Abstract
Background
The necessity for more effective therapies for chronic osteoarticular diseases has led to the development of treatments based on mesenchymal stem cells (MSCs), the natural precursors of musculoskeletal tissue. Treatments with autologous MSCs yielded excellent results, with nearly 70% improvement of pain and disability in osteoarthritis and degenerative disc disease. Using allogeneic MSCs is logistically more convenient and would widen the pool of eligible patients, but potential immune rejection should be considered. In this context, MSCs are purportedly immune evasive and better tolerated than other cell types.
Methods
We used samples collected during the performance of 2 randomized clinical trials using allogeneic bone marrow MSCs for treatment of osteoarthritis (NCT01586312) and degenerative disc disease (NCT01860417). Serum samples were used to determine anti-HLA antibodies, whereas either blood or MSC samples were used for HLA typing of recipients and donors, respectively. Algofunctional indexes were used as indicators of clinical evolution, and the correlation between the number of donor-host HLA mismatches and the efficacy of treatment was determined.
Results
Immune response was weak and transient, with reactivity decaying during the first year. Consistently, better donor-recipient HLA matching did not enhance efficacy.
Conclusions
This lack of reactivity is presumably due to the cooperation of 2 factors, (1) downregulation of the host immune responses by the transplanted MSCs and (2) effective insulation of these cells inside the articular cavity or the intervertebral disc, respectively. Interestingly, better HLA matching did not enhance efficacy. These observations have medical relevance as they support the clinical use of allogeneic cells, at least as a single-dose administration. Multiple-dose applications will require further research to exclude possible sensitization.
The necessity for more effective treatments for chronic osteoarticular diseases has led to the development of therapies with mesenchymal stem cells (MSCs), the natural precursors of musculoskeletal tissues. Treatment with autologous MSCs yielded excellent results, with nearly 70% improvement of pain and disability when used for degenerative disc disease (DDD)1 and knee osteoarthritis.2 The use of cheaper and more logistically convenient allogeneic MSCs would widen the pool of eligible patients, but the drawback of potential for immune rejection should be considered. With regard to the latter concern, MSCs are purportedly immune evasive and better tolerated than other cell types,3-5 and no serious adverse effects have been reported for allogeneic MSC treatments in more than 1000 cell-transplanted patients6,7; however, immune responses have not been studied in detail. Here we provide the data on HLA donor-host matching and recipient HLA sensitization results from 2 different clinical trials, and we establish a parallel to the clinical and functional outcomes.
MATERIALS AND METHODS
We used samples collected during 2 randomized clinical trials that tested the use of allogeneic bone marrow MSCs in the treatment of osteoarthritis (NCT01586312)6 and DDD (NCT01860417).7 Stored serum samples were used to determine anti-HLA antibodies, while blood samples were used for HLA typing of the hosts. Typing of the donors was performed using the retention samples collected during MSC manufacturing in both trials. The clinical results of the trials, including algofunctional indices and quantitative magnetic resonance imaging, were used for analysis. The human investigations were performed with informed consent and were preceded by local institutional review board approval.
RESULTS
Data on sensitization by allogeneic MSC infusion are scarce, with less than 100 patients studied in 3 different clinical trials.8-10 In all the cases, sensitization was poor, affected to only 5% to 30% of the patients, and no associated safety events were observed. Our new results come from prolonged follow-up in 2 different clinical trials6,7 and are summarized in Table 1. Table 1 compares the allelic HLA composition of recipients and donors for 23 patients that were treated with allogeneic MSC and computes the number of mismatches (from 3 to 6 in our cohort). The titers of anti-HLA antibodies in sera 1 to 6 months after the intervention and 12 to 18 months after intervention are also tabulated.
TABLE 1.
