Abstract
Background:
The optimum number of microvascular anastomoses for safe free tissue transfer is controversial. Although the case for 2 venous anastomoses versus 1 anastomosis has been argued, the use of an additional arterial anastomosis has not been examined in detail.
Methods:
Twelve patients who underwent 2 arterial anastomoses for a free flap transfer were identified retrospectively from the medical records of patients undergoing reconstruction for head and neck cancer. The free flaps were limited to anterolateral thigh (ALT) flaps.
Results:
All flaps survived. Complications included venous thrombosis (n = 1), reexploration (n = 1), and leakage (n = 3). The vascular patterns of dual-arterialized ALT flaps were classified into 3 groups. Types 1 and 2 were ALT flaps that had 2 vascular sources from the descending and lateral branches of the lateral circumflex femoral artery. The number of accompanying veins differed between type 1 (3 veins) and type 2 (2 veins). Type 3 differed from a conventional ALT flap nourished by the descending branch of the lateral circumflex femoral artery (1 vein) by the addition of anastomosis of an artery branching from the descending branch to the vastus medialis muscle. The total operation times for these 3 types of ALT were similar.
Conclusions:
An additional arterial anastomosis to the free cutaneous flap did not cause any congestion or disturb the balance between inflow and outflow. If the surgeon considers that the first arterial anastomosis is unreliable, an additional anastomosis might be an option in ALT transfer.
Transfer of a free flap should ideally be safe and reliable, but these flaps sometimes fail. An additional vascular anastomosis is often performed to minimize vascular complications such as thrombosis, congestion, or partial necrosis. It is controversial whether the use of 2 vascular anastomoses is superior to 1 anastomosis in terms of safe flap transfer.1–3 In theory, although 2 anastomoses are considered to be superior, thrombosis may occur in areas away from the vascular anastomosis. The occurrence of thrombosis may be influenced by external factors influencing the patient’s status and by intrinsic factors. We usually perform 2 vascular anastomoses when vessels other than 1 artery/vein pair are exposed in the surgical field without the need for any invasive undermining procedures. An additional anastomosis generally means a venous anastomosis; however, we have encountered cases where the additional anastomosis was arterial. The aim of this study was to propose the outcomes in a double-pedicled free flap for reconstruction of head and neck defects. In general, the success rate of a free flap transfer is reported over 95%1; however, it is very difficult to obtain 100% success. This procedure may provide an added benefit particularly to the more challenging cases such as the radiated, scarred neck or prior flap loss or fistula salvage.
MATERIALS AND METHODS
We retrospectively surveyed the patients who underwent free flap transfers in our hospital after ablative head and neck cancer surgery from 2012 to 2014. The main reconstructive surgeon was also the plastic surgeon (first author T.N.). Among these cases, we identified those in which more than 1 arterial anastomosis was performed. We analyzed the postoperative courses of these cases for factors including cutaneous flap color, free flap survival, thrombosis, ischemia, congestion, and leakage, together with the patient’s status. All the free flaps were anterolateral thigh (ALT) flaps.
