Abstract
Background
Infiltrative hepatocellular carcinoma with macrovascular invasion is a relatively rare presentation and usually fatal disease.
Methods
Both patients exceeded Milan and University of California-San Francisco (UCSF) criteria, and per Barcelona Clinic Liver Cancer group guidelines, they were enrolled in a prospective open-label radioembolization phase II trial that gave them optimized lobar doses of Yttrium-90 as solely the first-line therapy without concomitant or additional pharmacological or locoregional therapies.
Results
Three months after radioembolization, the patients demonstrated no residual viable disease on surveillance imaging. The patients were then followed up with serial imaging for 2 years in 3-month intervals, without documenting recurrence or extrahepatic disease. Finally, both patients underwent transplantation and after more than 20 months of imaging surveillance, no locoregional or systemic recurrence have been observed.
Conclusions
We present, to our knowledge, the first 2 reports of transplantation after successfully downstaging infiltrative disease with portal vein tumoral thrombosis, which traditionally poses as a relative contraindication for resection or transplantation.
Infiltrative hepatocellular carcinoma (HCC) is a relatively rare liver carcinoma subtype accounting for an estimated 7-20% of all diagnosed HCC cases. Compared with the more common nodular subtype, infiltrative HCC lesions usually progress aggressively and are associated with a worse prognosis. Morphologically, this subtype has been characterized by an ill-defined, diffuse and cirrhotomimetic phenotype which may be limited to 1 liver segment but also spread throughout the entire liver parenchyma.1,2 On magnetic resonance imaging (MRI), this variant usually manifests as a geographic area of increased signal on T2-weighted images with variable degrees of arterial enhancement, portal venous phase washout, and portal vein tumor thrombosis (PVTT).1,3
Given the advanced stages and aggressive nature of infiltrative HCC with a high likelihood of vascular invasion and extrahepatic metastatic disease, surgical resection and liver transplantation are usually not recommended for most patients due to decreased survival expectations.2-5 Thus, American Association for the Study of Liver Diseases guidelines recommend systemic treatment with sorafenib as treatment of choice which previously demonstrated a significant survival benefit compared with untreated patients.4,6-8 However, several studies investigated the safety and efficacy of locoregional therapies such as, for example, Yttrium-90 (Y90) radioembolization and concluded that this may be a safe and viable treatment option for these patients.9,10
We herein report on 2 patients diagnosed with infiltrative HCC with portal vein tumor thrombus PVTT (American Joint Committee on Cancer [AJCC] stage IIIB) and were therefore, beyond criteria for transplantation. Per Barcelona Clinic Liver Cancer group guidelines, they were enrolled in a prospective open-label radioembolization phase II trial and underwent orthotopic liver transplantation (OLT) after downstaging the disease and exhibiting complete response to therapy using a single high-dose lobar radioembolization followed by a 2-year observation period without concomitant or additional pharmacological or locoregional therapies.
MATERIALS AND METHODS
Case 1, a 58-year-old man (Eastern Cooperative Oncology Group [ECOG] 1), presented with a history of chronic hepatitis C (genotype 1a) and resultant cirrhosis (Child-Pugh A5). Case 2, a 65-year-old man (ECOG 0) presented with a history of alcoholic liver cirrhosis (Child-Pugh A5).
The relevant baseline laboratory test results for case 1 were as follows: platelet count, 170,000/μL; international normalized ratio, 1.2; serum albumin, 2.9 g/dL; aspartate aminotransferase, 271 IU/L; alanine aminotransferase, 120 IU/L; total bilirubin, 0.6 mg/dL; alkaline phosphatase, 112 IU/L; and α-fetoprotein (AFP), > 2000 IU/mL. The baseline laboratory test results for case 2 were as follows: platelet count, 125,000/μL; international normalized ratio, 1.21; serum albumin, 2.6 g/dL; aspartate aminotransferase, 65 IU/L; alanine aminotransferase, 44 IU/L; total bilirubin, 1.1 mg/dL; alkaline phosphatase, 201 IU/L; and AFP, > 2000 IU/mL.
Both underwent hepatology evaluation and screening MRI, which demonstrated a 7.3 × 10.3 cm HCC with infiltrative features predominantly involving segments 6 and 7 with minimal involvement of segments 5 and 8 in case 1; and a 4.5 × 8.3 cm HCC with infiltrative features predominantly involving segments 5 and 8 with minimal involvement of segment 6 and 7 in case 2. In addition, there was thrombosis of the right anterior and posterior portal veins, with imaging features of tumoral thrombosis present in both patients' tumors (Figures 1–3). An additional chest computer tomography performed in both patients was unremarkable.
