Abstract
Background:
Ischemic complications after nipple-sparing mastectomy (NSM) have been associated with numerous variables. However, the impact of NSM flap thickness has been incompletely evaluated.
Methods:
NSM flap thickness was determined for all NSMs from 2006 to 2016 with available pre- or postoperative breast magnetic resonance imaging (MRIs). Demographics and outcomes were stratified by those with and without ischemic complications.
Results:
Of 1,037 NSM reconstructions, 420 NSMs had MRI data available, which included 379 preoperative MRIs and 60 postoperative MRIs. Average total preoperative skin/subcutaneous tissue NSM flap thickness was 11.4 mm. Average total postoperative NSM flap thickness was 8.7 mm. NSMs with ischemic complications were found to have significantly thinner overall postoperative NSM flap thickness compared with those without ischemic complications (P = 0.0280). Average overall postoperative NSM flap thickness less than 8.0 mm was found to be an independent predictor of ischemic complications (odds ratio, 6.5263; P = 0.026). In NSMs with both pre- and postoperative MRIs, the overall average postoperative NSM flap thickness was 68.2% of preoperative measurements. Average overall postoperative NSM flap thickness was significantly less than average overall preoperative NSM flap thickness (P < 0.0001). NSMs with ischemic complications were found to have a significantly lower ratio of overall postoperative to preoperative flap thickness (52.0% versus 74.0%; P < 0.0001).
Conclusions:
Ischemic complications after NSM are significantly associated with thinner postoperative NSM flap thickness. Particularly, NSM flap thickness less than 8.0 mm is a positive independent predictor of ischemic complications. The ratio of postoperative to preoperative NSM flap thickness was significantly lower in reconstructions with ischemic complications.
INTRODUCTION
Nipple-sparing mastectomy (NSM) has become the mainstay in the treatment of oncologically appropriate breast cancer as well as surgical prophylaxis for high-risk patients.1–8 Preservation of the nipple-areolar complex (NAC) and breast skin envelope has allowed plastic surgeons to optimize cosmetic results with implant-based and autologous techniques.9–14 Maintaining the viability and form of the native breast skin envelope is therefore critical in obtaining ideal reconstructive and aesthetic outcomes. Ischemic complications, including mastectomy and NAC necrosis, can be devastating, affecting overall reconstruction and patient satisfaction after NSM.15,16
Reported rates of NAC and mastectomy flap necrosis range from 4.4% to 37.5% and 2% to 12.7% in the literature, respectively.9,11,14,17–20 Established risk factors for NAC and mastectomy flap include elevated body mass index (BMI), smoking, preoperative radiation, incision placement, and mastectomy specimen weight, among others.11,21–27 However, perfusion of the NAC and breast skin in NSM, perhaps the most critical factor, remains poorly studied.
The contribution of the superficial vasculature in the subdermal and subcutaneous tissues to the NAC is paramount after NSM.28–33 Most objective assessments of perfusion in NSM have focused on quantifying lack of perfusion after mastectomy with angiography to better predict and prevent ischemic complications.34–36 Conversely, anatomic factors that influence perfusion has been relatively overlooked. Although the importance of relative mastectomy flap thickness and anatomic dissection is emphasized by plastic surgeons, a large-scale, objective evaluation of preoperative breast skin and subcutaneous tissue thickness to the level of the superficial fascia, or breast capsule, and postoperative NSM flap thickness on ischemic complications is lacking.
Breast magnetic resonance imaging (MRI) is increasingly being used as a screening, diagnostic, and monitoring tool in the treatment of breast cancer.37–41 The availability of pre- and postoperative MRI provides a unique opportunity to evaluate anatomic parameters, including preoperative skin and subcutaneous thickness to the breast capsule level, and their resultant changes after NSM. We therefore aim to utilize pre- and postoperative breast MRIs to quantify mastectomy flap thickness before and after NSM while subsequently elucidating the influence of flap thickness on ischemic complications and outcomes in breast reconstruction after NSM.
METHODS
All NSMs performed from 2006 to June 2016 at NYU Langone Medical Center were reviewed. NSMs undergoing immediate implant-based and autologous reconstruction with available preoperative and/or postoperative breast MRIs were identified. NSMs that had any fat grafting procedures were excluded. All NSMs were performed using sharp dissection with electrocautery minimized to only as needed for hemostasis. Frozen subareolar NAC biopsies were routinely utilized.
A blinded reviewer measured NSM flap thickness for pre- and postoperative MRIs utilizing PACS software on sagittal and axial images acquired using a dedicated breast coil on 1.5-Tesla and 3-Tesla magnets with T1-weighted nonfat suppressed volumetric scans and high-resolution postcontrast scans as demonstrated in Figure 1. The thickness of skin and subcutaneous tissue to the breast capsule level was measured on preoperative MRIs in these NSMs; this measurement is here forward referred to as preoperative NSM flap thickness. On postoperative MRIs, NSM flap thickness was measured from the skin to the level of the pectoralis muscle, acellular dermal matrix, implant capsule, or plane of autologous donor site tissue depending on reconstruction. Measurements were averaged from 12 different locations on each breast (Fig. 1). Three measurements were taken on superior and inferior flaps each, in the sagittal plane, and on medial and lateral flaps in the axial plane. These 3 measurements corresponded with anterior, middle, and posterior locations taken at one-quarter, one-half, and three-quarters the length of the total anteroposterior distance of each breast.
Fig. 1.
