Abstract
Background:
The need to restore both the missing breast volume and breast surface area makes achieving excellent aesthetic outcomes in delayed breast reconstruction especially challenging. Autologous breast reconstruction can be used to achieve both goals. The aim of this study was to identify surgical maneuvers that can optimize aesthetic outcomes in delayed breast reconstruction.
Methods:
This is a retrospective review of operative and clinical records of all patients who underwent unilateral or bilateral delayed breast reconstruction with autologous tissue between April 2014 and January 2017. Three groups of delayed breast reconstruction patients were identified based on patient characteristics.
Results:
A total of 26 flaps were successfully performed in 17 patients. Key surgical maneuvers for achieving aesthetically optimal results were identified. A statistically significant difference for volume requirements was identified in cases where a delayed breast reconstruction and a contralateral immediate breast reconstruction were performed simultaneously.
Conclusions:
Optimal aesthetic results can be achieved with: (1) restoration of breast skin envelope with tissue expansion when possible, (2) optimal positioning of a small skin paddle to be later incorporated entirely into a nipple areola reconstruction when adequate breast skin surface area is present, (3) limiting the reconstructed breast mound to 2 skin tones when large area skin resurfacing is required, (4) increasing breast volume by deepithelializing, not discarding, the inferior mastectomy flap skin, (5) eccentric division of abdominal flaps when an immediate and delayed bilateral breast reconstructions are performed simultaneously; and (6) performing second-stage breast reconstruction revisions and fat grafting.
INTRODUCTION
Delayed breast reconstruction presents unique challenges beyond those that exist for immediate breast reconstruction. This is especially pronounced in cases where radiation therapy was a part of the patient’s treatment.1,2 The surgical plan must address both the absent breast volume and the deficient skin surface area.3,4 The application of autologous tissue flaps is ideally suited for these delayed breast reconstruction cases. However, the technical difficulty of operating in a scarred and radiated field, sometimes with inadequate recipient vessels, and significant breast asymmetry may narrow the surgical focus to simply achieving a living flap, with aesthetic considerations relegated to a secondary goal.5 With careful planning and an eye for detail, excellent aesthetic results for these most difficult breast reconstruction cases are achievable.
In our practice, we encounter 3 groups of patients who seek delayed breast reconstruction. Group 1 patients have adequate breast skin surface area and have completed tissue expansion or have completed implant-based reconstruction. Group 2 patients are candidates for tissue expansion but at the time of presentation lack the breast skin surface required for an aesthetically optimal result. Group 3 patients are not candidates for tissue expansion and will require breast skin resurfacing with autologous tissue.
This study reviews the author’s reconstructive algorithm, patient data, key technical points, and presents case studies for the 3 patient groups.
PATIENTS AND METHODS
Demographics
A retrospective review was conducted of 17 consecutively presenting patients who underwent 26 autologous unilateral or bilateral delayed breast reconstructions by W.D. from April 2014 to January 2017 (Table 1). Patients were divided into 3 groups based on the treatment they had received following mastectomy and available reconstructive options.
Table 1.
Delayed Breast Reconstruction Patient Data
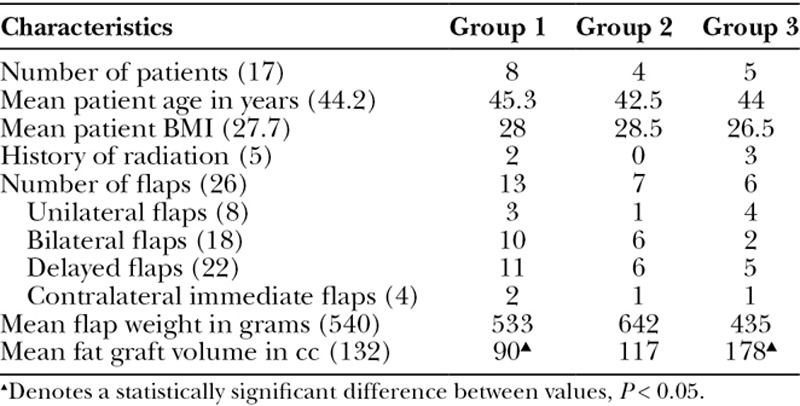
Group 1
Eight patients (13 flaps) who have completed tissue expansion or have completed implant-based reconstruction but are dissatisfied with the quality of their breast reconstruction (Fig. 1).
Fig. 1.