FIGURE 1.
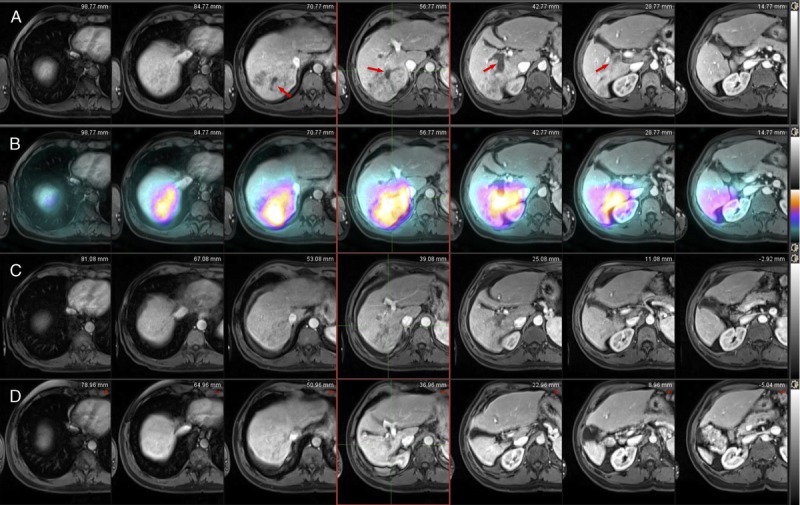
Case 1, Imaging findings. Pretreatment axial contrast enhanced T1-weighted MRI image on arterial phase (A) demonstrates infiltrative HCC in a geographic area of arterial enhancement involving the posterior segments of the right lobe that includes the right portal vein (arrow), which represents tumor thrombus. Post-Y90 Bremsstrahlung fused SPECT-CT scan (B) demonstrates increased Y90 tracer activity within the treated infiltrative HCC. On the same slices and on the 1-month postprocedural scan (C), note the reduction of enhancement of both the infiltrative mass. After 1 year, note the significant liver atrophy of the right liver lobe and the sustained complete treatment response without a recurrence (D). SPECT-CT, single-photon emission computed tomography.
FIGURE 3.
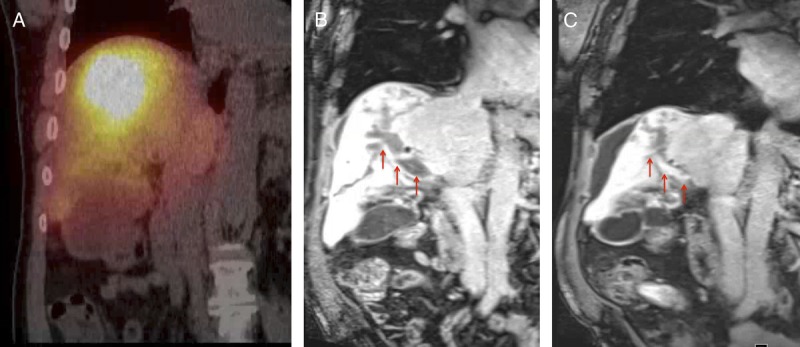
Case 2, Imaging findings. Postprocedural Bremsstrahlung fused SPECT-CT scan (A), demonstrates increased tracer activity within the expected location of the infiltrative lesion and within the right portal vein. On the correlative T1-weighted MRI image on portal venous phase on the preprocedural scan (B), the tumor thrombosis involves the right portal vein (arrow). In the correlative T1-weighted MRI image on portal venous phase on the 3-month postprocedural scan (c), note the partial recanalization of the portal vein (arrow).
FIGURE 2.
