Supplemental Digital Content is available in the text.
Abstract
Background:
Although numerous experimental models of arterialized venous flaps (AVFs) have been proposed, no single model has gained widespread acceptance. The main aim of this work was to evaluate the survival area of AVFs produced with different vascular constructs in the abdomen of the rat.
Methods:
Fifty-three male rats were divided into 4 groups. In group I (n = 12), a 5-cm-long and 3-cm-wide conventional epigastric flap was raised on the left side of the abdomen. This flap was pedicled on the superficial caudal epigastric vessels caudally and on the lateral thoracic vein cranially. In groups II, III, and IV, a similar flap was raised, but the superficial epigastric artery was ligated. In these groups, AVFs were created using the following arterial venous anastomosis at the caudal end of the flap: group II (n = 13) a 1-mm-long side-to-side anastomosis was performed between the femoral artery and vein laterally to the ending of the superficial caudal epigastric vein. In group III (n = 14), in addition to the procedure described for group II, the femoral vein was ligated medially. Finally, in group IV (n = 14), the superficial caudal epigastric vein was cut from the femoral vein with a 1-mm-long ellipse of adjacent tissue, and an end-to-side arterial venous anastomosis was established between it and the femoral artery.
Results:
Seven days postoperatively, the percentage of flap survival was 98.89 ± 1.69, 68.84 ± 7.36, 63.84 ± 10.38, 76.86 ± 13.67 in groups I–IV, respectively.
Conclusion:
An optimized AVF can be produced using the vascular architecture described for group IV.
INTRODUCTION
More than 30 years have passed since Vaubel’s 1975 description of an arterialized venous flap (AVF) to reconstruct the dorsum of the hand and the highly cited Nakayama’s 1981 article on the creation of an experimental AVF model in the abdomen of the rat.1,2 Initially, there was great enthusiasm with AVFs, since they allowed the transference from composite blocks of tissues based exclusively on the venous system. This in turn allowed the creation of thin, pliable, and versatile flaps, that could be tailored rapidly and with minimal morbidity in the donor zone.3 However, reports of high necrosis rates and a poorly understood physiology have been deterring many surgeons of using AVFs in clinical practice.3–8
Although numerous experimental models of AVFs have been proposed, no model has gained widespread acceptance, which hinders comparison of observations on the physiology and interventions on these flaps. Additionally, lack of a standardized model of AVF can be an obstacle for the novice in microsurgery while preparing to execute these flaps in a training environment.9 Therefore, the main aim of this work was to evaluate the flap survival area of AVFs produced with different vascular constructs in the abdomen of the rat. Secondary endpoints were determination of the time required to produce the flap, animal mortality, and surgical complications, as well as thermographic, histological, and microvascular characterization of the different constructs. The ultimate goal of all these evaluations was to define an optimized model of AVF that could be easily replicated for research and teaching purposes.
METHODS
Fifty-three male rats weighing 250–350 g were used. Only male rats were used to prevent potential confounding effects of cyclical hormonal changes in female rats.10 All the animals were housed under standard environmental conditions and given nothing by mouth 6 hours before surgical procedures. No antibiotic prophylaxis was given.
Rats were anesthetized with a mixture of ketamine (5 mg/kg) and diazepam (0.25 mg/kg) given intraperitoneally. The depth of anesthesia was evaluated by toe pinch and by observance of respiration rate throughout the entire procedure. Supplementary doses of the anesthetic mixture were provided as needed.11
After shaving the abdomen and placing the animals on the operation table, the skin was disinfected with an antiseptic solution (Cutasept, Hartmann, Heidenheim, Germany). Hypothermia was avoided by placing the rat over a heating pad for the duration of the surgery.
Under a surgical operating microscope, a 5-cm-long and 3-cm-wide fasciocutaneous flap was raised on the left side of the rat’s abdomen immediately deep to the panniculus carnosus layer (Fig. 1). This flap was initially pedicled on the superficial caudal vessels caudally and on the lateral thoracic vein cranially. All other vessels were carefully ligated.9,11
Fig. 1.