RESULTS
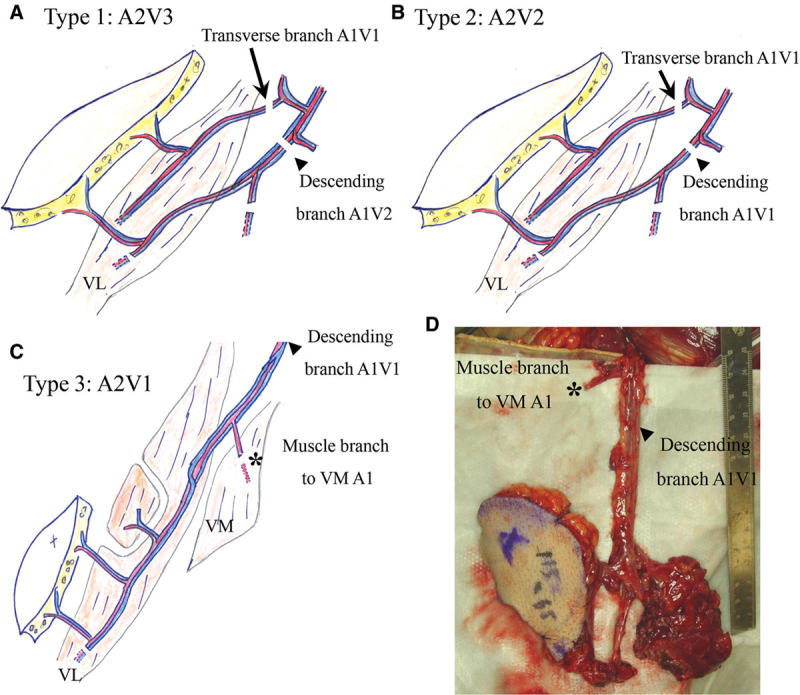
Twelve patients were included in this study. Their demographic data and outcomes are shown in Table 1. The patterns of vascular anastomoses were classified into 3 groups. In type 1, the ALT flap was nourished by 2 vascular systems, the descending branch (with an artery and 2 accompanying veins) and the transverse branch (with an artery and an accompanying vein) from the lateral circumflex femoral artery (LCFA) system (Fig. 1). In type 2, the nourishing system was similar to that of type 1, but the vessels from the descending branch included an artery and a single accompanying vein. In type 3, the nourishing vessels came only from the descending branch of the LCFA system and included an artery and an accompanying vein directly from the descending branch, and 1 branch artery to the vastus medialis muscle from the descending artery itself. This arterial branch was anastomosed in a retrograde flow fashion. Thus, 2 arteries and 1 vein were anastomosed. The breakdown of the multiple anastomoses were 2 arteries, 3 veins (n = 2, type 1); 2 arteries, 2 veins (n = 4, type 2); and 2 arteries, 1 vein (n = 6, type 3). The skin color of the type 3 flaps is shown in Figure 2.
Table 1.
Demographic Data and Outcomes of Patients
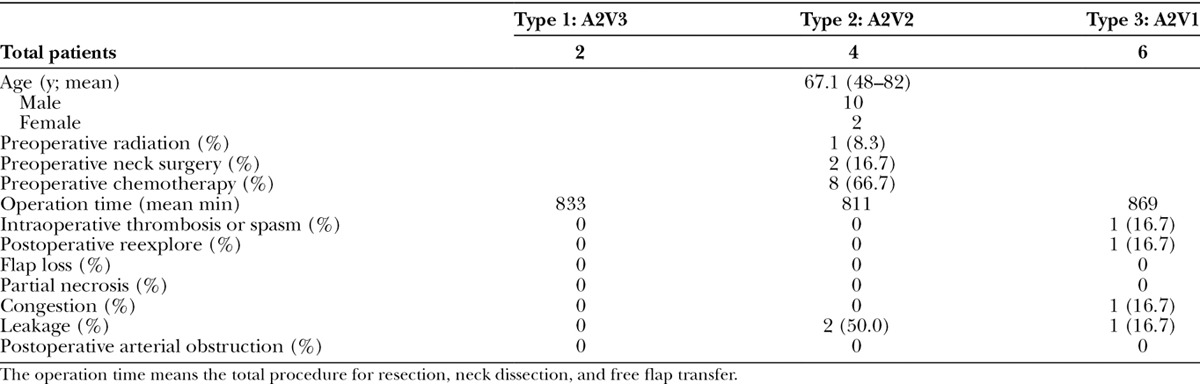
Fig. 1.