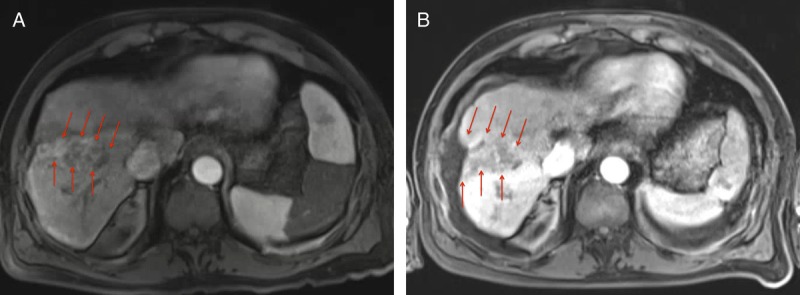
Case 2, Imaging findings. Pretreatment axial contrast enhanced T1-weighted MRI image on arterial phase (A) demonstrates a geographic area of arterial enhancement (arrows) involving predominantly the anterior segments of the right lobe. On the same slice and on the 3-month postprocedural scan (B), note the absence of enhancement of the infiltrative mass (arrow). Note the significant resultant liver atrophy.
Because of the advanced initial tumor stage of both patients with T3bN0M0 (stage IIIb) per the modified AJCC/Union for International Cancer Control staging system, the tumors were deemed nonresectable and noneligible for liver transplantation at a multidisciplinary tumor board meeting, and the decision to offer locoregional therapy was made. After interventional oncology consultation was performed, and per Barcelona Clinic Liver Cancer guidelines, the patients were enrolled in a prospective open-label radioembolization phase II trial immediately after presentation without undergoing any prior systemic or concomitant therapies.
After analyzing the pertinent imaging in conjunction with the nuclear medicine staff, a determination was made on the liver volume to be treated (case 1 = 900 mL, case 2 = 1350 mL) as well as an estimated involvement of the total liver volume (case 1 = 40%, case 2 = 20%). Technecium-99 m macroaggregated albumin hepatic shunt study was then performed demonstrating the pulmonary shunt fraction (case 1 = 12.3%, case 2 = 14.3%). The starting dose was calculated to deliver 120 Gy to the tumors in both case 1 and case 2. The estimated lung dose was calculated (case 1 = 15.9 Gy, case 2 = 27.8 Gy). Based on these calculations, a dose was ordered (case 1 = 69.5 mCi [2.57 GBq], case 2 = 106.1 mCi [3.93 GBq]) and subsequently, the patients' right hepatic artery was selectively catheterized delivering an approximated dose of Y90 microspheres (case 1 = 63.99 mCi, case 2 = 105.2 mCi). The administered radiation dose was equivalent to 112.1 Gy to the liver and 14.84 Gy to the lungs in case 1. The dose was equivalent to 193.5 Gy to the liver and 26.8 Gy to the lungs in case 2.
RESULTS
After radioembolization, the patients demonstrated no residual viable tumor by modified Response Evaluation Criteria in Solid Tumors and European Association for the Study of the Liver criteria (Figures 1–3). The AFP values dropped significantly 3 months post-radioembolization. Case 1 dropped to an AFP of 84 ng/mL, and case 2 dropped to 127 ng/mL. The patients were then followed clinically for 2 years (case 1) and 1.5 years (case 2), with MRI examinations performed every 3 months. During that period, no disease recurrence or extrahepatic disease progression was observed. In both cases, the right liver lobe became atrophic, and there was early response to therapy by modified Response Evaluation Criteria in Solid Tumors and European Association for the Study of the Liver criteria.
After these periods, the patients were both listed with a Model for End-Stage Liver Disease score of 22 exception points (as per the Organ Procurement and Transplantation Network allocation system in force in 2013-2014) and rapidly underwent OLT at our institution under the presumption of no tumor viability per imaging findings as well as no disease progression during the observation period. Both patients underwent OLT with standard technique undergoing no complications. Pathological explant (Figure 4) examination concurs with serial MR examinations demonstrating an atrophic right lobe with complete histopathologic tumor necrosis (Figures 5). Additionally, sphere deposition in the tumor bed (Figure 6A) and in the portal vein from case 2 (Figure 6B) was observed. After 24 months (case 1) and 20 months (case 2) of imaging surveillance after OLT, no locoregional or systemic recurrence have been observed (Figure 7).
FIGURE 4.
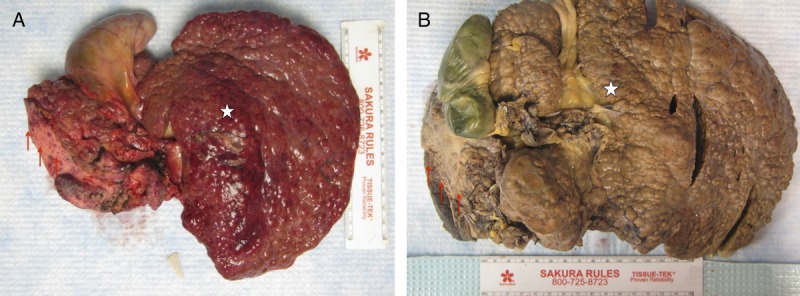
Gross pathologic specimens. The surface of both livers is diffusely cirrhotic (A) case 1; (B) case 2 with significant atrophy of the right liver lobe (arrows) and hypertrophy of the left liver lobe (star).