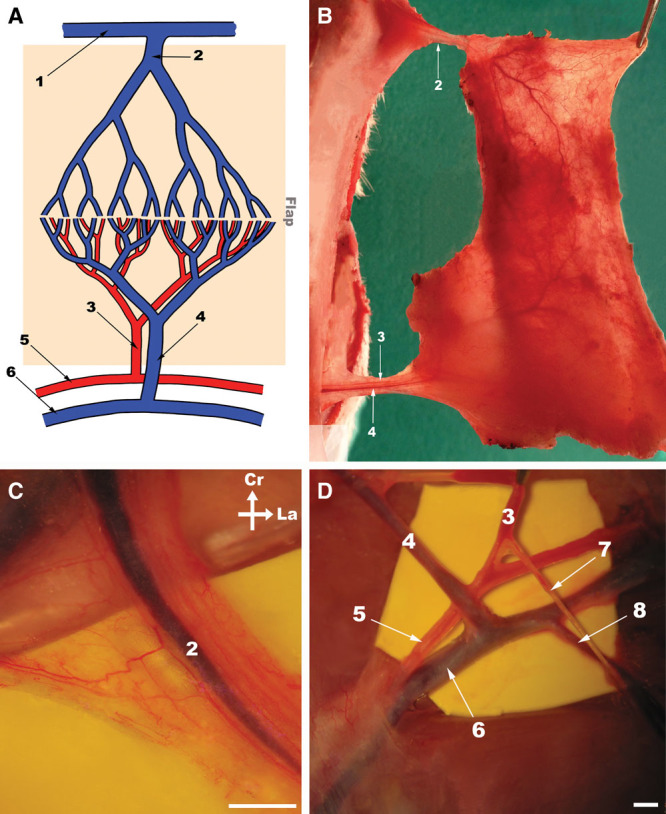
Epigastric flap surgical anatomy. A, Schematic drawing of the blood supply to the rat’s epigastric flap. The shaded area represents the flap. B, Photograph under transillumination of the flap’s deeps surface raised on the left side of the abdomen showing its largest vessels. C, High amplification photograph of the lateral thoracic vein before being dissected from the surrounding loose areolar tissue. D, Photograph showing the origin of the superficial caudal epigastric vessels from the femoral vessels. Cr, cranial; La, lateral; 1, axillary vein; 2, lateral thoracic vein; 3, superficial caudal epigastric artery; 4, SCEV; 5, femoral artery; 6, femoral vein; 7, superficial external pudendal artery; 8, superficial external pudendal vein. Calibration bar = 1 mm.
Rats were then randomly assigned to the following groups (Fig. 2):
Fig. 2.
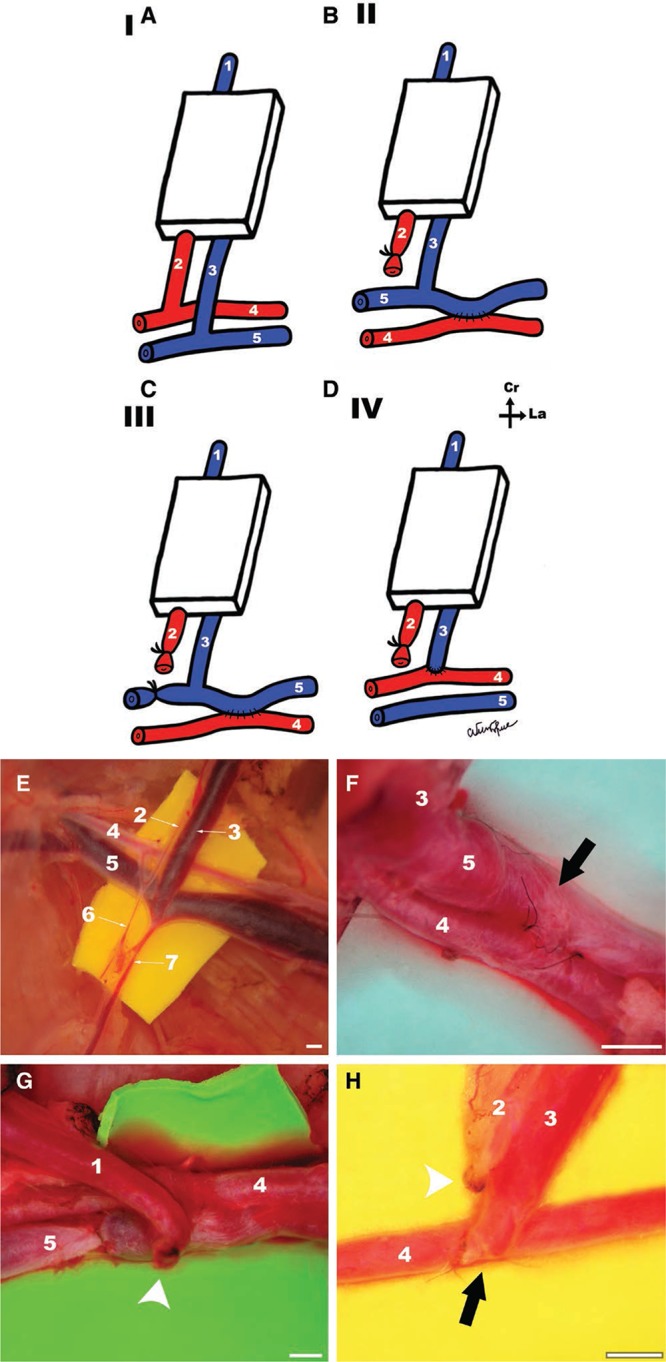
A,B,C,D, Schematic representation of the vascular patterns in the different experimental groups. A, Group I (CPF). B, Group II (AVF produced by femoral side-to-side anastomosis). C, Group III (AVF produced by femoral side-to-side anastomosis and proximal ligation of the femoral vein). D, Group IV (AVF produced by terminal-lateral anastomosis of the epigastric vein to the femoral artery). Cr, cranial; La, lateral. E, F, G, H, Surgical operating view of the inflow vessels of the flap in groups I, II, III, and IV, respectively. Arrows indicate place of arteriovenous anastomosis. Arrow heads indicate vessel ligation. Calibration bar = 1 mm.