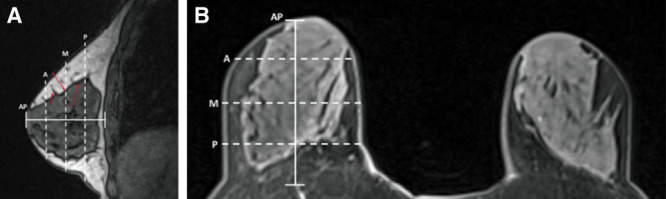
Measurement locations of anatomic breast flap thickness in a preoperative breast MRI. A, Sagittal nonfat saturated MRI. B, Axial high-resolution postcontrast MRI. A, anterior measurement at ¼ of AP distance; AP, anteroposterior distance of breast; M, middle measurement at ½ AP distance; P, posterior measurement and ¾ AP distance; short red arrow, Cooper’s ligament with breast tissue extending to dermis; long red arrow, anterior mammary fascia; double-headed red arrow, example of flap-thickness measurement at middle location.
Patient demographics, intraoperative variables, and reconstructive outcomes were collected and analyzed. Ischemic complications were defined as major and minor mastectomy flap necrosis or nipple-areola complex necrosis, defined as those managed with debridement or local wound care, respectively. Patients were grouped into 3 cohorts: those with preoperative imaging, those with postoperative imaging, and those with both pre- and postoperative imaging. Operative variables, including NSM flap thickness, were stratified by NSMs with and without ischemic complications.
Descriptive statistics and measures of central tendency were used to describe absolute and mean results. Student’s t tests were used to analyze binary data sets; chi-square analysis was used to compare proportional responses. All statistical analysis was performed using GraphPad Software, Inc. (La Jolla, CA). Univariate analysis with odds ratio calculation was utilized to identify specific NSM flap thickness as an independent risk factor for ischemic complications. P values of less than 0.05 were deemed significant.
RESULTS
A total of 1,037 NSM reconstructions were reviewed; 420 cases (243 patients) had MRI data available, which included 379 preoperative breast MRIs, 60 postoperative breast MRIs, and 19 cases with both pre- and postoperative breast MRIs. In cases with preoperative breast MRIs, average total preoperative NSM flap thickness was 11.4 mm (anterior, 6.6 mm; middle, 12.1 mm; posterior, 15.4 mm). In cases with postoperative breast MRIs, average total postoperative NSM flap thickness was 8.7 mm (anterior, 5.9 mm; middle, 8.3 mm; posterior, 11.8 mm).
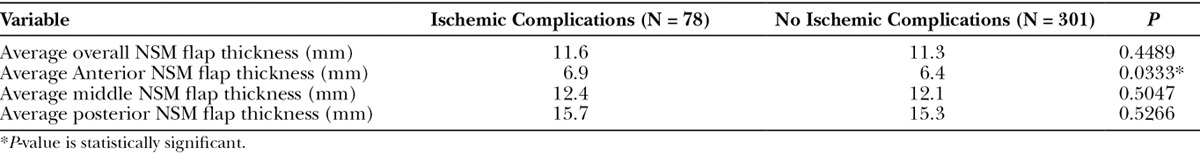
NSMs with preoperative breast MRI imaging who did and did not have ischemic postoperative complications were compared. Of the 379 NSMs with preoperative MRI imaging available, 78 (20.6%) had ischemic complications. Demographics are presented in Table 1. Preoperative NSM flap thickness based on MRI measurements were then compared between those NSMs with and without postoperative ischemic complications. NSMs with ischemic complications were found to have significantly greater average preoperative anterior NSM flap thickness (P = 0.0333; Table 2).
Table 1.
Demographics and Outcomes for NSMs with and without Ischemic Complications with Preoperative MRI Measurements Available
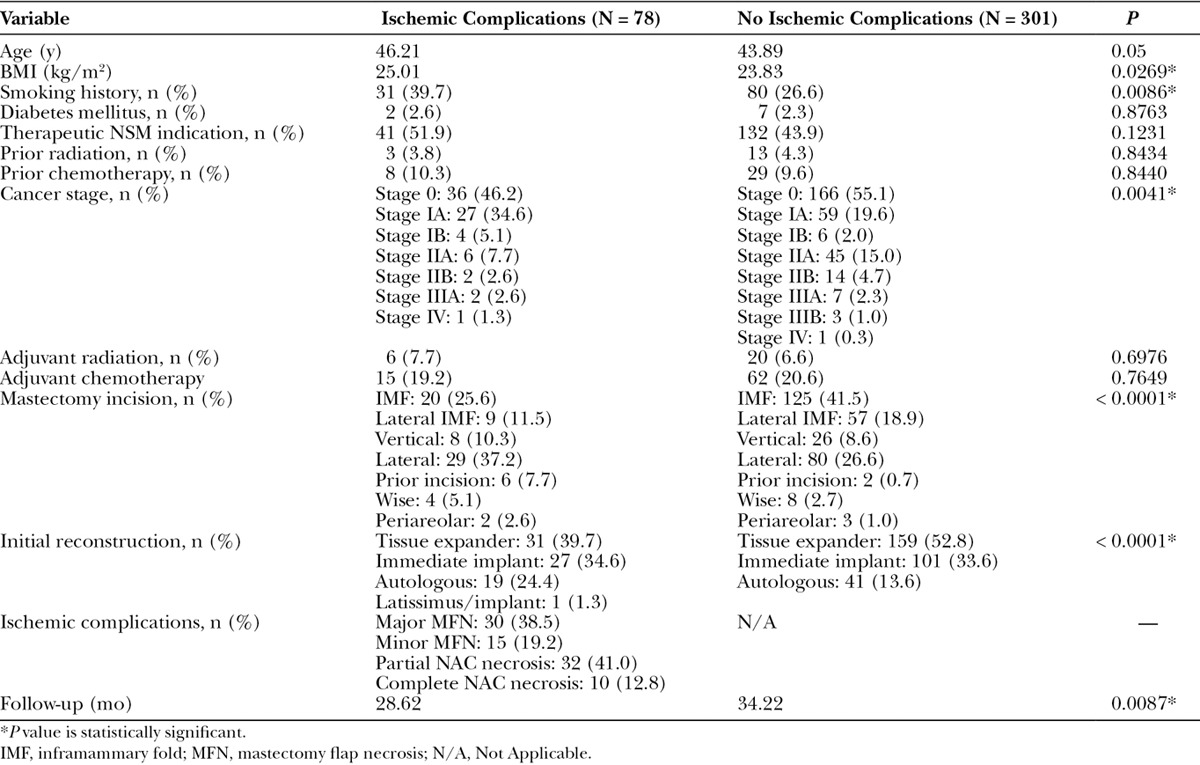
Table 2.
