Abstract
Background:
Diabetes is an increasingly prevalent comorbidity in patients presenting for surgery, impacting nearly 14% of adults in the United States. Although it is known that diabetic patients are at an increased risk for postoperative complications, there is a paucity of literature on the specific ramifications of diabetes on different surgical procedures.
Methods:
Using the American College of Surgeons National Surgical Quality Improvement Program dataset, demographics, outcomes, and length of in-patient hospitalization were examined for patients who underwent plastic surgery between 2007 and 2012. Adjusted multivariable logistic regression models were used to assess the relationship between diabetes status and a spectrum of medical and surgical postoperative outcomes.
Results:
Thirty-nine thousand four hundred seventy-five plastic surgery patients were identified, including 1,222 (3.10%) with insulin-dependent diabetes mellitus (IDDM) and 1,915 (4.75%) with non–insulin-dependent diabetes mellitus (NIDDM), who had undergone breast, hand/upper and lower extremity, abdominal, or craniofacial procedures. Logistic regression analyses showed that only insulin-dependent diabetics had a higher likelihood of surgical complications (IDDM: P value < 0.0001; NIDDM: P value < 0.103), whereas patients with both IDDM and NIDDM had increased likelihoods of medical complications (IDDM: P value < 0.001; NIDDM: P value = 0.0093) compared with nondiabetics. Average hospital stay for diabetics was also longer than for nondiabetics.
Conclusions:
Diabetes is associated with an increase in a multitude of postoperative complications and in hospital length of stay, in patients undergoing plastic surgery. Diabetes status should thus be evaluated and addressed when counseling patients preoperatively. Risks may be further stratified based on IDDM versus NIDDM status.
INTRODUCTION
Diabetes mellitus is an increasingly prevalent comorbidity in the United States impacting 9.3% (29.1 million) of Americans, as estimated by the Centers for Disease Control and Prevention in 2014.1 Alarmingly, 27.8% (8.1 million) of diabetic patients in the United States are undiagnosed. Additionally, approximately 30.0% (86 million) of Americans have prediabetes, and an estimated 15–30% of these prediabetic patients will develop type 2 diabetes mellitus within the next 5 years.1 Thus, diabetes has a pervasive health care impact on the United States, and furthermore, a substantial economic burden, incurring costs of 245 billion dollars in 2012 alone.1 In fact, diabetes has been reported as the leading contributor to inflation-adjusted increases in Medicare costs in the United States.2,3 A robust body of research has established diabetes as a risk factor for a myriad of chronic illnesses.3–10
Comparatively, there is substantially less research examining whether diabetes is associated with poorer surgical outcomes.11–15 It is known that diabetic patients are at an increased risk for postoperative complications, including infection.16–18 Poor tissue perfusion, which results in inadequate antibiotic penetration and underlying immune compromise, is cited to contribute to an increase in complicated skin and soft-tissue infections in diabetic patients.17 In terms of pathophysiology, polymorphonuclear lymphocytes are continually activated at baseline in hyperglycemic states and are thus deemed to be less responsive to infectious stimuli, thereby predisposing diabetic patients to surgical-site infections.17,19,20 Additionally, increased levels of pro-inflammatory cytokines result in an insufficient immune response to pathogens as well as vascular inflammation, further contributing to poorer surgical outcomes.17,19,20
In plastic surgery, specifically, numerous clinical studies have substantiated that current diabetics are at higher risk of wound-related complications; however, the majority of this research has been conducted among patients undergoing breast reconstruction procedures.21–23 To date, no known single study has examined the specific ramifications of diabetes mellitus on surgical outcomes for a range of plastic surgery procedures.
This study aims to identify how diabetes mellitus status (insulin-dependent diabetic, non–insulin-dependent diabetic, or nondiabetic) is associated with postoperative outcomes and wound-related complications in a full spectrum of plastic surgery procedures. It is the largest known study examining the link between diabetes mellitus and plastic surgery outcomes and uses data from the 2007–2012 American College of Surgeons National Surgery Quality Improvement Program (ACS-NSQIP) datasets.