The types of vascular pattern of the ALT flaps. A, The ALT flap has 2 nourishing sources, the transverse and descending branches from the LCFA. In type 1, the transverse branch artery has 1 associated vein (A1V1) and the descending branch has 2 veins (A1V2). Both nourishing sources are used for anastomoses, meaning that overall, 2 arteries and 3 veins are used (A2V3). B, Type 2 is similar to type 1 except that the descending branch artery has only 1 associated vein (A1V1). Therefore, in total, 2 arteries and 2 veins are anastomosed (A2V2). C, In type 3, the ALT flap is nourished only by the descending branch artery and associated vein (A1V1). The branch from the descending branch artery to the vastus medialis muscle is usually sacrificed during the raising of the ALT flap, but in type 3 it is harvested as an additional donor artery (A1). In total, 2 arteries and 1 vein are anastomosed (A2V1). The blood flow of the branch artery originally comes from the descending branch; however, after anastomosis in a retrograde fashion, the arterial inflow from the cervical recipient artery enters the descending branch. D, An actual type 3 ALT flap. The small muscle branch (*) is used as the second arterial anastomosis. Arrow: the transverse branch from the LCFA is divided. Arrowhead: the descending branch from the LCFA is divided. VL, vastus lateralis muscle; VM, vastus medialis muscle.
Fig. 2.
The immediate postoperative cutaneous colors of all 6 type 3 flaps (A-F). Type 3 has 2 arteries and 1 vein for circulation. None of the flaps showed congestion.
All flaps survived. Congestion and postoperative venous thrombosis were seen in one case (type 3) at postoperative day 1. Except for this case, no flaps showed congestion or ischemia. Other complications included intraoperative arterial spasm (n = 1) and leakage (n = 3). The operation time was similar for the 3 types. No statistical analysis was performed because of the small number of cases.
CASE REPORT (CASE 6)
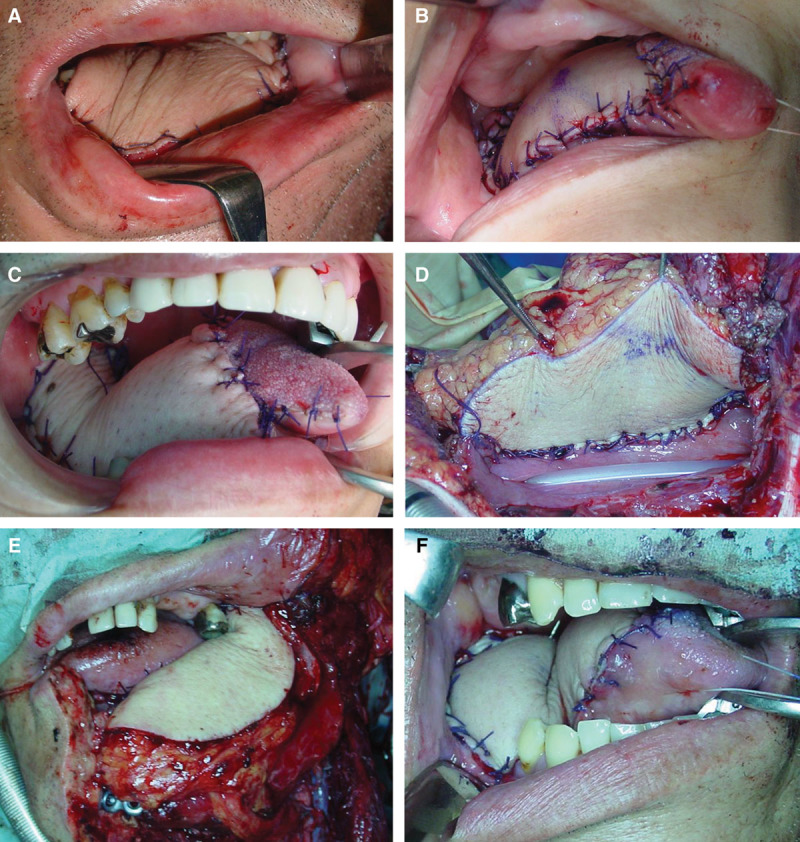
The patient was a 53-year-old man who had T4N2b lingual cancer but no other significant medical history. After the excision of the lingual cancer and neck dissection, reconstruction with an ALT flap was performed. The ALT flap was raised with 2 arteries and 1 vein (type 3). The arteries were anastomosed to the right superior thyroid artery and the lingual artery. The vein was anastomosed to the right internal jugular vein. The flap did not show any signs of congestion during the operation (Fig. 3). On postoperative day 1, the flap showed congestion, so we revised the venous anastomosis and removed a venous thrombosis from the recipient vein. After the revision surgery, the flap showed no congestion and survived. The patient’s postoperative course was uneventful except for this revision.