Average Preoperative MRI Measurements for NSMs with and without Ischemic Complications
NSMs with postoperative breast MRI imaging who did and did not have ischemic postoperative complications were then compared. Of the 60 NSMs with postoperative MRI imaging available, 10 (20.0%) had ischemic complications. Demographics are presented in Table 3. NSMs with ischemic complications were found to have significantly thinner overall (P = 0.0280) and posterior (P = 0.0208) postoperative NSM flap thickness (Table 4).
Table 3.
Demographics and Outcomes for NSMs with and without Ischemic Complications with Postoperative MRI Measurements Available
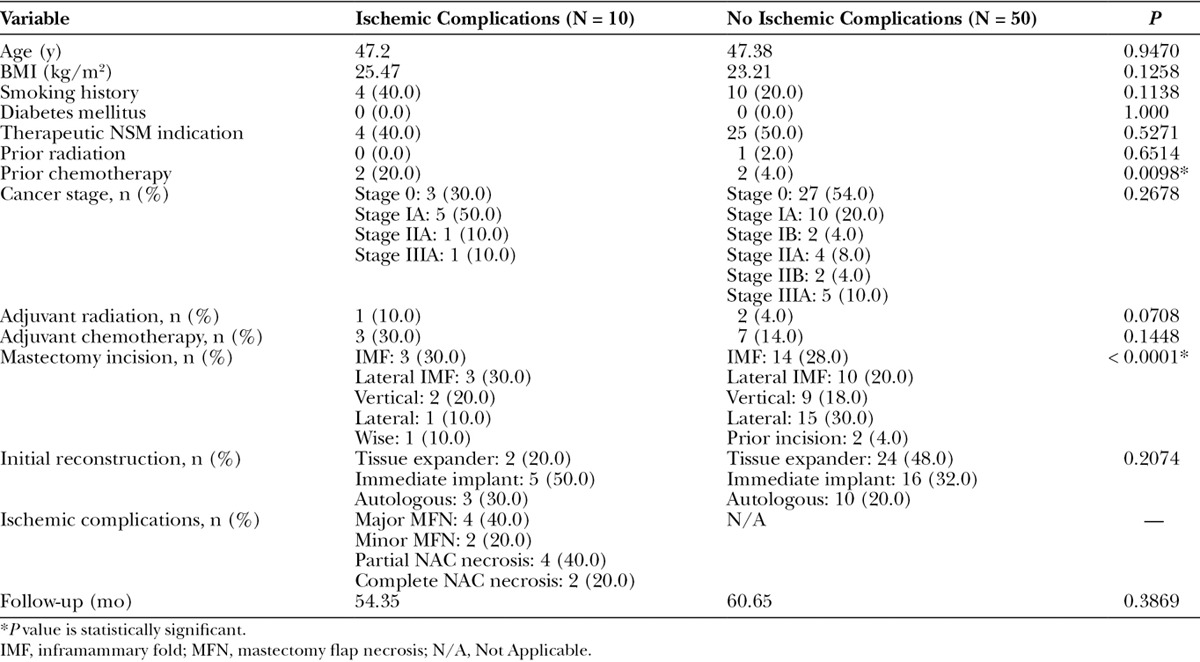
Table 4.
Average Postoperative MRI Measurements for NSMs with and without Ischemic Complications
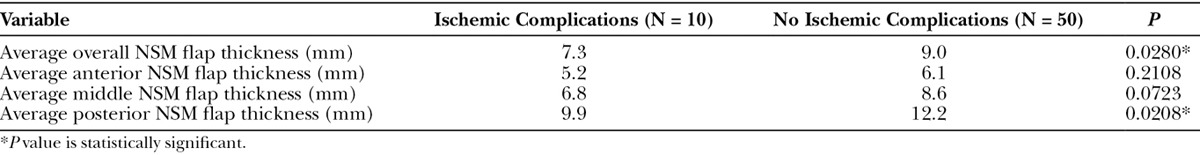
Scatter plots of overall postoperative NSM flap thickness measurements based on MRI imaging were created for NSMs with and without ischemic complications. These plots intersected at a value of approximately 8.0 mm (Fig. 2). Similarly, mean NSM flap-thickness measurements for both groups centered about a value of approximately 8.0 mm. A flap thickness of approximately 8.0 mm was thus identified as a potential threshold value for mastectomy flap thickness predicting ischemic events. Therefore, NSMs with postoperative MRI imaging were divided into those with average overall NSM flap thickness of greater than or less than 8.0 mm. Demographically, those with flap thicknesses of less than 8.0 mm were significantly more likely to undergo prior chemotherapy (P = 0.0135), have stage IA disease (P = 0.0297), undergo adjuvant chemotherapy (P = 0.0021), have an inframammary fold incision (P < 0.0001), and undergo immediate implant reconstruction (P < 0.0001). Other demographics were equivalent between the 2 groups. Ischemic complications were then compared between these 2 groups. Those with average overall postoperative NSM flap thickness less than 8.0 mm had significantly greater incidences of ischemic complications (P < 0.0001). Average overall postoperative NSM flap thickness less than 8.0 mm was also found to be an independent predictor of ischemic complications (odds ratio, 6.5263; P = 0.026).