FIGURE 5.
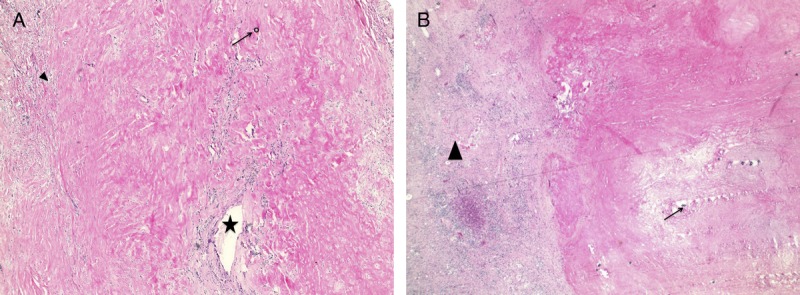
Histological examination of the treated lesions. Case 1 slides (A) Hematoxylin-eosin stain (10×) demonstrates complete pathologic necrosis (arrowhead) of the tumor bed with glass beads in the necrotic parenchyma (arrow) as well as a partially recanalized segmental portal vein (star). Case 2 slides (B) Hematoxylin-eosin stain (10×) demonstrates atrophic fibrotic parenchyma (arrowhead) with complete pathologic necrosis of the tumor bed with glass beads (arrow).
FIGURE 6.
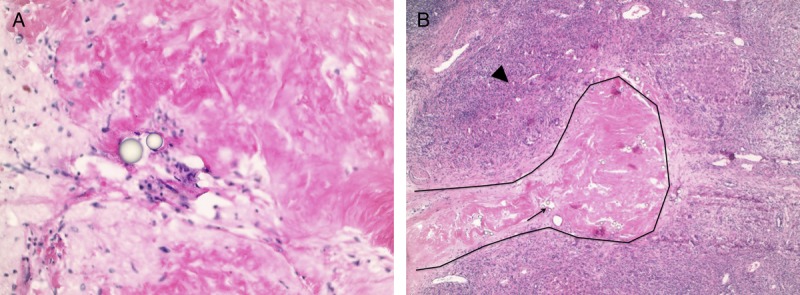
Histological examination of the treated lesions. Case 1 slides (A), hematoxylin-eosin stain (40×) demonstrates an area of fibrosis inside the tumor bed with glass beads (arrow). Case 2 slides (B), hematoxylin-eosin stain (10×) demonstrates a fibrotic portal vein (black contour) with glass beads inside the vessel lumen (arrow). The surrounding parenchyma is fibrotic.
FIGURE 7.
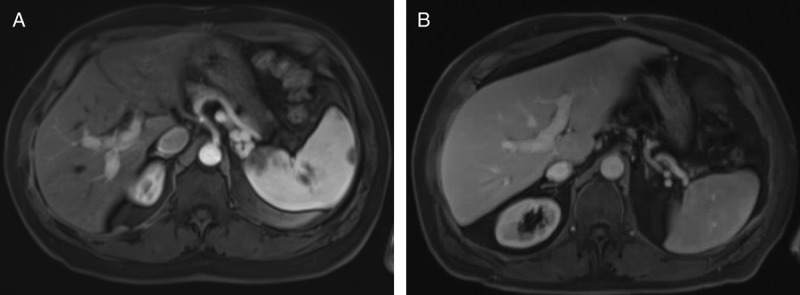
Posttransplant imaging surveillance. Posttransplant axial contrast enhanced T1-weighted MRI image on delayed arterial phase. (A) Case 1 at 24 months, and (B) case 2 at 20 months, demonstrates no areas of arterial enhancement as well as patent portal veins.
DISCUSSION
To the best of our knowledge, these 2 cases represent the first reports of OLT after successful downstaging of AJCC stage IIIB disease that included PVTT and extended disease-free survival with complete response to therapy using a single high-dose lobar radioembolization followed by a 2-year observation period (1.5 years in case 2) without concomitant or additional pharmacological or locoregional therapies.