Recipient and donor HLA typing and anti-HLA antibodies
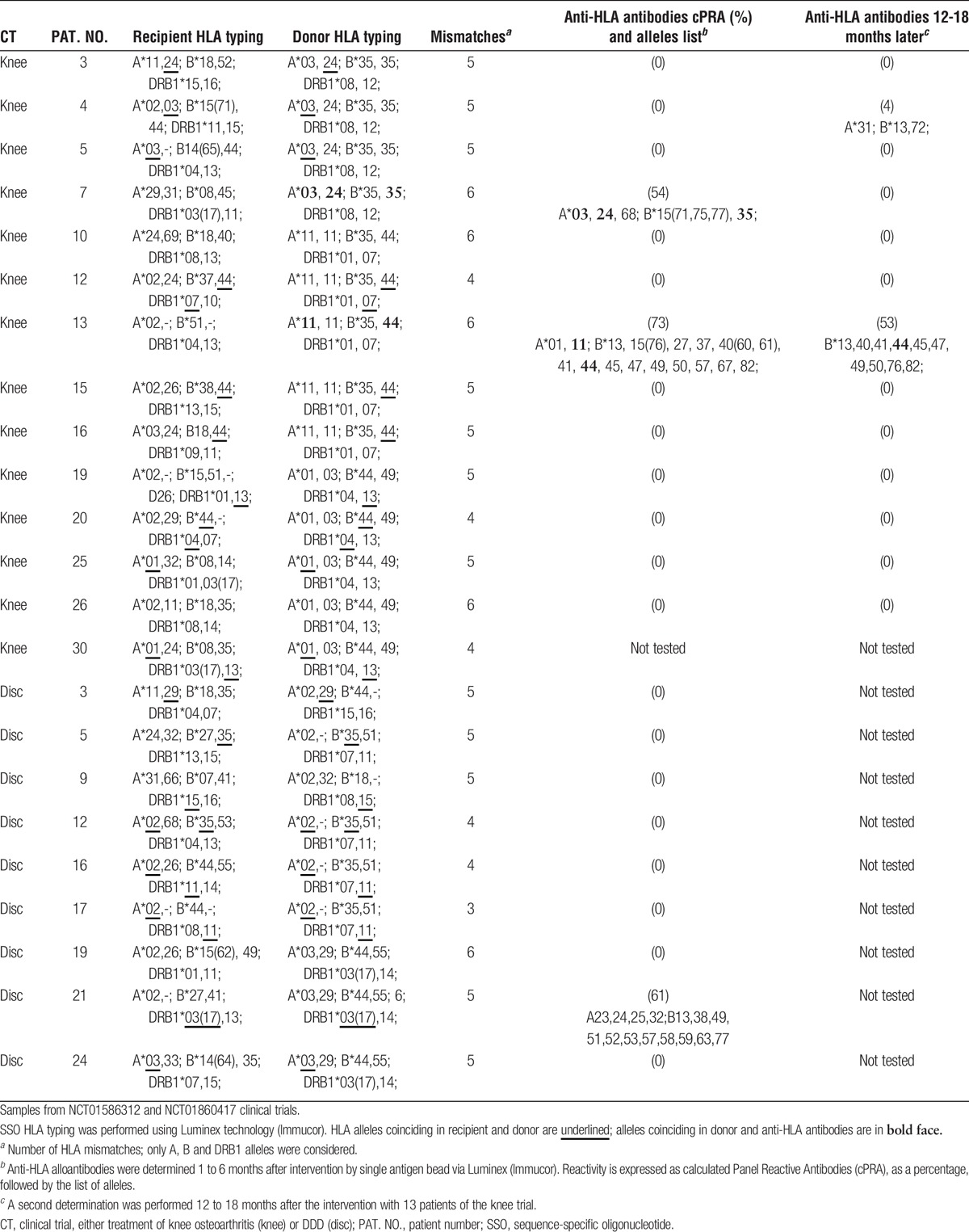
We could detect specific anti-HLA antibodies targeted to alleles present in the donor in only 2 of the 13 patients assessed during the knee osteoarthritis trial (Table 1). In these patients (patients 7 and 13), the reactivity decreased with time. The specific HLA reactivity also decreased during the first year in one of the previous reports.10 In the disc trial, the serum reactivity against MSCs was even smaller, and specific antibodies were not detected in any of the 9 patients tested. One patient (patient 21) displayed antibodies against antigenic determinants that were not present in the MSC donor. Finally, of 13 control patients analyzed, only 1 was positive for HLA antibodies.