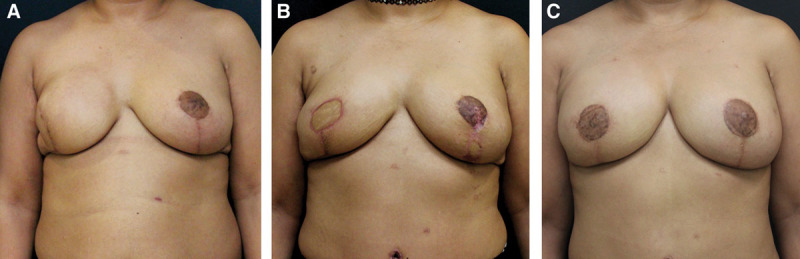
Group 1 patient. A, Patient dissatisfied with breast reconstruction following completion of right implant-based reconstruction and left breast reduction for symmetry. B, Following removal of right breast implant and left prophylactic mastectomy, and reconstruction with bilateral DIEP flaps. C, Following revision surgery and right nipple areola reconstruction and tattooing.
Group 2
Four patients (7 flaps) who lack the skin surface needed to complete an aesthetically optimal breast reconstruction, who were never treated with radiation and are candidates for tissue expansion (Fig. 2).
Fig. 2.
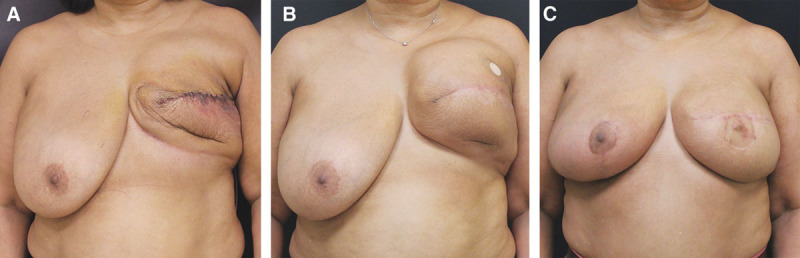
Group 2 patient. A, Patient following left mastectomy. B, Following placement and inflation of breast tissue expander to restore the breast skin surface area. C, Following delayed left breast reconstruction with a DIEP flap and right breast reduction for symmetry.
Group 3
Five patients (6 flaps) who failed implant-based reconstruction, or never underwent breast reconstruction, and were treated with radiation therapy, or cannot undergo additional expansion (Fig. 3).
Fig. 3.
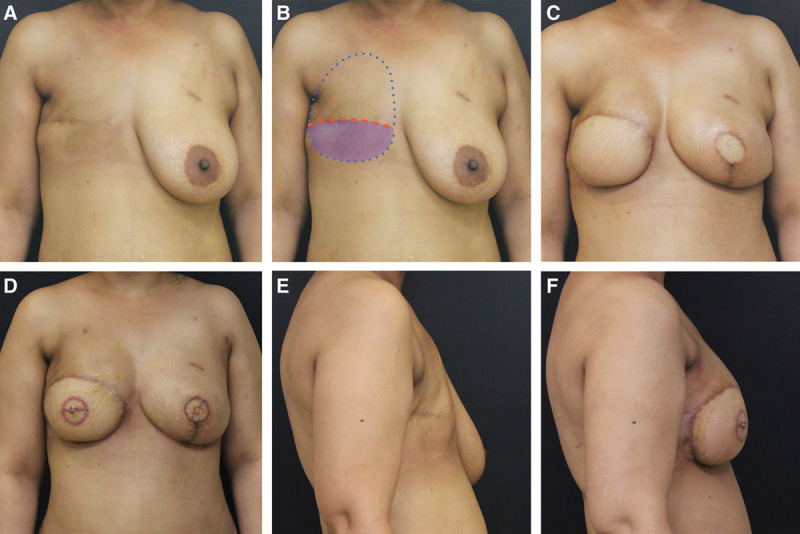
Group 3 patient. A, Patient following mastectomy and radiation therapy and no breast reconstruction. B, The breast footprint is marked. The upper mastectomy flap is elevated, and the lower mastectomy flap is deepithelialized to preserve maximum volume for the breast reconstruction. C, Following delayed right breast reconstruction with DIEP flap and left mastectomy and immediate breast reconstruction with DIEP flap. Although the abdominal tissue was divided eccentrically to achieve greater symmetry, some asymmetry persists. D, Following bilateral nipple reconstruction and fat grafting to further improve breast symmetry. E, F, Lateral photographs demonstrate breast projection optimized with preservation of lower mastectomy flap volume, eccentric distribution of DIEP flap tissues, and fat grafting.