Infiltrative HCC with PVTT is a rare presentation and typically yields poorer prognosis than the focal/nodular subtype and lower survival rates.1,3,11 Reported survivals yield 75.4% and 46.0% at 1 and 3 years in patients with focal/nodular HCC in comparison to 33.3% and 13.6% survival at 1 and 3 years in infiltrative HCC.11 Another study demonstrated a 4.0-month median overall survival (OS) and survival rates at 3 months, 6 months, and 1 year of 63%, 30%, and 8%, respectively.6 Current standard of care treatment for infiltrative HCC is systemic therapy with sorafenib per the American Association for the Study of Liver Diseases guidelines since surgical resection has shown a poor prognosis with a threefold increased risk of death compared with nodular HCC and a limited 5-year survival rate of 16%.2-5,8
Sorafenib has been shown to increase survival with reported median OS rates of 7.5 months in patients with infiltrated HCC in comparison to those who did not receive tumor therapy (3 months). However, sorafenib still cannot be deemed a curative therapy option because there have been no reports of effective downstaging and subsequent OLT.6,7 It should be noted that sorafenib has not been evaluated specifically in infiltrative HCC with PVTT, so no conclusion can be made regarding its use in this patient population.
Transarterial chemoembolization (TACE) has been previously studied in patients with infiltrative HCC demonstrating mixed results. One study showed no benefit in treating infiltrative HCC with chemoembolization, reporting increased morbidity and mortality with decreased OS.12 However, evidence does show TACE as well tolerated, extending median survival to 12 months as compared with 3 months with supportive measures. Better results have been achieved in patients with initial AFP levels less than 400 ng/mL and bilirubin levels less than 2.0 mg/dL.1 Others have also reported TACE to be a safe and effective treatment method in this patient population.13
Research has shown that the use of transarterial radioembolization for the treatment of HCC with PVTT is an effective therapy that yields a higher response rate (50-70%)14,15 with a median OS of 13.0 months14 and an improved median progression-free survival (11.0 months) as compared with similar patients treated with sorafenib (4.1 months).15,16 Recently, Y90 radioembolization therapy for infiltrative HCC with portal venous thrombosis demonstrated a median OS of 13 months and a median time to progression of 9 months.10 Here, ECOG performance status and Child-Pugh class have been deemed to be independent predictors of time to progression and in addition to hepatobiliary toxicity (grade 2 or higher), they were seen to be predictors of OS.10
Downstaging of infiltrative HCC to within Milan criteria has been controversial as some consider the criteria to be too stringent. Several studies have looked at downstaging and subsequent survival post-OLT with varying results.17-19 Despite the controversy, tumor downstaging to meet Milan criteria for OLT in selected patients has been associated with excellent post-transplant outcomes.17 Cohort studies comparing the use of TACE and Y90 radioembolization for downstaging showed that radioembolization was more successful than TACE (58% vs 31%).20 Although these data are encouraging, little is known about downstaging stage IIIB disease with radioembolization and the long-term outcomes.
These 2 presented cases support attempting to downstage infiltrative disease with PVTT using Y90 radioembolization before OLT as a potential curative treatment option. Possible rationale to explain the results in these 2 cases might be the degree of cirrhosis with relatively well-preserved liver function (Child-Pugh A5) as well as good preprocedural performance status, both conditions known to be favorable for response to treatment with radioembolization. These cases may also be optimal examples of advanced HCC with PVTT to be treated with high-dose radioembolization because both were unilobar, similar in size, and with ipsilateral branch PVTT. Limitation of this report includes the absence of pre-radioembolization pathology because all imaging and laboratory workup corroborated the diagnosis of infiltrative HCC before radioembolization therapy.
CONCLUSIONS
These 2 presented cases support that a single treatment of high-dose lobar radioembolization as the first-line therapy in patients with unilobar infiltrative HCC with ipsilateral PVTT could be a safe and efficacious treatment strategy to successfully downstage the tumor for successful liver transplantation and potential cure of the disease.
Footnotes
Published online 18 August, 2017.
The authors declare no funding or conflicts of interest.
M.S.D. and J.C.C. have contributed equally to the work.
M.S.D. conducted literature searches, interpreted data and wrote the article. J.C.C. compiled/evaluated patient data and wrote the article. J.M.L. helped to evaluate and edit the article. A.M.K. contributed all pathology-related data to the article, along with images. S.J.K. contributed surgical specimens and patient data from both case reports. H.S.K. supervised the development of work, research interpretation, and article development.