In group I (n = 12), a conventional perfusion flap (CPF) was raised as described above.
In groups II, III, and IV, the superficial epigastric artery was ligated with an 8/0 nylon suture. In these groups, AVFs were created using the following arterial venous anastomosis (AVA) at the caudal end of the flap:
In group II (n = 13), a 1-mm-long side-to-side anastomosis was performed between the femoral artery and vein laterally to the ending of the superficial caudal epigastric vein (SCEV) in the femoral vein. The AVA was performed after making a 1-mm-long ostium in adjacent flanks of the femoral artery and vein. A monofilament nylon 11/0–interrupted suture was used for the vascular anastomosis.12
In group III (n = 14), in addition to the procedure described for group II, the femoral vein was ligated immediately medial to the ending of the SCEV, to increase blood flow through the AVA.
Finally, in group IV (n = 14), the SCEV was cut from the femoral vein with a 1-mm-long ellipse of adjacent femoral vein tissue. The ostium in the femoral vein was closed with a monofilament nylon 11/0 continuous suture. The same suture line was used to perform a side-to-end AVA between the SCEV and the ventral flank of the femoral artery through a 1-mm-long ostium previously created in the latter vessel. Interrupted stitches were used for this anastomosis.
Surgical wounds were closed with 5/0 nylon stitches. No anticoagulants were administered pre-, intra-, or postoperatively.
After surgery, rats were kept in solitary rat cages and offered rat chow and water ad libitum.
Seven days after the surgery, rats were anesthetized as described above and AVA patency was noted. Only animals with patent AVAs were included in the study.
All surgical procedures were performed under aseptic conditions by the same microsurgeon (D.C.), to minimize intersurgeon variability. The operative time was registered in all animals by a blinded observer.
One hour postoperatively, 2 rats in each group were submitted to infrared thermography with a FLIR E6 camera (FLIR Systems, Wilsonville, Or.) placed 25 cm above the abdomen. Rats were placed on their backs for 10 minutes before this evaluation. Thermographic measurements were made at a constant room temperature (22 ± 0.05°C) and humidity (50%).13
Rats were assessed daily by the same researcher, to reduce interobserver bias and variability.8 The following parameters were evaluated: animal wellbeing, flap viability, flap ischemia, and presence of complications. Objective measurement of flap survival was performed on the third and seventh days postoperatively based on digital photographs, which were later analyzed by a blinded observer using the free Image J software (National Institutes of Health, Bethesda, Md.).11 AVF survival was expressed as a percentage of the total flap surface area.14
Half of the rats in each group were prepared for conventional histological examination, whereas the other half was submitted to processing to obtain vascular corrosion casts for scanning electron microscopy (SEM) evaluation. For histological processing, rats were submitted to axial sections in the caudal, middle, and cranial aspect of the AVF that were later stained using hematoxylin–eosin and Masson’s trichrome. These rats were euthanized by exsanguination after dividing the neck vessels under sedation.
The rats destined to SEM analysis were submitted to intravascular injection of a resin cast (Mercox, Ladd Research, Williston, Vt.) and latter processed.15 SEM images were obtained using a JEOL JSM-7001F with an acceleration voltage of 2–30 kV. Vascular cast interpretation of microvascular findings was made according to Aharinejad and Lametschwandtner.15 These rats were euthanized by exsanguination after left and right ventricular catheterization.
All in vivo studies involving rats were carried out in strict accordance with the recommendations in the Guide for Proper Conduct of Animal Experiments and Related Activities in Academic Research and Technology.16
The protocol was approved by the Institutional Animal Care and Use Committee and Ethical Committee at the authors’ institution (CEFCM/08/2012).