Fig. 3.
The time course of the events for case 6. After the operation, the flap did not show congestion (A). However, congestive color developed on postoperative day 1 (B). Immediate revision surgery was performed, and a venous thrombosis from the recipient vein to the internal jugular vein was revealed (D). After removing the thrombosis, reanastomosis was performed (E). After the revision surgery, the flap color became pink and healthy (C). Arrow: the recipient vein that was anastomosed to the right internal jugular vein (in an end-to-side fashion) showed sign of thrombosis. After the reanastomosis, the flap color was improved.
DISCUSSION
An alternative procedure for free flap transfer is to perform vascular anastomoses in 2 areas. The purpose of anastomosing vessels in addition to 1 artery and 1 vein is to perform free flap transfer more safely by reducing the occurrence of flap failure caused by thrombosis or congestion and to enlarge the perfused area. Deleyiannis et al.4 reported, if the defect size is too large for covering with using a single perforator ALT flap, additional supercharging anastomoses are used successfully in 5 patients.
In general, 2 vascular anastomoses refer to 2 venous anastomoses. Both the (superficial) cephalic vein system and the radial (deep) vein systems are often anastomosed in radial forearm flap transfers.5 The superficial inferior epigastric vein is sometimes anastomosed in addition to the deep inferior epigastric vein in a free deep inferior epigastric perforator (DIEP) flap transfer. These 2 veins are anastomosed because the cutaneous area of the transferred flap is usually irrigated by both venous systems and sometimes the flap becomes congested when only 1 venous system is anastomosed. If the irrigation area of a single venous system does not include the whole area of the flap, another venous system will provide rescue to avoid congestion or partial necrosis. Thus, the question for this type of anastomosis is whether the cutaneous area of the flap is irrigated or compensated by only 1 venous system. Two vascular anastomoses function as a safety valve for any error in estimating the area irrigated by anastomosis of a single venous system. However, these considerations do not address the question of whether 2 vascular anastomoses are better for avoiding free flap necrosis caused by thrombosis at the anastomosis site.
Another consideration is whether both veins should be anastomosed when 2 associated veins are available. If 2 associated veins that irrigate the same area are available, and there are also 2 recipient veins that can be anastomosed to the donor veins, should we anastomose both or only a single vein?
Hanasono et al.1 recommended a single vein anastomosis rather than 2 because venous stasis was better with a single venous anastomosis. They considered that blood flow velocity and stasis were more important than the number of anastomoses.
However, reports describing 2 arterial anastomoses are scarce,6 and although arterial supercharging of a pedicled jejunal transfer to the neck or free jejunum flap has been reported,7 its use is rare in cutaneous flap transfer. Sekido8 reported that arterial supercharge is more important than venous superdrainage for pedicled jejunal transfer in animals. In contrast, Ueda et al.7 stressed that both artery and vein are important in clinical cases. It has been reported that if arterial blood flow is increased in a free cutaneous flap transfer, the nourished area of the flap is extended.9 Zheng et al.10 reported that venous additional superdrainage is superior in terms of survived flap territory than arterial supercharge in the rat model. Arterial supercharge was better than the control group.10 Arata et al.11 recommended the use of multiple arterial and venous anastomoses if possible when reattaching a crushed fingertip, to increase the patency rate, because vascular anastomoses in a damaged fingertip are technically demanding.
