleotides by polymerization: a 10 μL reaction mix consisting of 4 μL template DNA (0.05 μg/μL), 5 μL SYBR Green (TAKARA, Dalian, China), 0.2 μL of each forward and reverse oligonucleotide (10 μM each), and 0.6 μL deionized water was subjected to heating at 95 °C for 3 min, 30 cycles of 94 °C for 30 s, 50 °C for 30 s, and elongation for 30 s at 72 °C, then 60 °C for 30 min for data acquisition.
Table 1.
Primer used in the analysis of gene copy number and mRNA expression levels.
| Primer designation | Type of analysis | Primer sequence (5′-3′) |
|---|---|---|
| EGFP-Fwd | qRT-PCR/qPCR | 5′-CTACGTCCAGGAGCGCACCATCT-3′ |
| EGFP-Rev | qRT-PCR/qPCR | 5′-GTTCTTCTGCTTGTCGGCCATGATAT-3′ |
| GAPDH-Fwd | qRT-PCR/qPCR | 5′-CGACCCCTTCATTGACCTC-3′ |
| GAPDH-Rev | qRT-PCR/qPCR | 5′-CTCCACGACATACTCAGCACC-3′ |
Recombinant mRNA expression analysis
After approximately 10 passages, the expression levels of recombinant mRNA were analyzed. We isolated total RNA from cells transfected with each plasmid, and reverse transcription reaction was performed with a HiFiScript first strand cDNA synthesis kit (CWBIO, Beijing, China) according to the manufacturer’s protocol. qRT-PCR was undertaken using a PikoReal™ Software 2.2 Real-Time PCR System (Thermo Scientific, Waltham, MA, USA). cDNA template (40 ng/reaction) was quantified by using a Multiscan Spectrum spectrophotometer (SpectraMax i3x, Silicon Valley, CA, USA). PCR reactions were performed according to standard procedures using primer sets designed for the eGFP and GAPDH sequences (Table 1). The mRNA expression level of the target gene was calculated by comparison with that of the internal reference gene.
ELISA analysis
Cells transfected vectors were screened using G418 (800 μg/mL) after 48 h post-tranfection. Colonies arose after 10–14 days. Briefly, like the method demonstrated in stable expression of transfected cells and flow cytometry analysis. 30 stable clones were picked out and transferred to 24-well plates. Volumetric EPO production (mg/L) for initial CHO-EPO cell lines cloned were scaled up to 125 mL Corning shake flasks. Cells were grown in protein-free, serum-free, chemically-defined CD CHO medium supplemented with 8 mM L-glutamine in 125 mL Corning shake flasks with 30 mL medium; at 60% density compared with 1.5 × 107. Cells supernatant were collected for analysis by ELISA to determine volumetric EPO production as previously described47.
Western blot analysis
The cells transfected with EPO-containing vector were suspended. When the cell number reached 8 × 106/mL, the supernatant was collected and EPO was detected by immunoblotting. The supernatant containing EPO mixed with 5× SDS sample buffer was boiled. Ten microliters of sample were subjected to electrophoresis on a 15% SDS–polyacrylamide gel and transferred to a nitrocellulose membrane by electro-blotting. A 1:1500 dilution of an anti-EPO (EPO resistance protein) rabbit antiserum (Baoankang Biotechnology Co., Ltd., Shenzhen, China) was incubated with the membrane followed by a secondary incubation with a 1:2000 dilution of goat anti-rabbit antibody conjugated to alkaline phosphatase (Jackson Immuno Research Lab, West Grove, PA, USA). Densitometric analysis was performed by using ImageJ v2.1.4.7 software (National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
All experimental data were analyzed by using SPSS 18.0 software (SPSS Inc., Chicago, IL, USA). Data are reported as mean ± standard deviation. All experiments were performed three times and t-tests were used for comparisons. Differences with P values <0.05 were considered statistically significant.
Electronic supplementary material
Acknowledgements
This work was supported by the Grants from the National Natural Science Foundation of China (No. 81673337), Plan for Scientific Innovation Talent of Henan Province, China (No. 164200510003)and the Key Scientific Research Projects in Universities of Henan Province (No. 18A350008).