Fig. 2.
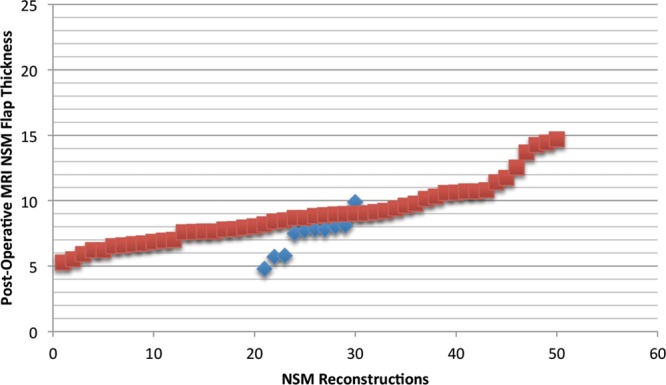
Scatter plot of average overall postoperative NSM flap thickness based on MRI imaging for those with and without ischemic complications (each red box represents an NSM with postoperative MRI data without ischemic complications; blue boxes represent NSMs with postoperative MRI data with ischemic complications).
NSMs with preoperative and postoperative breast MRIs in which both imaging studies had been performed were then compared. NSMs with both preoperative and postoperative breast MRIs (19 total cases; 15 patients) had an average age of 45.44 years, whereas average BMI was 24.16 kg/m2. Seven NSMs were in patients with a smoking history, whereas no patients had diabetes mellitus. Thirteen cases (68.4%) were for a therapeutic indication. Three cases had undergone neoadjuvant chemotherapy, whereas 1 underwent preoperative radiation. Five and 3 NSMs underwent adjuvant chemotherapy and radiation, respectively. Eight NSMs underwent tissue expander–based reconstruction, 9 underwent immediate, permanent implant reconstruction, and 2 underwent abdominally based autologous reconstruction. Average follow-up time was 42.92 months.
In these cases, average total preoperative NSM flap thickness was 11.9 mm (anterior, 7.3 mm; middle, 12.7 mm; posterior, 15.6 mm). Meanwhile, average total postoperative NSM flap thickness was 8.0 mm (anterior, 5.7 mm; middle, 7.0 mm; posterior, 11.4 mm). The overall average postoperative NSM flap thickness was 68.2% of the overall average preoperative NSM flap thickness and was significantly thinner than average overall preoperative NSM flap thickness (P < 0.0001). Moreover, average postoperative flap thickness was significantly less than average preoperative flap thickness with regard to anterior (P = 0.0154), middle (P < 0.0001), and posterior (P < 0.0001) breast planes.
In this group, 5 NSMs (26.3%) had ischemic complications: 2 incidences each of major mastectomy flap necrosis, minor mastectomy flap necrosis, and partial NAC necrosis (10.5%) with 1 incidence of complete NAC necrosis (5.3%). Cases with ischemic complications had a significant difference noted in terms of overall (13.4 versus 11.3 mm; P = 0.0422) and anterior (9.3 versus 6.6 mm; P = 0.0060) NSM flap thickness on preoperative MRIs. Based on postoperative MRIs in this group, NSMs with ischemic complications trended toward thinner flaps (7.0 versus 8.4 mm; P = 0.1144). However, NSMs with ischemic complications were found to have a significantly lower ratio of overall postoperative to preoperative flap thickness based on MRI measurements (52.0% versus 74.0%; P < 0.0001).
DISCUSSION
NSM is associated with excellent aesthetic results and oncologic outcomes that seem equivalent to traditional mastectomy techniques, such as total and skin-sparing mastectomy.1,2,6,7,10,20,42 However, reconstructive outcomes and risk factors for reconstructive complications in NSM continue to be defined.9–11,14 Smoking, obesity, periareolar mastectomy incisions, radiation, and chemotherapy, among other factors, have all been identified as risk factors for adverse outcomes, specifically ischemic complications, after NSM.9,11,19,43 Meanwhile, NSM flap quality at the time of breast extirpation and reconstruction may be considered the principal determinant of subsequent ischemic sequela.9 However, mastectomy flap thickness as related to ischemic complications has not yet been fully evaluated in NSM.