Eight patients had unilateral breast reconstruction and 9 had bilateral breast reconstruction. Additionally, 4 patients spanning all 3 groups underwent unilateral immediate breast reconstruction contralateral to the delayed breast reconstruction. Data regarding patient characteristics, treatment history, details of surgical interventions, and photographs were collected.
Breast Reconstruction Technique
All patients reviewed in the study underwent autologous breast reconstruction with Deep Inferior Epigastric Perforator (DIEP; 24) or Profunda Artery Perforator (PAP; 2) flaps. PAP flaps were chosen for 1 patient who was slender and had an inadequate abdominal donor site for a bilateral breast reconstruction. Thirteen patients underwent second-stage revision surgery, which involved fat grafting, adjustment of the skin envelope, nipple reconstruction, and donor-site revisions. Nipple reconstruction was performed using the CV flap technique. Fat harvest was performed using tumescent technique with a 4-mm basket cannula without power assistance. Fat was processed with the aid of the Revolve system and aliquoted into 10 cc syringes. Adherent scars, such as those which frequently occur in the area of the axilla were released by subcision using an 18-gauge hypodermic needle. Fat injection was performed with a high number of cannula passes to limit fat clumping using 18 gauge single port microcannulas.
Statistical Analysis
A two-tailed student t test was used to compare flap weight and fat graft volume differences between groups and between breasts in bilateral breast reconstruction cases. A value of P < 0.05 was used to determine statistical significance.
RESULTS
Seventeen consecutively presenting patients who underwent unilateral or bilateral delayed breast reconstructions with 26 free flaps were included in the study. The average patient age was 44.2 years old (range, 25–66). The original reason to undergo mastectomy was invasive breast cancer (9 patients) and ductal carcinoma in situ (8 patients). The mean flap weight for all patients was 540 g (range, 342–944 g). There was no significant difference in flap weights between the 3 groups.
Four patients in the group underwent contralateral mastectomy and immediate breast reconstruction at the time of their delayed reconstruction. The mean difference in flap weight between contralateral breasts in the 4 patients who underwent immediate and delayed breast reconstruction was 60 g (range, 20–79 g). In all cases, the heavier flap was placed on the delayed side.
The mean tissue expander fill volume was 523 cc (range, 330–780 cc) before performing delayed autologous reconstruction. The mean fat graft volume was 132 g (range, 70–240 g) per breast; group 3 patients required a larger mean volume of fat graft than group 1 and group 2 patients (Table 1). The mean difference in fat graft volume between contralateral breasts in the patients who simultaneously underwent immediate and delayed breast reconstruction was 175 g (range, 130–220 g); this difference was statistically significant (Table 2).
Table 2.
Bilateral Breast Reconstruction Data for Delayed-Immediate Versus Delayed-Delayed Combination Reconstructions
No flaps were lost; complications were limited to minor wound healing complications, which occurred in 3 patients and did not require return to the operating room, and 1 hospital readmission for cellulitis.
Thirteen patients underwent a second-stage breast reconstruction revision, which included nipple reconstruction, fat grafting, and modifications of the skin envelope, as well as modification of the donor sites. A mean period of 15 weeks (range, 12–28 weeks) elapsed between the original delayed breast reconstruction and the second-stage reconstruction revision.
DISCUSSION
Group 1 Patients: Adequate Breast Skin Surface Area
Patients who have completed tissue expansion or completed implant-based reconstruction either with or without radiation therapy but are dissatisfied with the quality of their breast reconstruction represent a common scenario.1 Frequently, these patients complain about an unnatural appearance or feel of their breasts and breast pain, which is secondary to capsular contracture, or deformity related to pectoralis muscle animation or implant malposition.
In these patients, there is sufficient skin surface to perform delayed autologous reconstruction without the need for resurfacing the breast with additional skin from the flap. Existing mastectomy scars are opened to recreate the mastectomy defect. The filled tissue expander or breast implant and its capsule are removed, and the pectoralis muscle is replaced to its native position. The ideal breast footprint is recreated by modifying the mastectomy pocket with tissue undermining or suturing. A tissue flap is placed and secured into the mastectomy pocket as in immediate breast reconstruction. The ideal nipple location is identified on the breast surface, and a small slightly elliptical skin paddle is externalized in this location for monitoring (Fig. 1B right breast). The skin paddle is later used to complete a nipple reconstruction. In cases where the native nipple was preserved during the mastectomy, the skin paddle is externalized as a small ellipse along the access incision (Fig. 1B left breast) and removed in the subsequent stage.