Statistical Analysis
Qualitative variables were expressed as percentages. Quantitative variables were expressed as means ± SD. IBM SPSS Version 21.0 software (Armonk, N.Y.) was used for descriptive and inferential statistical analysis. The Kolmogorov-Smirnov test was used to assess whether variables were distributed normally. Analysis of variance and t test were used to compare averages in normally distributed data. Kruskal-Wallis and Mann-Whitney tests were used to compare means in nonnormally distributed data. Proportions were analyzed with the chi-square test or Fisher’s exact test. Kaplan Meier survival analysis was performed to identify differences in mortality between groups.
A 2-tail value of P < 0.05 was considered to be statistically significant.
RESULTS
Contrarily to CPFs (group I), all AVFs presented venous congestion, marked edema, epidermolysis, and areas of necrosis (see figure, Supplemental Digital Content 1, which shows representative photographs of CPFs and AVFs from the end of surgery to the seventh postoperative day, http://links.lww.com/PRSGO/A494).
Most of the necrotic areas were clearly defined on the third day after surgery (Fig. 3; see figures, Supplemental Digital Content 1, http://links.lww.com/PRSGO/A494 and Supplemental Digital Content 2, which shows box plot graphics illustrating flap survival in the different experimental groups 3 days postoperatively. Horizontal lines over boxplots indicate statistically significant differences, http://links.lww.com/PRSGO/A495). On this day, the percentage of flap survival was 99.16 ± 1.46, 71.48 ± 7.80, 68.01 ± 12.39, and 83.21 ± 11.36 for groups I, II, III, and IV, respectively. Seven days postoperatively, the percentage of flap survival in these groups was 98.89 ± 1.69, 68.84 ± 7.36, 63.84 ± 10.38, 76.86 ± 13.67. Necrotic areas were more extensive in the caudal third of the flap (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A494). Flap survival was higher in the CPF group than in any of the AVF groups (P < 0.01). Among AVFs, group IV presented a higher flap survival than groups II and III (P < 0.05). There were no statistically significant differences between groups II and III.
Fig. 3.
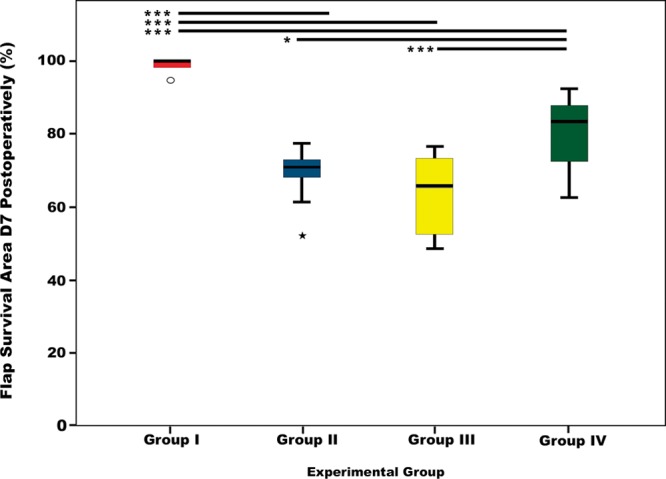
Box plots graphics illustrating flap survival in the different experimental groups 7 days postoperatively. Horizontal lines over boxplots indicate statistically significant differences. *P < 0.05; **P < 0.01; ***P < 0.001.
Average operating time was increasing longer in groups I, II, III, and IV (Table 1; P < 0.0001). On average, it took twice the time to produce a group IV AVF compared with a CPF (group I).
Table 1.
Comparison of the Different Vascular Constructs for Producing Arterialized Venous Fasciocutaneous Epigastric Flaps in the Rat
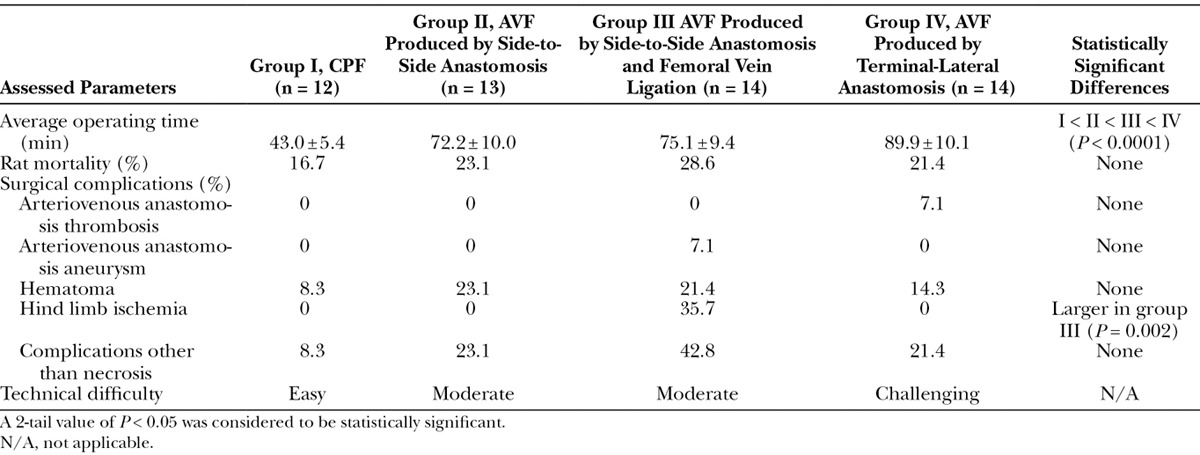
There were no statistically significant differences in rat mortality rates among the different groups (Table 1).