In our study, we performed additional anastomoses only when the additional donor and recipient vessels were already exposed during the routine excision of the malignant tumor and neck dissection. Additional dissection around the exposed vessels was not performed to reduce the invasiveness of the procedure and to save time, because the purpose of the additional arterial anastomosis was to perform the least invasive free flap transfer safely. This was also true for the additional venous anastomoses, that is, we performed additional venous anastomoses only when there was more than 1 accompanying vein and when the same number of recipient site veins were already exposed. Thus, in this series of patients, 3 venous anastomoses were performed if 3 veins were available, whereas if only 1 accompanying vein was exposed, only 1 vein was anastomosed.
When the harvested cutaneous flap had multiple arteries and veins, and when the same number of vessels were exposed in the recipient site, we placed the flap and anastomosed all these vessels during reconstruction. If an additional invasive procedure was necessary to perform multiple anastomoses, we did not perform them because of our policy of minimally invasive surgery. Therefore, there is a possible selection bias in this study.
We classified the flaps into 3 groups according to the number of the vessels anastomosed, because type 1 and 2 have essentially different meaning from type 3 in terms of a style of back-up anastomoses.
In the type 1 and type 2 cases with 2 arterial anastomoses, the ALT flaps are nourished by both the transverse and descending branches of the LCFA; therefore, these cases are considered to be similar to the double irrigation system from the deep inferior epigastric vein and superficial inferior epigastric vein systems using DIEP flap transfer, and performing additional arterial anastomoses means adding a second vascular source of irrigation. In this series, none of the flaps were considered to be an insufficiently perfused flap intraoperatively. Therefore, the main reason for the second anastomosis is not enlarging the perfused skin territory. Because type 1 and 2 include dual vascular supply both in artery and vein from different vascular sources (transverse and descending branches), these flaps theoretically lived with rich blood flow supply, which might be given over the minimum requirement. These types of flaps also have safety valves for thrombotic obstruction because the number of the anastomosis is not single.
However, in type 3 cases, the arterial branch to the vastus medialis muscle is anastomosed in a retrograde flow fashion, and the only arterial source to the ALT flap is the descending branch of the LCFA. Therefore, in type 3 cases, performing additional arterial anastomoses means adding to the number of vascular anastomoses. Type 3 multiple anastomoses are expected only to act as a safety valve if 1 of the anastomoses is obstructed by thrombosis. This is similar to the situation in breast reconstruction with a DIEP flap in which a second vein is sometimes anastomosed in a retrograde fashion to the cut end of the internal mammary vein.
Unlike the situation for double venous anastomoses, in which blood velocity is considered to be decreased, double arterial anastomoses will theoretically decrease the rate of obstruction in the anastomosed area because arterial blood flow will increase. Because a patency rate of over 95% is generally reported for 1 arterial and 1 venous anastomosis, it is considered to be very difficult to demonstrate statistically any superiority of double anastomoses.
Previously, we reported a type 3 case (not included in this study) that developed an arterial obstruction and was rescued by the additional arterial flow.12 This indicates that a second arterial anastomosis can function as a safety valve for flap failure caused by the obstruction of a single vessel.