Author Contributions
Tian-Yun Wang designed, analyzed the experiments and revised the manuscript. Wen Wang and Yan-long Jia performed the experiments and wrote the manuscript. Yi-chun Li and Chang-qin Jing performed experiments for stable expression of transfected cells. Xue-fang Shang performed experiments for Figure 5, interpreted these data and edited the manuscript. Xiao Guo and Chun-peng Zhao performed the experiment for vector construction and cultured cells.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Wen Wang and Yan-long Jia contributed equally to this work.
Change history
9/4/2018
This paper has been retracted at the request of the authors
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-10966-y
References
- 1.Hoban MD, Orkin SH, Bauer DE. Genetic treatment of a molecular disorder: gene therapy approaches to sickle cell disease. Blood. 2016;18:839–848. doi: 10.1182/blood-2015-09-618587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garcia-Gomez M, et al. Safe and Efficient Gene Therapy for Pyruvate Kinase Deficiency. Mol. Ther. 2016;24:1187–1198. doi: 10.1038/mt.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Flynn R, et al. CRISPR-mediated genotypic and phenotypic correction of a chronic granulomatous disease mutation in human iPS cells. Exp. Hematol. 2015;43:838–848. doi: 10.1016/j.exphem.2015.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang, T. Y. et al. Cell compatibility of an eposimal vector mediated by the characteristic motifs of matrix attachment regions. Curr. Gene Ther. (2016). [DOI] [PubMed]
- 5.Hansen HG, Pristovšek N, Kildegaard HF, Lee GM. Improving the secretory capacity of Chinese hamster ovary cells by ectopic expression of effector genes: lessons learned and future directions. Biotechnol. Adv. 2017;35:64–76. doi: 10.1016/j.biotechadv.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Walsh G. Biopharmaceutical benchmarks. Nat. Biotechnol. 2014;32:992–1000. doi: 10.1038/nbt.3040. [DOI] [PubMed] [Google Scholar]
- 7.Wang XY, et al. Impact of Different Promoters on Episomal Vectors Harbouring Characteristic Motifs of Matrix Attachment Regions. Sci. Rep. 2016;6:26446. doi: 10.1038/srep26446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santilli G, et al. Biochemical correction of X-CGD by a novel chimeric promoter regulating high levels of transgene expression in myeloid cells. Mol. Ther. 2011;19:122–132. doi: 10.1038/mt.2010.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stein S, et al. Genomic instability and myelodysplasia with monosomy 7 consequent to EVI1 activation after gene therapy for chronic granulomatous disease. Nat. Med. 2010;16:198–204. doi: 10.1038/nm.2088. [DOI] [PubMed] [Google Scholar]
- 10.Girod PA, et al. Genome-wide prediction of matrix attachment regions that increase gene expression in mammalian cells. Nat. Methods. 2007;4:747–753. doi: 10.1038/nmeth1076. [DOI] [PubMed] [Google Scholar]
- 11.Allen ML, Antoniou M. Correlation of DNA methylation with histone modifications across the HNRPA2B1-CBX3 Ubiquitously-Acting Chromatin Open Element (UCOE) Epigenetics. 2007;2:227–236. doi: 10.4161/epi.2.4.5231. [DOI] [PubMed] [Google Scholar]
- 12.Brown AJ, Sweeney B, Mainwaring DO, James DC. Synthetic promoters for CHO cell engineering. Biotechnol. Bioeng. 2014;111:1638–1647. doi: 10.1002/bit.25227. [DOI] [PubMed] [Google Scholar]
- 13.Kim SY, Lee JH, Shin HS, Kang HJ, Kim YS. The human elongation factor 1 alpha (EF-1 alpha) first intron highly enhances expression of foreign genes from the murine cytomegalovirus promoter. J. Biotechnol. 2002;93:183–187. doi: 10.1016/S0168-1656(01)00388-1. [DOI] [PubMed] [Google Scholar]
- 14.Ho SC, Mariati, Yeo JH, Fang SG, Yang Y. Impact of using different promoters and matrix attachment regions on recombinant protein expression level and stability in stably transfected CHO cells. Mol. Biotechnol. 2015;57:138–144. doi: 10.1007/s12033-014-9809-2. [DOI] [PubMed] [Google Scholar]