The glandular breast tissue is encapsulated by a superficial breast fascia.44 This fascia separates the breast tissue from the overlying skin and subcutaneous tissue, representing the ideal plane of dissection during a mastectomy. The superficial subcutaneous anatomy of the breast is particularly important to the perfusion of the native breast skin and NAC after mastectomy, which relies on perfusion from the subdermal plexus and perforators in the subcutaneous plane.38–40 Dissecting the breast in a plane deep to this fascia risks incomplete oncologic resection while overaggressive suprafascial dissection thins the mastectomy flap, compromising blood flow and risking mastectomy or nipple-areola complex necrosis. Prior studies on breast anatomy have suggested the variability of this subcutaneous tissue layer thickness. Larson et al.45,46 demonstrated that the dermal thickness ranged from 3.0 to 16 mm and subcutaneous tissue from 0 to 29 mm, correlating well with the preoperative tissue thickness of 2.2–34.1 mm found in this study. Disruptions in this subcutaneous plane, particularly by breast-tissue containing Cooper’s ligaments extending to the dermis should be considered and may contribute to the incidence of breast tissue observed much closer to the dermis on histological specimens. Given the variable nature of the location, thickness, and macroscopic appearance of this encapsulating breast fascia, great care must be taken intraoperatively to ensure that this proper plane is established and maintained throughout the mastectomy procedure.47
In breast reconstruction, mastectomy flap quality has long been considered a primary, and perhaps the most important, factor contributing to ischemic postoperative complications.9 This is an especially significant consideration is NSM during which the maximal breast skin envelope is preserved, including the NAC. Prior studies concerning NSM have attempted to evaluate flap thickness as a risk factor for complications.25,47 Both studies identified mastectomy flap thickness less than 5 mm as a significant risk factor for ischemic complications.25,47 However, neither study discusses the method of assessing intraoperative NSM flap thickness or the rationale for selecting 5 mm as a threshold value.25,47
MRI presents the opportunity to assess pre- and postoperative mastectomy flap thickness at multiple locations in a controlled setting. Moreover, comparisons between preoperative and postoperative measurements can be made. These represent advantages over intraoperative assessment at a single intraoperative time point as has been previously employed.25,47 Although NSM breast vascularity patterns on MRI have been studied, direct measurements of flap thickness using MRI has not yet been investigated.48 We therefore sought to fully evaluate the impact of mastectomy flap thickness on outcomes in NSM utilizing MRI.
In greater than 1,000 NSMs, over 40% had pre- or postoperative MRI data available. NSMs were over six times more likely to have undergone preoperative MRI compared with postoperative MRI. This is not surprising, given the utilization of MRI in the management of breast cancer patients. Preoperative breast MRI may be utilized in breast cancer screening or in the diagnostic work-up of known breast cancer.49,50 Postoperative MRI after surgical management of breast cancer is less commonly utilized in infrequent cases of potential residual breast tissue or in the work-up of palpable masses.51,52
Overall, the average preoperative NSM flap thickness was 11.4 mm, whereas the average postoperative flap thickness was 8.7 millimeters, or 76.3% of the preoperative thickness. Although direct comparison of these groups is precluded, given the lack of postoperative MRI data for a significant portion of NSMs, this discrepancy foreshadows the finding that the ratio of preoperative to postoperative NSM flap thickness in NSM with both pre- and postoperative MRIs was only 68.2%. These results strongly suggest that the plane of dissection during the NSM was above the level of the superficial breast fascia, on average, by greater than 30%.
To evaluate the importance of these findings, the impact of mastectomy flap thickness on ischemic complications in NSM must be established. Notably, though preoperative thickness of the subcutaneous tissue layer was variable among patients based on MRI measurements, average thickness was observed to increase predictably moving from anterior to posterior (Fig. 3). This information may be utilized to guide 3-dimensional intraoperative flap dissection. Moreover, utilizing preoperative measurements of NSM flap thickness, there was found to be no difference in overall flap thickness between those NSMs with and without ischemic complications. In fact, NSMs with ischemic complications were found to have a thicker average anterior mastectomy flap compared with NSMs with ischemic complications. Along with findings that postoperative NSM flap thickness is thinner on average than preoperative anatomic flap thickness, these results serve to further confirm that preoperative measurements are not reliable predictors of ischemic complications.
Fig. 3.
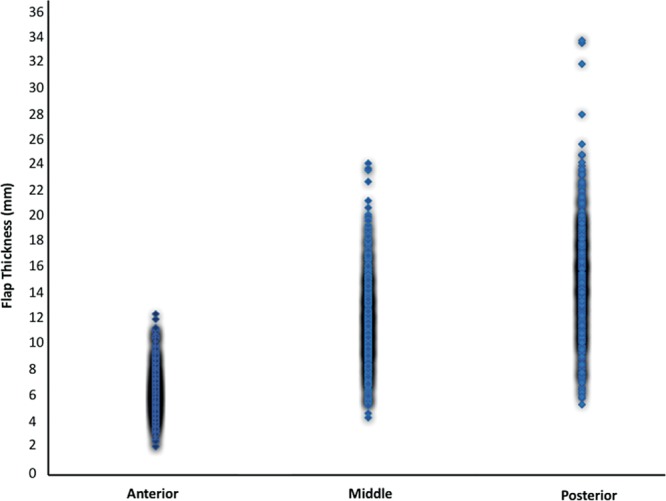
Scatter plot of preoperative thickness of breast skin and subcutaneous tissue at anterior, middle, and posterior locations showing increasing relative thickness moving from anterior to posteriorly. Density of shadowing correlates to the number of measurements at corresponding values.
When postoperative MRI data were stratified by NSMs with and without ischemic complications, those with ischemic complications were found to have significantly thinner overall mastectomy flaps. This significant findings appear to have been particularly driven by thinner anterior and poster flap thicknesses in NSMs with ischemic complications. As would be expected, postoperative mastectomy flap thickness appears to more reliably forecast ischemic outcomes in NSM.