Group 2 Patients: Amenable to Breast Skin Expansion
Patients who failed implant-based reconstruction, or never underwent breast reconstruction, and were never treated with radiation therapy may be candidates for expansion of their skin envelope before undergoing definitive reconstruction with autologous tissue (Fig. 2).6 When a tissue expander is already present in a subpectoral plane, then the skin envelope is simply expanded. If the patient does not have a tissue expander in place, but the skin envelope is amenable to expansion, then the tissue expander is placed in a subcutaneous plane. This scenario requires a commitment from the patient to undergo an additional surgical step of placing a tissue expander and a time delay for expansion. This additional surgical step obviates the need for a large skin paddle, which limits the scars to the original mastectomy scar, and significantly improves the aesthetic result of the final reconstruction. As in group 1 patients, the flap skin paddle is externalized for monitoring in an ideal nipple position or along one of the mastectomy incisions.
Group 3 Patients: Require Breast Skin Resurfacing
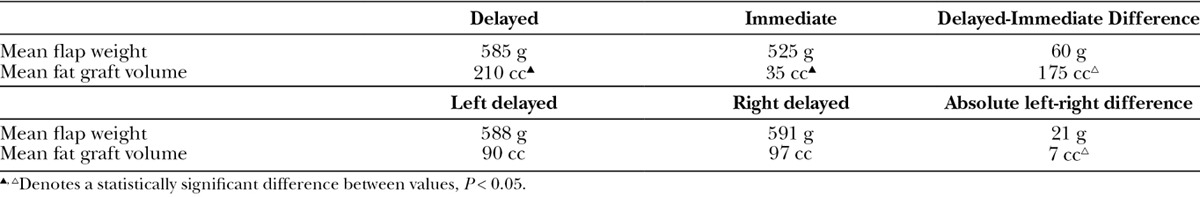
Patients who failed implant-based reconstruction, or never underwent breast reconstruction, and were treated with radiation therapy or cannot undergo expansion represent the greatest challenge to achieving an excellent aesthetic result. In all instances, there is a paucity of not only breast volume but also breast surface area, which requires the transfer of a significant amount of flap skin. Skin paddle design is paramount to optimizing aesthetic results. Optimal results are obtained when the flap skin replaces the entire skin of the lower breast resulting in a 2 tone breast mound (superior mastectomy skin and flap skin). The alternative is to use a smaller elliptical skin paddle placed between the upper and lower mastectomy flaps. Although this approach may restore the breast skin surface area, it results in a less attractive 3 tone breast mound (inferior mastectomy skin, flap skin, and superior mastectomy skin; Fig. 4).
Fig. 4.
A delayed breast reconstruction with a suboptimal aesthetic result. The flap skin restores the breast skin surface area but creates a less aesthetic 3 tone breast.
It is the author’s preference to resurface the lower pole of the breast with flap skin (Fig. 3D). The ideal breast footprint is marked preoperatively (Fig. 3B); the upper mastectomy flap is elevated to recreate the mastectomy defect and provide access to the internal mammary recipient vessels. The lower mastectomy flap is deepithelialized, not discarded, so that the additional volume of the lower mastectomy flap remains to simulate the appearance of natural ptosis and maximize volume (Fig. 3E, F). This is especially useful in bilateral reconstructions where options to maximize volume are limited. Additionally, in the event of a flap failure, the preservation of this tissue may facilitate wound management. Whether burying a mastectomy skin flap under a breast reconstruction will affect the ability to detect future breast cancer recurrences remains to be seen.7,8 Every patient is aware of this limitation as part of their informed consent.
Furthermore, group 3 patients require a greater volume of fat graft at the time of revision surgery compared with the 2 other patient groups. This difference achieved statistical significance when compared with group 1 patients.
Bilateral Breast Reconstruction
Cases of bilateral breast reconstruction, in which one of the breasts is reconstructed in a delayed fashion and the other breast is reconstructed immediately after a mastectomy, represent the most challenging and interesting reconstructions in this series. Achieving breast symmetry in these cases requires consideration. Unlike a bilateral breast reconstruction performed in the immediate setting or a bilateral delayed breast reconstruction where the abdominal tissue can be divided in the midline to achieve a symmetric result, delayed breast reconstruction performed at the same time as an immediate breast reconstruction requires asymmetric tissue distribution. In this series, the delayed flap was on average larger by 60 g and required a mean of 175 cc of fat graft to achieve breast symmetry. This translates to an additional 200–300 cc of tissue on the delayed side to achieve symmetry. Future flap designs will anticipate these volume requirements to decrease reliance on large volume fat grafting, which may be less reliable long term because of fat graft resorption. In a typical DIEP flap, which may be 15 cm tall and 3 cm thick, the line of division between the left and right flap needs to be shifted 2–3 cm to account for the different volume requirements between breasts. Options for asymmetric tissue distribution may be limited when both flaps are based on medial row perforators. In those cases, symmetry will be achieved during the secondary procedure with excision, liposuction, or fat grafting.