- 15.Rotondaro L, Mele A, Rovera G. Efficiency of different viral promoters in directing gene expression in mammalian cells: effect of 3′-untranslated sequences. Gene. 1996;168:195–198. doi: 10.1016/0378-1119(95)00767-9. [DOI] [PubMed] [Google Scholar]
- 16.Addison CL, Hitt M, Kunsken D, Graham F. L.Comparison of the human versus murine cytomegalovirus immediate early gene promoters for transgene expression by adenoviral vectors. J. Gen. Virol. 1997;78:1653–1661. doi: 10.1099/0022-1317-78-7-1653. [DOI] [PubMed] [Google Scholar]
- 17.Rita Costa A, Elisa Rodrigues M, Henriques M, Azeredo J, Oliveira R. Guidelines to cell engineering for monoclonal antibody production. Eur. J. Pharm. Biopharm. 2010;74:127–138. doi: 10.1016/j.ejpb.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Bailey LA, Hatton D, Field R, Dickson AJ. Determination of Chinese hamster ovary cell line stability and recombinant antibody expression during long-term culture. Biotechnol. Bioeng. 2012;109:2093–2103. doi: 10.1002/bit.24485. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CC, et al. Targeted methylation of CMV and E1A viral promoters. Biochem. Biophys. Res. Comm. 2010;402:228–234. doi: 10.1016/j.bbrc.2010.09.131. [DOI] [PubMed] [Google Scholar]
- 20.Deer JR, Allison DS. High-level expression of proteins in mammalian cells using transcription regulatory sequences from the Chinese hamster EF-1 alpha gene. Biotechnol. Progr. 2004;20:880–889. doi: 10.1021/bp034383r. [DOI] [PubMed] [Google Scholar]
- 21.Byun HM, et al. Plasmid vectors harboring cellular promoters can induce prolonged gene expression in hematopoietic and mesenchymal progenitor cells. Biochem. Biophys. Res. Comm. 2005;332:518–523. doi: 10.1016/j.bbrc.2005.04.155. [DOI] [PubMed] [Google Scholar]
- 22.Chan KK, Wu SM, Nissom PM, Oh SK, Choo AB. Generation of high-level stable transgene expressing human embryonic stem cell lines using Chinese hamster elongation factor-1 alpha promoter system. Stem cells Dev. 2008;17:825–836. doi: 10.1089/scd.2007.0233. [DOI] [PubMed] [Google Scholar]
- 23.Dighe N, et al. Long-term reproducible expression in human fetal liver hematopoietic stem cells with a UCOE-based lentiviral vector. PLoS One. 2014;9:e104805. doi: 10.1371/journal.pone.0104805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farokhimanesh S, et al. Hybrid promoters directed tBid gene expression to breast cancer cells by transcriptional targeting. Biotechnol. Prog. 2010;26:505–511. doi: 10.1002/btpr.353. [DOI] [PubMed] [Google Scholar]
- 25.Yew NS, Przybylska M, Ziegler RJ, Liu D, Cheng SH. High and sustained transgene expression in vivo from plasmid vectors containing a hybrid ubiquitin promoter. Mol. Ther. 2001;4:75–82. doi: 10.1006/mthe.2001.0415. [DOI] [PubMed] [Google Scholar]
- 26.Sladitschek HL, Neveu PA. Bidirectional Promoter Engineering for Single Cell MicroRNA Sensors in Embryonic Stem Cells. PLoS One. 2016;11:e0155177. doi: 10.1371/journal.pone.0155177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo HW, et al. Evaluation of combinatorial cis-regulatory elements for stable gene expression in chicken cells. BMC. Biotechnol. 2010;19(10):69. doi: 10.1186/1472-6750-10-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlabach MR, Hu JK, Li M, Elledge SJ. Synthetic design of strong promoters. Proc. Natl. Acad. Sci. USA. 2010;107:2538–2543. doi: 10.1073/pnas.0914803107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Estes B, et al. Uncovering Methods for the Prevention of Protein Aggregation and Improvement of Product Quality in a Transient Expression System. Biotechnol. Prog. 2015;31:258–267. doi: 10.1002/btpr.2021. [DOI] [PubMed] [Google Scholar]
- 30.Pham PL, Kamen A, Durocher Y. Large-scale transfection of mammalian cells for the fast production of recombinant protein. Mol. Biotechnol. 2006;34:225–237. doi: 10.1385/MB:34:2:225. [DOI] [PubMed] [Google Scholar]