DISCUSSION
The influence of HLA mismatches on renal graft survival has been questioned recently.11-13 However, an extensive study with 189 184 patients demonstrated that every mismatch cumulatively increases the probability of rejection by a modest 13%.14 In our cohort, MSC treatment improved pain for 17 of the 23 patients studied here and the relative efficacy was (mean ± SEM) 32 ± 10%, a value significantly different from 0 (P < 0.005, t test). To study the effects of HLA mismatch, we represented the relative pain improvement against the number of mismatches (Figure 1). The theoretical expectations would be that the pain improvement would decrease with the number of mismatches. In contrast, pain improvement appeared to increase with increasing MSC-host mismatch, although the mean improvement values obtained with 4, 5, and 6 mismatches were not significantly different.
FIGURE 1.
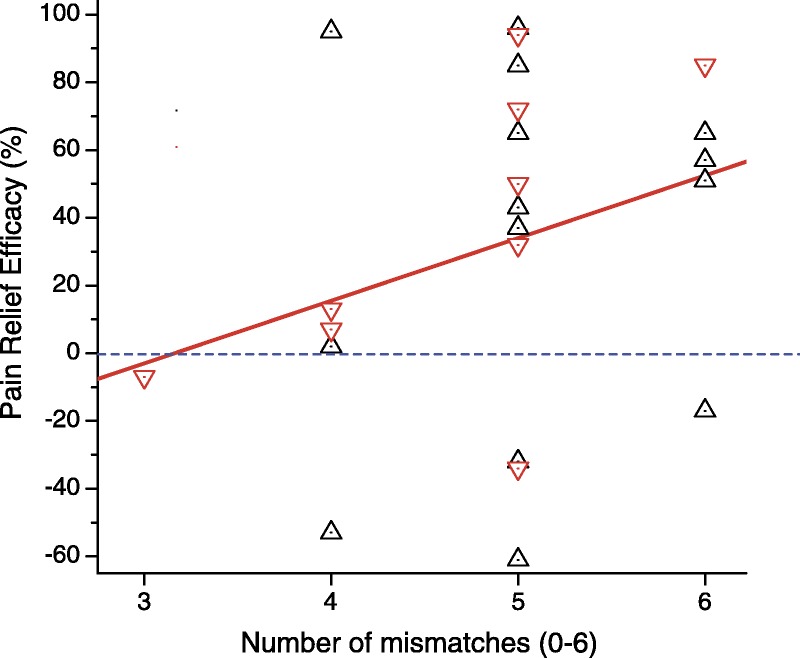
Correlation between pain relief and the number of HLA mismatches. Data from 2 randomized trials, MSC treatments of osteoarthritis (triangles) and DDD (inverted triangles), were pooled. Pain was measured with the Visual Analogue Scale and is quantified as relative pain relief, (pain at baseline minus pain 1 year after the intervention)/(pain at baseline), and expressed as percentage. The number of mismatches between alleles A, B and DRB1 included values from 3 to 6 in our cohort, gathering 23 patients from both the osteoarthritis and the DDD trials. The red line was adjusted using the least squares method. Note that pain improvement happened in all the values above the blue dotted line. Variation among the values obtained with the 4, 5, and 6 mismatches was not significantly greater than that expected by chance (ANOVA, P = 0.53). ANOVA, analysis of variance.
In conclusion, our results indicate that real-life immune responses to allogeneic MSC treatments of knee osteoarthritis and DDD are weak. This is probably due to the cooperation of 2 factors: (i) down regulation of the immune responses by the own MSCs and (ii) effective insulation of these cells inside the articular cavity or the intervertebral disc, respectively. Consistently, it seems that HLA matching does not enhance the efficacy of the treatment. These observations have medical relevance, as they support clinical use of allogeneic cells not matching to the recipient, at least as a single-dose administration. Multiple-dose applications will require further research to exclude possible sensitizations.
ACKNOWLEDGMENTS
The authors thank Dr Antonio Orduña for help with the use of the Immunology Platform facilities of the Valladolid University Hospital and Dr. Alberto Acedo, from AC-Gen, for help with the preparation of DNA extracts. The authors thank Ms. Sandra Güemes and Ms. Virginia Gordillo for technical support.