REFERENCES
- 1.Kneuertz PJ, Demirjian A, Firoozmand A, et al. Diffuse infiltrative hepatocellular carcinoma: assessment of presentation, treatment, and outcomes. Ann Surg Oncol. 2012;19:2897–2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynolds AR, Furlan A, Fetzer DT, et al. Infiltrative hepatocellular carcinoma: what radiologists need to know. Radiographics. 2015;35:371–386. [DOI] [PubMed] [Google Scholar]
- 3.Demirjian A, Peng P, Geschwind JF, et al. Infiltrating hepatocellular carcinoma: seeing the tree through the forest. J Gastrointest Surg. 2011;15:2089–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yopp AC, Mokdad A, Zhu H, et al. Infiltrative hepatocellular carcinoma: natural history and comparison with multifocal, nodular hepatocellular carcinoma. Ann Surg Oncol. 2015;22(Suppl 3):S1075–S1082. [DOI] [PubMed] [Google Scholar]
- 5.Ochiai T, Sonoyama T, Ichikawa D, et al. Poor prognostic factors of hepatectomy in patients with resectable small hepatocellular carcinoma and cirrhosis. J Cancer Res Clin Oncol. 2004;130:197–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mehta N, Fidelman N, Sarkar M, et al. Factors associated with outcomes and response to therapy in patients with infiltrative hepatocellular carcinoma. Clin Gastroenterol Hepatol. 2013;11:572–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim HY, Park JW, Nam BH, et al. Survival of patients with advanced hepatocellular carcinoma: sorafenib versus other treatments. J Gastroenterol Hepatol. 2011;26:1612–1618. [DOI] [PubMed] [Google Scholar]
- 8.Bruix J, Sherman M. American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53:1020–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Golfieri R, Mosconi C, Cappelli A, et al. Efficacy of radioembolization according to tumor morphology and portal vein thrombosis in intermediate-advanced hepatocellular carcinoma. Future Oncol. 2015;11:3133–3142. [DOI] [PubMed] [Google Scholar]
- 10.Kokabi N, Camacho JC, Xing M, et al. Open-label prospective study of the safety and efficacy of glass-based yttrium 90 radioembolization for infiltrative hepatocellular carcinoma with portal vein thrombosis. Cancer. 2015;121:2164–2174. [DOI] [PubMed] [Google Scholar]
- 11.Benvegnu L, Noventa F, Bernardinello E, et al. Evidence for an association between the aetiology of cirrhosis and pattern of hepatocellular carcinoma development. Gut. 2001;48:110–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lopez RR, Jr, Pan SH, Hoffman AL, et al. Comparison of transarterial chemoembolization in patients with unresectable, diffuse vs focal hepatocellular carcinoma. Arch Surg. 2002;137:653–657, discussion 657–658. [DOI] [PubMed] [Google Scholar]
- 13.Jang ES, Yoon JH, Chung JW, et al. Survival of infiltrative hepatocellular carcinoma patients with preserved hepatic function after treatment with transarterial chemoembolization. J Cancer Res Clin Oncol. 2013;139:635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826–1837. [DOI] [PubMed] [Google Scholar]
- 15.Pracht M, Edeline J, Lenoir L, et al. Lobar hepatocellular carcinoma with ipsilateral portal vein tumor thrombosis treated with yttrium-90 glass microsphere radioembolization: preliminary results. Int J Hepatol. 2013;2013:827649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bruix J, Raoul JL, Sherman M, et al. Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol. 2012;57:821–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao FY, Kerlan RK, Jr, Hirose R, et al. Excellent outcome following down-staging of hepatocellular carcinoma prior to liver transplantation: an intention-to-treat analysis. Hepatology. 2008;48:819–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Otto G, Herber S, Heise M, et al. Response to transarterial chemoembolization as a biological selection criterion for liver transplantation in hepatocellular carcinoma. Liver Transpl. 2006;12:1260–1267. [DOI] [PubMed] [Google Scholar]
- 19.Millonig G, Graziadei IW, Freund MC, et al. Response to preoperative chemoembolization correlates with outcome after liver transplantation in patients with hepatocellular carcinoma. Liver Transpl. 2007;13:272–279. [DOI] [PubMed] [Google Scholar]
- 20.Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant. 2009;9:1920–1928. [DOI] [PubMed] [Google Scholar]