- 31.Veith N, Ziehr H, MacLeod RA, Reamon-Buettner SM. Mechanisms underlying epigenetic and transcriptional heterogeneity in Chinese hamster ovary (CHO) cell lines. BMC. Biotechnol. 2016;16:6. doi: 10.1186/s12896-016-0238-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lin Y, et al. MAR characteristic motifs mediate episomal vector in CHO cells. Gene. 2015;559:137–143. doi: 10.1016/j.gene.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 33.Kelly LE, Martinez-De Luna RI, El-Hodiri HM. Autoregulation of retinal homeobox (rax) gene promoter activity through a highly conserved genomic element. Genesis. 2016;54:562–567. doi: 10.1002/dvg.22983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ford KL, Baumgartner K, Henricot B, Bailey AM, Foster GD. A native promoter and inclusion of an intron is necessary for efficient expression of GFP or mRFP in Armillaria mellea. Sci. Rep. 2016;6:29226. doi: 10.1038/srep29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brown AJ, Sweeney B, Mainwaring DO, James DC. NF-κB, CRE and YY1 elements are key functional regulators of CMV promoter-driven transient gene expression in CHO cells. Biotechnol. J. 2015;10:1019–1028. doi: 10.1002/biot.201400744. [DOI] [PubMed] [Google Scholar]
- 36.Román R, et al. Enhancing heterologous protein expression and secretion in HEK293 cells by means of combination of CMV promoter and IFN2 signal peptide. Biotechnol. J. 2015;10:1019–1028. doi: 10.1002/biot.201400744. [DOI] [PubMed] [Google Scholar]
- 37.Humby MS, O’Connor CM. Human Cytomegalovirus US28 Is Important for Latent Infection of Hematopoietic Progenitor Cells. J. Virol. 2015;90:2959–2970. doi: 10.1128/JVI.02507-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moritz B, Becker PB, Göpfert U. CMV promoter mutants with a reduced propensity to productivity loss in CHO cells. Sci. Rep. 2015;5:16952. doi: 10.1038/srep16952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montiel-Equihua CA, et al. The β-globin locus control region in combination with the EF1α short promoter allows enhanced lentiviral vector-mediated erythroid gene expression with conserved multilineage activity. Mol. Ther. 2012;20:1400–1409. doi: 10.1038/mt.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roodbari F, et al. Immune responses against a new HIV-1p24-gp41/pCAGGS-IL-12 DNA vaccine in Balb/c mice. Iran. J. Immunol. 2012;9:86–97. [PubMed] [Google Scholar]
- 41.Yang C, et al. DNMT 1 maintains hypermethylation of CAG promoter specific region and prevents expression of exogenous gene in fat-1 transgenic sheep. PLoS One. 2017;12:e0171442. doi: 10.1371/journal.pone.0171442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wippermann A, Rupp O, Brinkrolf K, Hoffrogge R, Noll T. The DNA methylation landscape of Chinese hamster ovary (CHO) DP-12 cells. J. Biotechnol. 2015;199:38–46. doi: 10.1016/j.jbiotec.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 43.Tornøe J, Kusk P, Johansen TE, Jensen PR. Generation of a synthetic mammalian promoter library by modification of sequences spacing transcription factor binding sites. Gene. 2002;297:21–32. doi: 10.1016/S0378-1119(02)00878-8. [DOI] [PubMed] [Google Scholar]
- 44.Magnusson T, Haase R, Schleef M, Wagner E, Ogris M. Sustained, high transgene expression in liver with plasmid vectors using optimized promoter-enhancer combinations. J. Gene Med. 2011;13:382–391. doi: 10.1002/jgm.1585. [DOI] [PubMed] [Google Scholar]
- 45.Ho SC, et al. IRES-mediated Tricistronic vectors for enhancing generation of high monoclonal antibody expressing CHO cell lines. J.Biotechnol. 2012;157:130–139. doi: 10.1016/j.jbiotec.2011.09.023. [DOI] [PubMed] [Google Scholar]
- 46.Kim CH, Oh Y, Lee TH. Codon optimization for high-level expression of human erythropoietin (EPO) in mammalian cells. Gene. 1997;199:293–301. doi: 10.1016/S0378-1119(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 47.Yang M, Butler M. Effect of ammonia on the glycosylation of human recombinant erythropoietin in culture. Biotechnol. Prog. 2000;16:751–759. doi: 10.1021/bp000090b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.