Footnotes
Published online 17 August, 2017.
J.G.-S. and A.S. are members of the Board of Directors of Citospin, a spin-off the University of Valladolid that specializes in Good Manufacturing Practices (GMP)-compliant cell production. No other disclosures are reported.
Funding/Support: Financial support from the Red de Terapia Celular of the Instituto de Salud Carlos III (RD16/0011/0003) and from the Centro en Red de Medicina Regenerativa de Castilla y León are gratefully acknowledged. EU cofinanced these grants through the European Regional Development Fund. The sponsors had no role in the design and conduct of the study; the collection, management, analysis, and interpretation of the data; or the preparation, review, or approval of the article.
J.G-S. had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the analysis. J.G-S. and M.N. participated in the conception and design of the article. A.V.N. and M.N. were primarily responsible for the clinical and analytical work, and A.S. for the cell production. All authors participated in analysis, discussion, and interpretation of data, revised the report, and gave final approval of the version to be published. J.G-S. assembled all data and wrote the final form of the article.
The authors declare no conflicts of interest.
REFERENCES
- 1.Orozco L, Munar A, Soler R, et al. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 2013;95:1535–1541. [DOI] [PubMed] [Google Scholar]
- 2.Orozco L, Soler R, Morera C, et al. Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011;92:822–828. [DOI] [PubMed] [Google Scholar]
- 3.Ankrum JA, Ong JF, Karp JM. Mesenchymal stem cells: immune evasive, not immune privileged. Nat Biotechnol. 2014;32:252–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Le Blanc K, Ringden O. Immunomodulation by mesenchymal stem cells and clinical experience. J Intern Med. 2007;262:509–525. [DOI] [PubMed] [Google Scholar]
- 5.Murphy MB, Moncivais K, Caplan AI. Mesenchymal stem cells: environmentally responsive therapeutics for regenerative medicine. Exp Mol Med. 2013;45:e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vega A, Martin-Ferrero MA, Del Canto F, et al. Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 2015;99:1681–1690. [DOI] [PubMed] [Google Scholar]
- 7.Noriega DC, Ardura F, Hernández-Ramajo R, et al. Intervertebral disc repair by allogeneic mesenchymal bone marrow cells: a randomized controlled trial. Transplantation. 2017;101:1945–1951. [DOI] [PubMed] [Google Scholar]
- 8.Ascheim DD, Gelijns AC, Goldstein D, et al. Mesenchymal precursor cells as adjunctive therapy in recipients of contemporary left ventricular assist devices. Circulation. 2014;129:2287–2296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hare JM, DiFede DL, Rieger AC, et al. Randomized comparison of allogeneic versus autologous mesenchymal stem cells for nonischemic dilated cardiomyopathy: POSEIDON-DCM trial. J Am Coll Cardiol. 2017;69:526–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hare JM, Fishman JE, Gerstenblith G, et al. Comparison of allogeneic vs autologous bone marrow-derived mesenchymal stem cells delivered by transendocardial injection in patients with ischemic cardiomyopathy: the POSEIDON randomized trial. JAMA. 2012;308:2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ashby VB, Port FK, Wolfe RA, et al. Transplanting kidneys without points for HLA-B matching: consequences of the policy change. Am J Transplant. 2011;11:1712–1718. [DOI] [PubMed] [Google Scholar]
- 12.Morales JM, Marcén R, Andrés A, et al. Renal transplantation in the modern immunosuppressive era in Spain: four-year results from a multicenter database focus on post-transplant cardiovascular disease. Kidney Int Suppl. 2008;S94–S99. [DOI] [PubMed] [Google Scholar]
- 13.Su X, Zenios SA, Chakkera H, et al. Diminishing significance of HLA matching in kidney transplantation. Am J Transplant. 2004;4:1501–1508. [DOI] [PubMed] [Google Scholar]
- 14.Williams RC, Opelz G, McGarvey CJ, et al. The risk of transplant failure with HLA mismatch in first adult kidney allografts from deceased donors. Transplantation. 2016;100:1094–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]