In sum, what are the drawbacks of performing 2 arterial anastomoses? In this case series, there were no significant harmful events, and multiple vascular anastomoses did not increase the operation time. If 2 arteries and 1 vein are anastomosed, will congestion of the flap or necrosis occur because only the inflow is increased? In the type 3 transfers in this series, no flap showed congestion as a result of the additional arterial anastomosis (Fig. 2). Even if a second artery is anastomosed, inflow of arterial blood is not unlimited because of the vascular resistance of the peripheral artery. Moreover, if the inflow increases, the vein will dilate as a matter of course. Therefore, if arterial inflow increases, the outflow venous velocity will be greater and the flow stasis may be improved. One case in our series showed venous thrombosis and congestion that required revision. However, after removing the thrombosis and reanastomosing the vessel, the flap survived and showed no congestion. Therefore, this 1 case of thrombosis was an ordinary venous thrombosis that was not related to the 2 arterial anastomoses. This means that none of the ALT flaps with 2 arterial and 1 venous anastomosis showed any congestion caused by an inflow–outflow imbalance, suggesting that even if the number of arteries is greater than the number of veins in a free cutaneous transfer, this does not cause an insufficiency or imbalance between the artery and vein. Therefore, for the purpose of easily increasing the number of anastomoses, the type 3 procedure is more rational than type 1 and type 2. Thus, we started the back-up anastomoses from type 1 and 2 cases; however, our preferred procedure became type 3 recently. If the main purpose of the double anastomoses is only to obtain the back-up anastomoses, the type 3 is ideal because it is less invasive and hand-manipulative.
CONCLUSIONS
An additional arterial anastomosis for free ALT flaps did not cause any harmful events such as congestion and did not require much surgical time. If the surgeon considers that an arterial anastomosis is unreliable, an additional anastomosis might be an option in ALT transfer. However, this should be confirmed in a larger study because of the small number of cases in our study.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Hanasono MM, Kocak E, Ogunleye O, et al. One versus two venous anastomoses in microvascular free flap surgery. Plast Reconstr Surg. 2010;126:1548–1557.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enajat M, Rozen WM, Whitaker IS, et al. A single center comparison of one versus two venous anastomoses in 564 consecutive DIEP flaps: investigating the effect on venous congestion and flap survival. Microsurgery. 2010;30:185–191.. [DOI] [PubMed] [Google Scholar]
- 3.Ross GL, Ang ES, Golger A, et al. Which venous system to choose for anastomosis in head and neck reconstructions? Ann Plast Surg. 2008;61:396–398.. [DOI] [PubMed] [Google Scholar]
- 4.Deleyiannis FW, Badeau AM, Leem TH, et al. Supercharging and augmenting venous drainage of an anterolateral thigh free flap: options and indications. Plast Reconstr Surg Glob Open. 2014;2:e135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ichinose A, Tahara S, Yokoo S, et al. Fail-safe drainage procedure in free radial forearm flap transfer. J Reconstr Microsurg. 2003;19:371–376.. [DOI] [PubMed] [Google Scholar]
- 6.Ueda K, Harashina T, Oba S, et al. Which vessel is more important in the supercharged flap—artery, vein, or both? An experimental study. J Reconstr Microsurg. 1994;10:153–155.. [DOI] [PubMed] [Google Scholar]
- 7.Ueda K, Kajikawa A, Suzuki Y, et al. Blood gas analysis of the jejunum in the supercharge technique: to what degree does circulation improve? Plast Reconstr Surg. 2007;119:1745–1750.. [DOI] [PubMed] [Google Scholar]
- 8.Sekido M. A study on blood flow in pedicled jejunum after supercharge using a dog model. J Jpn S.R.M. 2005;18:1–8.. [Google Scholar]
- 9.Miyamoto S, Minabe T, Harii K. Effect of recipient arterial blood inflow on free flap survival area. Plast Reconstr Surg. 2008;121:505–513.. [DOI] [PubMed] [Google Scholar]
- 10.Zheng J, Xi S, Ding M, et al. Effects of venous superdrainage and arterial supercharging on dorsal perforator flap in a rat model. PLoS One. 2016;11:e0160942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arata J, Ishikawa K, Shigeyoshi N, et al. Effect of multiple vascular anastomoses for replantation of amputated fingers. J Jpn S.R.M. 2007;20:402–405.. [Google Scholar]
- 12.Numajiri T, Sowa Y, Nishino K, et al. Successful retrograde arterial inflow through a muscular branch in a free anterolateral thigh chimeric flap transfer. Microsurgery. 2012;32:318–321.. [DOI] [PubMed] [Google Scholar]