The most common complication was hematoma, which occurred in 8.3%, 23.1%, 21.4%, and 14.3% of cases of groups I, II, III, and IV, respectively. Five rats (35.7%) in group III developed venous congestion and a swollen left hind limb. Four of these rats died within the first 48 hours after surgery. At the end of the experiment, surgical inspection of the AVA revealed an aneurysm in 1 of the rats in group III and thrombosis in 1 of the rats in group IV. No infections were noted. Overall, complications were more common in group III, although this difference was not statistically significant.
Thermographic evaluation revealed that all flaps, including CPFs, presented a lower temperature than the contralateral nonoperated region (Fig. 4). However, the temperature difference was higher in the AVFs, being of at least of 2°C in these flaps. In all AVFs, temperature was lower in the caudal third of the flap. No significant difference was found among the different AVF groups.
Fig. 4.
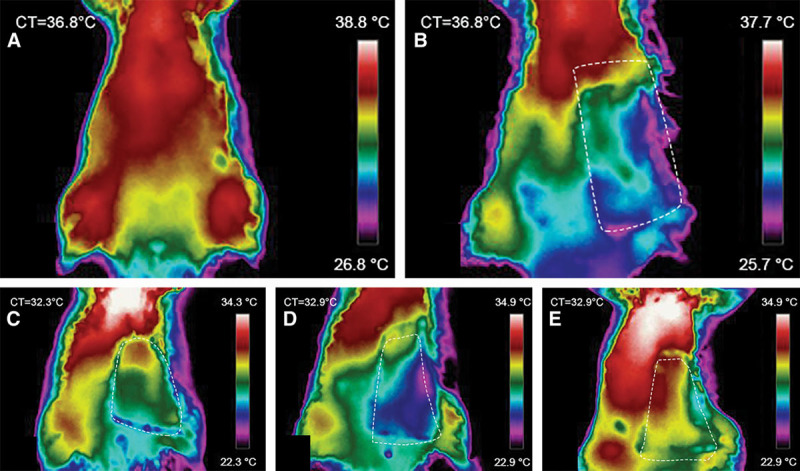
Representative direct infrared thermography images of the ventrolateral aspect of the abdomen of the rat 1 hour postoperatively. Flap boundaries are highlighted with the interrupted lines. A, Rats before surgery. This image shows the location of the dominant perforator vessels in the central and cranial aspect of the abdomen. B, Group I (conventional flap). C, Group II (AVF produced by femoral lateral-lateral anastomosis). D, Group III (AVF produced by femoral latero-lateral anastomosis and proximal ligation of the femoral vein). E, Group IV (AVF produced by terminal-lateral anastomosis of the epigastric vein to the femoral artery). CT, rat core temperature.
Histological and SEM evaluation of vascular corrosion casts revealed great morphological homogeneity among the different AVFs (see figure, Supplemental Digital Content 3, which displays a comparison of the histological features of AVFs compared with the CPFs controls, http://links.lww.com/PRSGO/A496; see figure, Supplemental Digital Content 4, which displays a comparison of the microvasculature of the conventional flap and the AVF using scanning electron microscope images of vascular corrosion casts, http://links.lww.com/PRSGO/A497). In fact, the authors were not able to identify distinctive morphological patterns for any of the AVFs groups, based on qualitative and/or quantitative features. Nevertheless, from a histological standpoint, comparatively to CPFs, AVFs presented greater flap edema, epidermolysis, loss of skin appendages, venous congestion and rupture, subcutaneous hematoma, and necrosis. In AVFs, there were regions of necrosis scattered throughout all integumentary layers. These histological features were more prominent in sections taken from the caudal third of AVFs. Moreover, all AVFs presented signs of SCEV arterialization and significant increment of the lumen diameter of the lateral thoracic vein (Supplemental Digital Content 3, http://links.lww.com/PRSGO/A496).