Flap Monitoring
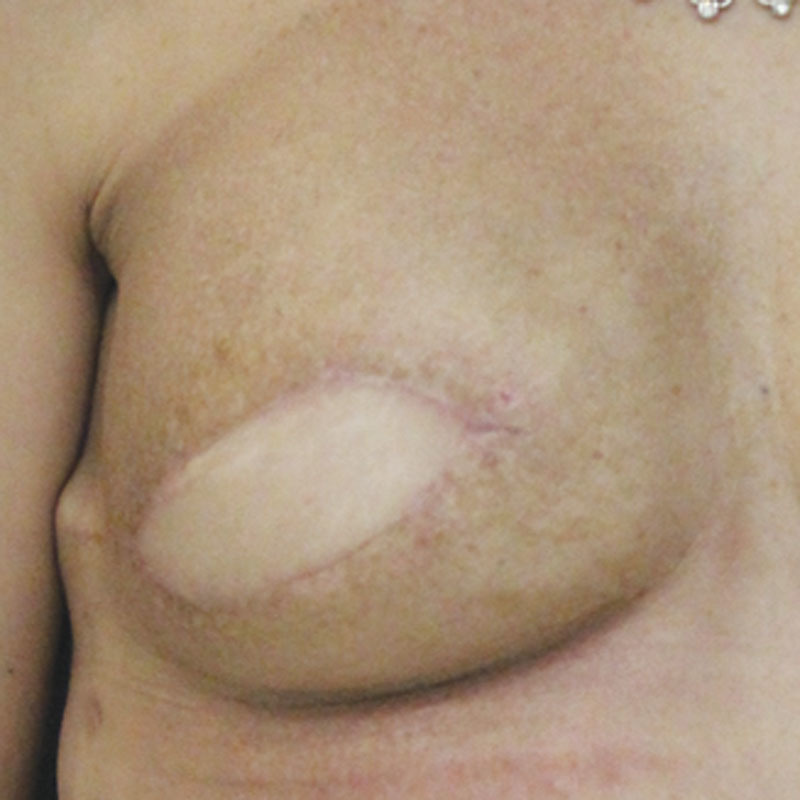
It is the author’s preference to monitor the flap with a skin paddle. In group 3 patients, this is simple to accomplish because of the large skin surface area required to complete the reconstruction. In cases where the native breast skin envelope is preserved, a small skin paddle is designed to be later incorporated into a nipple areola reconstruction. In cases where no Doppler signal is captured over the externalized portion of the flap, an implantable Doppler is used to monitor the arterial portion of the flap, and the flap skin paddle color is monitored to assess the venous outflow. This is preferred to leaving an excessively large or poorly positioned skin paddle to capture a skin perforator signal. In patients with an adequate breast skin surface area, the skin paddle should fall entirely within the area of the tattooed areola at the completion of the reconstruction (Fig. 5C).
Fig. 5.
A, DIEP flap elliptical skin paddle design in anticipation of nipple reconstruction. B, Round flap skin paddle following nipple reconstruction. C, All scars are covered following nipple areola tattooing.
Nipple Reconstruction and Additional Procedures
A second-stage breast reconstruction revision operation is recommended to all patients to be performed at the time of the nipple reconstruction to optimize aesthetic outcomes. Nipple reconstruction is performed by utilizing CV flaps. Even when the original DIEP flap skin paddle is designed to be slightly elliptical to account for the skin that will be used for the nipple reconstruction, some distortion of the skin paddle perimeter results in less than a perfectly round areola (Fig. 5B). As long as the final skin paddle is smaller than the eventual areola, this deformity can be concealed within the tattoo (Fig. 5C). In cases of a large skin paddle, as in group 3 patients, it is the author’s preference to incise a 38–42 mm areola perimeter at the time of the nipple reconstruction. This results in an immediate illusion of the entire nipple areola complex and creates a guide for future tattooing (Fig. 3D). The eventual tattoo overlaps the scars from this step resulting in a scarless appearance of the reconstructed nipple areola (Fig. 5C).