Utilizing these significant data, a mean value for NSM flap thickness about which data clustered for both NSMs with and without ischemic complications were recognized. This threshold value was identified as approximately 8.0 mm. NSMs with flap thickness less than 8.0 mm experienced significantly more ischemic complications, whereas flap thickness less than 8.0 mm was found to be an independent risk factor for ischemic complications. It is notable that this threshold value was greater than the 5.0 mm value utilized in prior studies.25,47 Therefore, NSM flaps thicker than the previous standard value of 5.0 mm still remain prone to clinically significant mastectomy and NAC ischemia. NSM flaps should be evaluated intraoperatively in all cases. Areas of flap thickness less than 8.0 mm warrant consideration for excision, as possible. Moreover, such NSM thickness may warrant less tissue expander fill or conversion from planned immediate implant to tissue expander reconstruction in implant-based breast reconstruction as well as for banking donor-site skin in autologous breast reconstruction.53
Finally, the comparison between NSMs with both pre- and postoperative MRI data available revealed that postoperative NSM flap thickness was significantly thinner compared with preoperative anatomic breast flap thickness. This was true in the anterior, middle, and posterior breast planes and with regard to the overall averaged postoperative flap thickness, which, as discussed above, was 68.2% of the preoperative anatomic breast flap thickness. Within this group, there were no significant differences when postoperative NSM flap thickness was compared between those with and without ischemic complications. However, NSMs in this group with ischemic complications were found to have a postoperative flap thickness that was only approximately 50% as thick as the corresponding average preoperative anatomic breast flap thickness. This ratio of overall postoperative to preoperative flap thickness was significantly lower compared with the group without ischemic complications. Although each patient’s unique breast anatomy will vary, this signifies the importance, regardless of absolute mastectomy flap thickness, of dissecting the breast at the level of the superficial fascia during the mastectomy to minimize risk of ischemic complications.
Limitations of this study include its retrospective nature. Ischemic complications were defined by methods of treatment and are therefore influenced by providers’ choices in treatment. Follow-up time was also shorter in the group with preoperative MRI data available compared with the group with postoperative MRI data. This is predicted as patients who underwent postoperative MRI would be expected to have progressed further since their NSM. However, this follow-up compare favorably with the literature.9,43 Moreover, variations of physical positioning of the NAC precluded accurate measurement of the NAC thickness. Finally, mastectomy flap measurements were calculated postoperatively, given the nature of MRI timing and may therefore be influenced by postoperative skin and soft-tissue changes. Although these flap measurements primarily rely on the subcutaneous layer, there was minimal postoperative radiation in this cohort (3 cases) and multiple points of measurement were used per breast; however, these measurements still likely reflect some difference from actual intraoperative values. Correlation with intraoperative flap thickness measurements in a prospective manner is a future area of investigation.
In conclusion, utilizing MRI, thinner postoperative NSM flaps were found to be significantly associated with mastectomy flap and NAC necrosis. Postoperative NSM flap thickness less than 8.0 mm was identified as a positive independent risk factor of these ischemic complications. The ratio of overall postoperative to preoperative NSM flap thickness was significantly lower in reconstructions with ischemic complications, emphasizing the importance of dissection at the level of the superficial breast fascia during mastectomy.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Adam H, Bygdeson M, de Boniface J. The oncological safety of nipple-sparing mastectomy—a Swedish matched cohort study. Eur J Surg Oncol. 2014;40:1209–1215.. [DOI] [PubMed] [Google Scholar]
- 2.Benediktsson KP, Perbeck L. Survival in breast cancer after nipple-sparing subcutaneous mastectomy and immediate reconstruction with implants: a prospective trial with 13 years median follow-up in 216 patients. Eur J Surg Oncol. 2008;34:143–148.. [DOI] [PubMed] [Google Scholar]
- 3.Burdge EC, Yuen J, Hardee M, et al. Nipple skin-sparing mastectomy is feasible for advanced disease. Ann Surg Oncol. 2013;20:3294–3302.. [DOI] [PubMed] [Google Scholar]
- 4.Cemal Y, Albornoz CR, Disa JJ, et al. A paradigm shift in U.S. breast reconstruction: Part 2. The influence of changing mastectomy patterns on reconstructive rate and method. Plast Reconstr Surg. 2013;131:320e–326e.. [DOI] [PubMed] [Google Scholar]
- 5.Coopey SB, Tang R, Lei L, et al. Increasing eligibility for nipple-sparing mastectomy. Ann Surg Oncol. 2013;20:3218–3222.. [DOI] [PubMed] [Google Scholar]