The study of the microvasculature through SEM vascular corrosion casts revealed higher vascular density in AVFs. In addition, these flaps also presented loss of venular valves and/or venous valve incompetency particularly in the caudal half of the flap. Signs of sprouting angiogenesis were present in both CPFs and AVFs, although more markedly in the latter group. In AVFs, capillary vessels sprouted more commonly from venules, whereas in CPFs, new capillary vessels sprouted mostly from neighboring capillaries. In AVFs, it was also frequent to find evidence of intussusceptive angiogenesis (Supplemental Digital Content 4, http://links.lww.com/PRSGO/A497).
DISCUSSION
Average flap survival area of the best AVF model in this work (group IV) was 76.86 ± 13.67%. This value is slightly inferior to that reported on a recent systematic review and meta-analysis on the clinical application of AVFs. In fact, it was estimated that the survival area of unconventional perfusion flaps used in the clinical context varies between 87.30% and 91.30% (P < 0.001).3 However, as the cited authors mention, this estimation may be affected by several biasses associated with any meta-analysis, namely publication bias, which tend to overestimate positive outcomes.3
AVF survival in the present work was worse than that described in the early description of Nakayama et al.2 These authors described a mean area of survival of 98% in nondelayed AVFs. However, they left a superior skin pedicle that no doubt contributed arterial axial and random perfusion to the flap.2 Nevertheless, the results herein presented concerning AVF survival are similar to those reported by other authors in the rat.17–20
The impact of different anastomotic layouts in flap survival had already been tested in CPFs.21,22 In these flaps, it was shown that side-to-end and end-to-end arterial anastomoses of similarly sized arteries guaranteed comparable survivals.21 However, as far as the authors could determine, this is the first time that the impact of microvascular anastomotic type on the survival of AVFs is studied. In the present work, the vascular layout used in group IV (side-to-end AVA) proved to be superior regarding AVF survival than that used in groups II and III (side-to-side AVAs). Many authors have used side-to-side AVAs of the femoral rats to obtain AVFs of the rat’s abdomen. In this way, they avoid the technical challenging AVAs of very small vessels such as the SCEV and the homonymous artery, which are considerably prone to thrombosis.12 Moreover, Nakayama et al.2 had already demonstrated in a pilot study that direct end-to-end AVA of the femoral artery and SCEV was associated with significant hemodynamic disturbance and death of 13 of 15 rats.
The data herein presented suggest that the incorporation of a 1-mm-ellipse of the femoral vein adjacent to the draining point of the SCEV (group IV) allows a safe AVA. This pattern probably ensures a more direct blood flow into the SCEV and thus greater venous valves’ incompetency, in comparison with AVAs in groups II and III. Furthermore, it is reasonable to expect that after removing the vascular clamps, the AVA in group IV is pulled by the distension of walls of the femoral artery, resulting in radial distraction of the SCEV. This, in turn, will further lead to venous valve incompetency in the later vessel, thus facilitating the entry of blood through the afferent vein of the group IV AVF.
Interestingly, mathematical modeling using Laplace’s law has suggested that the fine structure of venules under 100 µm in diameter renders the valves in these vessels readily incompetent in the presence of venous arterialization of ischemic lower limbs in humans.23 Our SEM data give empirical support to this assertion in the rat model, as in all AVFs groups there were signs of loss valve competency particularly in venules located in the afferent half of the flap (Supplemental Digital Content 4, http://links.lww.com/PRSGO/A497). Similar findings have been reported by other authors.12
Multiple examples of intussusceptive angiogenesis in the vascular molds of AVFs were observed in the present study. These may be justified by the fact that increases in blood pressure inside small vessels have been shown to be associated with transluminal tissue pillar formation and subsequent vascular splitting and neovessel formation.24 This mechanism has been largely neglected in the literature and may have a pivotal role in AVF hemodynamic adaptation.12
To the best of the authors’ knowledge, thermography imaging had never been employed before to study perfusion of AVFs. Notwithstanding, skin temperature has been used as surrogate marker of perfusion in hind limb vein arterialization in rats. The rationale for this is that skin temperature is proportional to integumentary perfusion.25 Our study lends support to the use of infrared thermography imaging for AVF perfusion evaluation, because it confirmed an inferior temperature in these flaps comparatively to CPFs and to the contralateral side of the abdomen. Additionally, in all AVFs, temperature was lower in the caudal third of the flap, where necrosis was more commonly found.
Recently, it has been shown that this region of the abdomen of the rat can be used to produce axial flaps simulating arterial ischemia or venous congestion, readily observed macroscopically by a pale and dark violet color, respectively.14 Remarkably, in the present study, all AVFs groups presented a dark bluish color suggestive of venous congestion (Supplemental Digital Content 1, http://links.lww.com/PRSGO/A494). Another common finding in these 2 studies was that necrotic areas were clearly defined in both CPFs and AVFs on the third postoperative day.14 This information may be of great interest for future research works using similar flaps in this region of the rat.