Fat grafting is commonly utilized at the time of the secondary procedure. Fat graft is used to disguise surface contour irregularities, which commonly occur along the perimeter of the flap. In the case of a significant breast asymmetry, fat graft is placed diffusely into the breast flap to increase its volume. Typically 50–100 cc of fat is used to camouflage irregularities and 200 cc or more to augment the volume of a breast to correct an asymmetry. The abdominal donor site frequently benefits by obtaining the fat graft from areas of excessive fullness.
Future Directions
It is currently the author’s practice to externalize a skin paddle for monitoring. Even in cases where no arterial signal is present over the skin paddle, it provides valuable information about the venous drainage of the flap. Some surgeons prefer to monitor breast flaps with an implantable Doppler only, which obviates the need for a skin island and may further optimize the aesthetics of the eventual nipple reconstruction.
The author utilizes stacked DIEP flaps for immediate unilateral or stacked DIEP/PAP flaps for immediate bilateral reconstructions in slender patients. None of the patients included in this study underwent a stacked flap procedure, but a stacked DIEP flap can restore a significant amount of breast surface area and would be ideal for unilateral delayed breast reconstruction in a slender patient with a large contralateral breast.
Predicting volume discrepancies between 2 breasts can be challenging. The use of 3D imaging and volume subtraction can help guide eccentric flap volume distribution and fat grafting decisions.9
CONCLUSIONS
Aesthetic outcomes of delayed breast reconstruction can be optimized with several key surgical maneuvers including:
Restoration of breast skin envelope with tissue expansion when possible.
Optimal positioning of a small skin paddle to be later incorporated entirely into a nipple areola reconstruction when adequate breast skin surface area is present.
Limiting the reconstructed breast mound to 2 skin tones when a large area skin resurfacing is required.
Increasing breast volume by deepithelializing, not discarding, the inferior mastectomy flap skin.
Eccentric division of abdominal flaps when an immediate and delayed bilateral breast reconstruction are performed simultaneously.
Performing second-stage breast reconstruction revisions and fat grafting.
Footnotes
Disclosure: The author has no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the author.
REFERENCES
- 1.Kronowitz SJ, Hunt KK, Kuerer HM, et al. Delayed-immediate breast reconstruction. Plast Reconstr Surg. 2004;113:1617–1628.. [DOI] [PubMed] [Google Scholar]
- 2.Kraemer O, Andersen M, Siim E. Breast reconstruction and tissue expansion in irradiated versus not irradiated women after mastectomy. Scand J Plast Reconstr Surg Hand Surg. 1996;30:201–206.. [DOI] [PubMed] [Google Scholar]
- 3.Albino FP, Patel KM, Smith JR, et al. Delayed versus delayed-immediate autologous breast reconstruction: a blinded evaluation of aesthetic outcomes. Arch Plast Surg. 2014;41:264–270.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ribuffo D, Vaia N, Petrianni GM. Comparison of delayed and immediate tissue expander breast reconstruction in the setting of postmastectomy radiation therapy. Ann Plast Surg. 2016;76:743–744.. [DOI] [PubMed] [Google Scholar]
- 5.Baumann DP, Crosby MA, Selber JC, et al. Optimal timing of delayed free lower abdominal flap breast reconstruction after postmastectomy radiation therapy. Plast Reconstr Surg. 2011;127:1100–1106.. [DOI] [PubMed] [Google Scholar]
- 6.Tadiparthi S, Alrawi M, Collis N. Two-stage delayed breast reconstruction with an expander and free abdominal tissue transfer: outcomes of 65 consecutive cases by a single surgeon. J Plast Reconstr Aesthet Surg. 2011;64:1608–1612.. [DOI] [PubMed] [Google Scholar]
- 7.Lindford AJ, Siponen ET, Jahkola TA, et al. Effect of delayed autologous breast reconstruction on breast cancer recurrence and survival. World J Surg. 2013;37:2872–2882.. [DOI] [PubMed] [Google Scholar]
- 8.Isern AE, Manjer J, Malina J, et al. Risk of recurrence following delayed large flap reconstruction after mastectomy for breast cancer. Br J Surg. 2011;98:659–666.. [DOI] [PubMed] [Google Scholar]
- 9.Tepper OM, Small K, Rudolph L, et al. Virtual 3-dimensional modeling as a valuable adjunct to aesthetic and reconstructive breast surgery. Am J Surg. 2006;192:548–551.. [DOI] [PubMed] [Google Scholar]