- 6.De La Cruz L, Moody AM, Tappy EE, Blankenship SA, Hecht EM. Overall survival, disease-free survival, local recurrence, and nipple-areolar recurrence in the setting of nipple-sparing mastectomy: a meta-analysis and systematic review. Ann Surg Oncol. 2015;22:3241–3249.. [DOI] [PubMed] [Google Scholar]
- 7.Frey JD, Alperovich M, Kim JC, et al. Oncologic outcomes after nipple-sparing mastectomy: a single-institution experience. J Surg Oncol. 2016;113:8–11.. [DOI] [PubMed] [Google Scholar]
- 8.Metcalfe KA, Cil TD, Semple JL, et al. Long-term psychosocial functioning in women with bilateral prophylactic mastectomy: does preservation of the nipple-areolar complex make a difference? Ann Surg Oncol. 2015;22:3324–3330.. [DOI] [PubMed] [Google Scholar]
- 9.Choi M, Frey JD, Alperovich M, et al. “Breast in a Day”: examining single-stage immediate, permanent implant reconstruction in nipple-sparing mastectomy. Plast Reconstr Surg. 2016;138:184e–191e.. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez-Feliz J, Codner MA. Embrace the change: incorporating single-stage implant breast reconstruction into your practice. Plast Reconstr Surg. 2015;136:221–231.. [DOI] [PubMed] [Google Scholar]
- 11.Colwell AS, Tessler O, Lin AM, et al. Breast reconstruction following nipple-sparing mastectomy: predictors of complications, reconstruction outcomes, and 5-year trends. Plast Reconstr Surg. 2014;133:496–506.. [DOI] [PubMed] [Google Scholar]
- 12.Fujimoto H, Ishikawa T, Satake T, et al. Donor site selection and clinical outcomes of nipple-areola skin-sparing mastectomy with immediate autologous free flap reconstruction: a single-institution experience. Eur J Surg Oncol. 2016;42:369–375.. [DOI] [PubMed] [Google Scholar]
- 13.Levine SM, Snider C, Gerald G, et al. Buried flap reconstruction after nipple-sparing mastectomy: advancing toward single-stage breast reconstruction. Plast Reconstr Surg. 2013;132:489e–497e.. [DOI] [PubMed] [Google Scholar]
- 14.Tanna N, Broer PN, Weichman KE, et al. Microsurgical breast reconstruction for nipple-sparing mastectomy. Plast Reconstr Surg. 2013;131:139e–147e.. [DOI] [PubMed] [Google Scholar]
- 15.Andrade WN, Baxter N, Semple JL. Clinical determinants of patient satisfaction with breast reconstruction. Plast Reconstr Surg. 2001;107:46–54.. [DOI] [PubMed] [Google Scholar]
- 16.Guyomard V, Leinster S, Wilkinson M. Systematic review of studies of patients’ satisfaction with breast reconstruction after mastectomy. Breast. 2007;16:547–567.. [DOI] [PubMed] [Google Scholar]
- 17.Cordeiro PG, McCarthy CM. A single surgeon’s 12-year experience with tissue expander/implant breast reconstruction: part I. A prospective analysis of early complications. Plast Reconstr Surg. 2006;118:825–831.. [DOI] [PubMed] [Google Scholar]
- 18.Endara M, Chen D, Verma K, et al. Breast reconstruction following nipple-sparing mastectomy: a systematic review of the literature with pooled analysis. Plast Reconstr Surg. 2013;132:1043–1054.. [DOI] [PubMed] [Google Scholar]
- 19.Moyer HR, Ghazi B, Daniel JR, et al. Nipple-sparing mastectomy: technical aspects and aesthetic outcomes. Ann Plast Surg. 2012;68:446–450.. [DOI] [PubMed] [Google Scholar]
- 20.Orzalesi L, Casella D, Santi C, et al. Nipple sparing mastectomy: surgical and oncological outcomes from a national multicentric registry with 913 patients (1006 cases) over a six year period. Breast. 2016;25:75–81.. [DOI] [PubMed] [Google Scholar]
- 21.Lee TJ, Oh TS, Kim EK, et al. Risk factors of mastectomy skin flap necrosis in immediate breast reconstruction using low abdominal flaps. J Plast Surg Hand Surg. 2016;50:302–306.. [DOI] [PubMed] [Google Scholar]
- 22.Wang F, Alvarado M, Ewing C, et al. The impact of breast mass on outcomes of total skin-sparing mastectomy and immediate tissue expander-based breast reconstruction. Plast Reconstr Surg. 2015;135:672–679.. [DOI] [PubMed] [Google Scholar]
- 23.Carlson GW, Chu CK, Moyer HR, et al. Predictors of nipple ischemia after nipple sparing mastectomy. Breast J. 2014;20:69–73.. [DOI] [PubMed] [Google Scholar]
- 24.Gould DJ, Hunt KK, Liu J, et al. Impact of surgical techniques, biomaterials, and patient variables on rate of nipple necrosis after nipple-sparing mastectomy. Plast Reconstr Surg. 2013;132:330e–338e.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Algaithy ZK, Petit JY, Lohsiriwat V, et al. Nipple sparing mastectomy: can we predict the factors predisposing to necrosis? Eur J Surg Oncol. 2012;38:125–129.. [DOI] [PubMed] [Google Scholar]
- 26.Chirappapha P, Petit JY, Rietjens M, et al. Nipple sparing mastectomy: does breast morphological factor related to necrotic complications? Plast Reconstr Surg Glob Open. 2014;2:e99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reintgen C, Leavitt A, Pace E, et al. Risk factor analysis for mastectomy skin flap necrosis: implications for intraoperative vascular analysis. Ann Plast Surg. 2016;76:S336–S339.. [DOI] [PubMed] [Google Scholar]
- 28.Seitz IA, Nixon AT, Friedewald SM, et al. “NACsomes”: a new classification system of the blood supply to the nipple areola complex (NAC) based on diagnostic breast MRI exams. J Plast Reconstr Aesthet Surg. 2015;68:792–799.. [DOI] [PubMed] [Google Scholar]