Rats were chosen in the present study because they have been the most widely used animal model in the realm of experimental flap surgery.26 This is certainly due to the fact they are readily available in most countries, they are easy to keep, and they are among the cheapest animals to obtain and to maintain.26 Nonetheless, it should be noted that AVFs performed in humans are usually based on vessels of a larger caliber.3 Hence, extrapolation of data obtained with the AVFs used in this article to humans must be done with this limitation in mind. In fact, it would be interesting to study the various vascular constructions described in these articles in other animal species, where larger flaps could be produced.
Furthermore, it is well known that in loose-skinned animals, the well-developed panniculus carnosus in the deep aspect of the integument leads to significant contraction of wounds and flaps.27 To tackle this problem, multiple strategies have been devised, namely choosing anatomical sites where the integument is firmly adherent to the deep structures (e.g., rabbit ear) or using various devices or splints to fixate the integumentary layer and the surgical flaps.27 Despite these limitations, in plastic surgery experimental research, it is customary to consider flap’s survival and necrosis as a percentage of flap’s total area, as the authors did in the present article.9,11,14,26 However, this difference in flap and wound behavior between rodents and humans should be taken into consideration when generalizing the data presented in this article to the clinical scenario.
Finally, the authors believe that the AVFs in group IV represent an optimized model of unconventional perfusion flap that can be easily replicated for research and teaching purposes. However, further studies are warranted to confirm or dismiss their usefulness in these contexts.
CONCLUSIONS
An optimized AVF can be reliably produced in the ventrolateral aspect of the abdomen of the rat by performing an end-to-side AVA between the femoral artery and the SCEV including the adjacent portion of the femoral vein, to produce a 1-mm-wide afferent vein. This model presented an average flap survival area of 76.86% ± 13.67%.
ACKNOWLEDGMENTS
The authors are very grateful to Mr. Carlos Lopes and Mr. Octávio Chaveiro for their help in producing and observing the scanning electron microscope specimens. The authors also thank Mr. Nuno Folque for producing all the drawings contained in this article.
Supplementary Material
Footnotes
Disclosure: Supported by a grant from “The Programme for Advanced Medical Education” (D.C.) sponsored by “Fundação Calouste Gulbenkian, Fundação Champalimaud, Ministério da Saúde and Fundação para a Ciência e Tecnologia, Portugal.” The Article Processing Charge was paid for by the authors.
Supplemental digital content is available for this article. Clickable URL citations appear in the text.
REFERENCES
- 1.Vaubel W. Indikationen und Technik des arterialisierten Lappens zur Deckung großer Defekte im Handbereich. Hefte Unfallheilkd. 1975;126:381. [PubMed] [Google Scholar]
- 2.Nakayama Y, Soeda S, Kasai Y. Flaps nourished by arterial inflow through the venous system: an experimental investigation. Plast Reconstr Surg. 1981;67:328–334.. [DOI] [PubMed] [Google Scholar]
- 3.Casal D, Cunha T, Pais D, et al. Systematic review and meta-analysis of unconventional perfusion flaps in clinical practice. Plast Reconstr Surg. 2016;138:459–479.. [DOI] [PubMed] [Google Scholar]
- 4.Goldschlager R, Rozen WM, Ting JW, et al. The nomenclature of venous flow-through flaps: updated classification and review of the literature. Microsurgery. 2012;32:497–501.. [DOI] [PubMed] [Google Scholar]
- 5.Yan H, Brooks D, Ladner R, et al. Arterialized venous flaps: a review of the literature. Microsurgery. 2010;30:472–478.. [DOI] [PubMed] [Google Scholar]
- 6.Yan H, Zhang F, Akdemir O, et al. Clinical applications of venous flaps in the reconstruction of hands and fingers. Arch Orthop Trauma Surg. 2011;131:65–74.. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Fan C, Zhang F, et al. Reconstruction of large dorsal digital defects with arterialized venous flaps: our experience and comprehensive review of literature. Ann Plast Surg. 2013;70:666–671.. [DOI] [PubMed] [Google Scholar]
- 8.Weng W, Zhang F, Zhao B, et al. The complicated role of venous drainage on the survival of arterialized venous flaps. Oncotarget. 2017;8:16414–16420.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirase Y. Tamai S, Usui M, Yoshizu T. Skin and muscle flaps in the rat. In: Experimental and Clinical Reconstructive Microsurgery. Vol. 2004:1, 1st ed Japan: Springer-Verlag; 111–114.. [Google Scholar]
- 10.Thatte M, Healy C, McGrouther D. Laser Doppler and microvascular pulsed Doppler studies of the physiology of venous flaps. Eur J Plast Surg. 1993;16:134–138.. [Google Scholar]
- 11.Casal D, Pais D, Iria I, et al. A model of free tissue transfer: the rat epigastric free flap. J Vis Exp. 2017;1:e55281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wungcharoen B, Pradidarcheep W, Santidhananon Y, et al. Pre-arterialisation of the arterialised venous flap: an experimental study in the rat. Br J Plast Surg. 2001;54:621–630.. [DOI] [PubMed] [Google Scholar]
- 13.Sheena Y, Jennison T, Hardwicke JT, et al. Detection of perforators using thermal imaging. Plast Reconstr Surg. 2013;132:1603–1610.. [DOI] [PubMed] [Google Scholar]
- 14.Matsumoto NM, Aoki M, Nakao J, et al. Experimental rat skin flap model that distinguishes between venous congestion and arterial ischemia: the reverse U-shaped bipedicled superficial inferior epigastric artery and venous system flap. Plast Reconstr Surg. 2017;139:79e–84e.. [DOI] [PubMed] [Google Scholar]
- 15.Aharinejad SH, Lametschwandtner A. Aharinejad SH, Lametschwandtner A. Identification and interpretation of cast vessel structures. In: Microvascular Corrosion Casting in Scanning Electron Microscopy: Tecnhiques and Applications. 1992:1st ed New York, N.Y.: Springer-Verlag; 103–115.. [Google Scholar]
- 16.National Research Council (U.S.). Committee for the update of the guide for the care and use of laboratory animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.). Guide for the Care and Use of Laboratory Animals. 2011:xxv:8th ed Washington, D.C.: National Academies Press; 220. [Google Scholar]
- 17.Başer NT, Silistreli OK, Sişman N, et al. Effects of surgical or chemical delaying procedures on the survival of proximal predicled venous island flaps: an experimental study in rats. Scand J Plast Reconstr Surg Hand Surg. 2005;39:197–203.. [DOI] [PubMed] [Google Scholar]
- 18.Chow SP, Chen DZ, Gu YD. A comparison of arterial and venous flaps. J Hand Surg Br. 1992;17:359–364.. [DOI] [PubMed] [Google Scholar]
- 19.Miles DA, Crosby NL, Clapson JB. The role of the venous system in the abdominal flap of the rat. Plast Reconstr Surg. 1997;99:2030–2033.. [DOI] [PubMed] [Google Scholar]
- 20.Mutaf M, Tasaki Y, Fujii T. Expansion of venous flaps: an experimental study in rats. Br J Plast Surg. 1998;51:393–401.. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto S, Takushima A, Okazaki M, et al. Relationship between microvascular arterial anastomotic type and area of free flap survival: comparison of end-to-end, end-to-side, and retrograde arterial anastomosis. Plast Reconstr Surg. 2008;121:1901–1908.. [DOI] [PubMed] [Google Scholar]
- 22.Parsa FD, Spira M. Evaluation of anastomotic techniques in the experimental transfer of free skin flaps. Plast Reconstr Surg. 1979;63:696–699.. [DOI] [PubMed] [Google Scholar]
- 23.Koyama T, Sugihara-Seki M, Sasajima T, et al. Swartz HM, Harrison DK, Bruley DF. Venular valves and retrograde perfusion. In: Oxygen Transport to Tissue XXXVI. 2014;1:1st ed New York, N.Y.: Springer; 317–323.. [DOI] [PubMed] [Google Scholar]
- 24.Makanya AN, Hlushchuk R, Djonov VG. Intussusceptive angiogenesis and its role in vascular morphogenesis, patterning, and remodeling. Angiogenesis. 2009;12:113–123.. [DOI] [PubMed] [Google Scholar]
- 25.Sasajima T, Kikuchi S, Ishikawa N, et al. Swartz HM, Harrison DK, Bruley DF. Skin temperature in lower hind limb subjected to distal vein arterialization in rats. In: Oxygen Transport to Tissue XXXVI. 2014;1:1st ed New York, N.Y.: Springer; 361–368.. [DOI] [PubMed] [Google Scholar]
- 26.Dunn RM, Mancoll J. Flap models in the rat: a review and reappraisal. Plast Reconstr Surg. 1992;90:319–328.. [PubMed] [Google Scholar]
- 27.Davidson JM, Yu F, Opalenik SR. Splinting strategies to overcome confounding wound contraction in experimental animal models. Adv Wound Care (New Rochelle). 2013;2:142–148.. [DOI] [PMC free article] [PubMed] [Google Scholar]