- 29.Cunningham L. The anatomy of the arteries and veins of the breast. J Surg Oncol. 1977;9:71–85.. [DOI] [PubMed] [Google Scholar]
- 30.van Deventer PV. The blood supply to the nipple-areola complex of the human mammary gland. Aesthetic Plast Surg. 2004;28:393–398.. [DOI] [PubMed] [Google Scholar]
- 31.O’Dey Dm, Prescher A, Pallua N. Vascular reliability of nipple-areola complex-bearing pedicles: an anatomical microdissection study. Plast Reconstr Surg. 2007;119:1167–1177.. [DOI] [PubMed] [Google Scholar]
- 32.Başaran K, Ucar A, Guven E, et al. Ultrasonographically determined pedicled breast reduction in severe gigantomastia. Plast Reconstr Surg. 2011;128:252e–259e.. [DOI] [PubMed] [Google Scholar]
- 33.Hall-Findlay EJ, Shestak KC. Breast reduction. Plast Reconstr Surg. 2015;136:531e–544e.. [DOI] [PubMed] [Google Scholar]
- 34.Duggal CS, Madni T, Losken A. An outcome analysis of intraoperative angiography for postmastectomy breast reconstruction. Aesthet Surg J. 2014;34:61–65.. [DOI] [PubMed] [Google Scholar]
- 35.Phillips BT, Lanier ST, Conkling N, et al. Intraoperative perfusion techniques can accurately predict mastectomy skin flap necrosis in breast reconstruction: results of a prospective trial. Plast Reconstr Surg. 2012;129:778e–788e.. [DOI] [PubMed] [Google Scholar]
- 36.Kanuri A, Liu AS, Guo L. Whom should we SPY? A cost analysis of laser-assisted indocyanine green angiography in prevention of mastectomy skin flap necrosis during prosthesis-based breast reconstruction. Plast Reconstr Surg. 2014;133:448e–454e.. [DOI] [PubMed] [Google Scholar]
- 37.Stout NK, Nekhlyudov L, Li L, et al. Rapid increase in breast magnetic resonance imaging use: trends from 2000 to 2011. JAMA Intern Med. 2014;174:114–121.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuhl C. The current status of breast MR imaging. Part I. Choice of technique, image interpretation, diagnostic accuracy, and transfer to clinical practice. Radiology. 2007;244:356–378.. [DOI] [PubMed] [Google Scholar]
- 39.Kuhl CK. Current status of breast MR imaging. Part 2. Clinical applications. Radiology. 2007;244:672–691.. [DOI] [PubMed] [Google Scholar]
- 40.Ponzone R, Maggiorotto F, Carabalona S, et al. MRI and intraoperative pathology to predict nipple-areola complex (NAC) involvement in patients undergoing NAC-sparing mastectomy. Eur J Cancer. 2015;51:1882–1889.. [DOI] [PubMed] [Google Scholar]
- 41.Steen ST, Chung AP, Han SH, et al. Predicting nipple-areolar involvement using preoperative breast MRI and primary tumor characteristics. Ann Surg Oncol. 2013;20:633–639.. [DOI] [PubMed] [Google Scholar]
- 42.Didier F, Radice D, Gandini S, et al. Does nipple preservation in mastectomy improve satisfaction with cosmetic results, psychological adjustment, body image and sexuality? Breast Cancer Res Treat. 2009;118:623–633.. [DOI] [PubMed] [Google Scholar]
- 43.Frey JD, Choi M, Karp NS. The effect of neoadjuvant chemotherapy compared to adjuvant chemotherapy in healing after nipple-sparing mastectomy. Plast Reconstr Surg. 2017;139:10e–19e.. [DOI] [PubMed] [Google Scholar]
- 44.Robertson SA, Rusby JE, Cutress RI. Determinants of optimal mastectomy skin flap thickness. Br J Surg. 2014;101:899–911.. [DOI] [PubMed] [Google Scholar]
- 45.Beer GM, Varga Z, Budi S, et al. Incidence of the superficial fascia and its relevance in skin-sparing mastectomy. Cancer. 2002;94:1619–1625.. [DOI] [PubMed] [Google Scholar]
- 46.Larson DL, Basir Z, Bruce T. Is oncologic safety compatible with a predictably viable mastectomy skin flap? Plast Reconstr Surg. 2011;127:27–33.. [DOI] [PubMed] [Google Scholar]
- 47.De Vita R, Zoccali G, Buccheri EM, et al. Outcome evaluation after 2023 nipple-sparing mastectomies: our experience. Plast Reconstr Surg. 2017;139:335e–347e.. [DOI] [PubMed] [Google Scholar]
- 48.Bahl M, Pien IJ, Buretta KJ, et al. Can vascular patterns on preoperative magnetic resonance imaging help predict skin necrosis after nipple-sparing mastectomy? J Am Coll Surg. 2016;223:279–285.. [DOI] [PubMed] [Google Scholar]
- 49.Kuhl CK, Strobel K, Bieling H, et al. Supplemental breast MR imaging screening of women with average risk of breast cancer. Radiology. 2017;283:361–370.. [DOI] [PubMed] [Google Scholar]
- 50.McCray DK, Grobmyer SR, Pederson HJ. Impact of value based breast cancer care pathway implementation on pre-operative breast magnetic resonance imaging utilization. Gland Surg. 2017;6:57–63.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang Y, Du H. Role of short-term follow-up magnetic resonance imaging in the detection of post-operative residual breast cancer. Mol Clin Oncol. 2016;5:388–394.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis NE, Morley C, Lotfi P, et al. Update on imaging of the postsurgical breast. Radiographics. 2014;34:642–660.. [DOI] [PubMed] [Google Scholar]
- 53.Singh M, Carty M, Nuutila K, et al. Saved by de-epithelialization: DIEP flap dermal skin regeneration salvage after mastectomy skin flap loss. Plast Reconstr Surg Glob Open. 2015;3:e511. [DOI] [PMC free article] [PubMed] [Google Scholar]