METHODS
ACS-NSQIP Datasets
Data for the ACS-NSQIP datasets were collected by trained research nurses employed at participating institutions. For each patient, there were approximately 240 variables collected, including demographics, preexisting comorbidities, intraoperative variables, and outcomes affecting morbidity and mortality. Patients were contacted by letter or telephone 30 days after discharge to collect information on surgical-related outcomes up to this period. A complete list of the variables collected as part of ACS-NSQIP dataset is publicly available (http://www.acsnsqip.org/).
Data used in the current analyses were extracted from all plastic surgery procedures identified in the 2007–2012 ACS-NSQIP datasets. The 2012 Current Procedural Terminology (CPT) codes were used to identify these procedures.
Dependent Variables
The dependent variables examined included a range of surgical complications: superficial wound infections, deep wound infections, organ space infections, wound dehiscence, pneumonia, reintubation, pulmonary embolism, urinary tract infection, postoperative bleeding/transfusion, graft or flap loss, deep venous thrombosis, sepsis, and return to operating room. Three composite outcome variables “major surgical complications,” “medical complications,” and “wound complications” were created by combining certain specific outcomes. A “major surgical complication” comprised a deep wound infection, a graft/prosthetic loss, or an unplanned return to the operating room within the 30-day postoperative period. “Medical complications” included any of the following: pneumonia, pulmonary embolism, postoperative renal insufficiency (creatinine > 2 mg/dL), urinary tract infection, stroke, myocardial infarction, symptomatic deep vein thrombosis, or sepsis. “Wound complications” comprised superficial surgical-site infections, deep incisional wound infections, organ space infections, and wound dehiscence. “Wound infections” comprised superficial surgical-site infections and deep incisional wound infections.
Independent Variables
The principal independent variable was the diabetic status of the patient at the time of the procedure. Patients were categorized as either insulin-dependent diabetic (IDDM), non–insulin-dependent diabetic (NIDDM), or nondiabetic. The ACS-NSQIP dataset defines a diabetic as an individual who has required a treatment regimen with exogenous parenteral insulin or oral antidiabetic agents for more than 2 weeks. Patients with insulin resistance who routinely take antidiabetic agents were also included. However, those whose diabetes was controlled by diet alone were not classified as diabetic in the current study. Of note, the laboratory value hemoglobin A1c is not a variable available or included within the ACS-NSQIP dataset.
Statistical Analyses
The baseline sociodemographic and surgical characteristics of the sample were summarized using number and percentage in each group: IDDM, NIDDM, or nondiabetic.
Logistic regression models were used to examine associations between diabetic status and the various adverse outcomes. These analyses were adjusted for sex, race, age group, operation year, CPT code, body mass index (BMI), smoking, congestive heart failure, use of antihypertensives, renal failure, and dyspnea. The statistic of interest in these analyses was the odds ratios [95% confidence interval (CI)] and their P values of the IDDM and NIDDM groups compared with the nondiabetic group. Logistic regression was also used with an interaction term for CPT categories and the outcome variables to examine if diabetic status was associated with adverse outcomes for each of the CPT categories.
A negative binomial model was used for the hospital length of stay (LOS), adjusting for sex, race, age group, operation year, CPT code, BMI, smoking, history of congestive heart failure, use of antihypertensives, renal failure, and dyspnea. The statistics of interest were the least square mean values (95% CI), the rate ratio (95% CI), the P value for the test that each rate ratio was different from one, and the overall P value for the test that at least one of the rate ratios was different from one.
RESULTS
Study Sample
The baseline characteristics of the study sample as well as details of the surgeries performed are shown in Table 1. During the study period, 39,475 plastic surgery patients were identified who had undergone breast, hand/upper and lower extremity, abdominal, or craniofacial procedures. The cohort included 1,222 (3.10%) insulin-dependent diabetics and 1,915 (4.75%) non–insulin-dependent diabetics. In terms of baseline demographics, 32,239 patients (81.67%) were female, and 27,324 (69.22%) were Caucasian. Age analyses showed that 50.09% of the patients were between 40 and 59 years of age at the time of surgery, as 9,578 (24.26%) were 40–49 years old, and 10,198 (25.83%) were 50–58 years old.
Table 1.
Baseline Characteristics
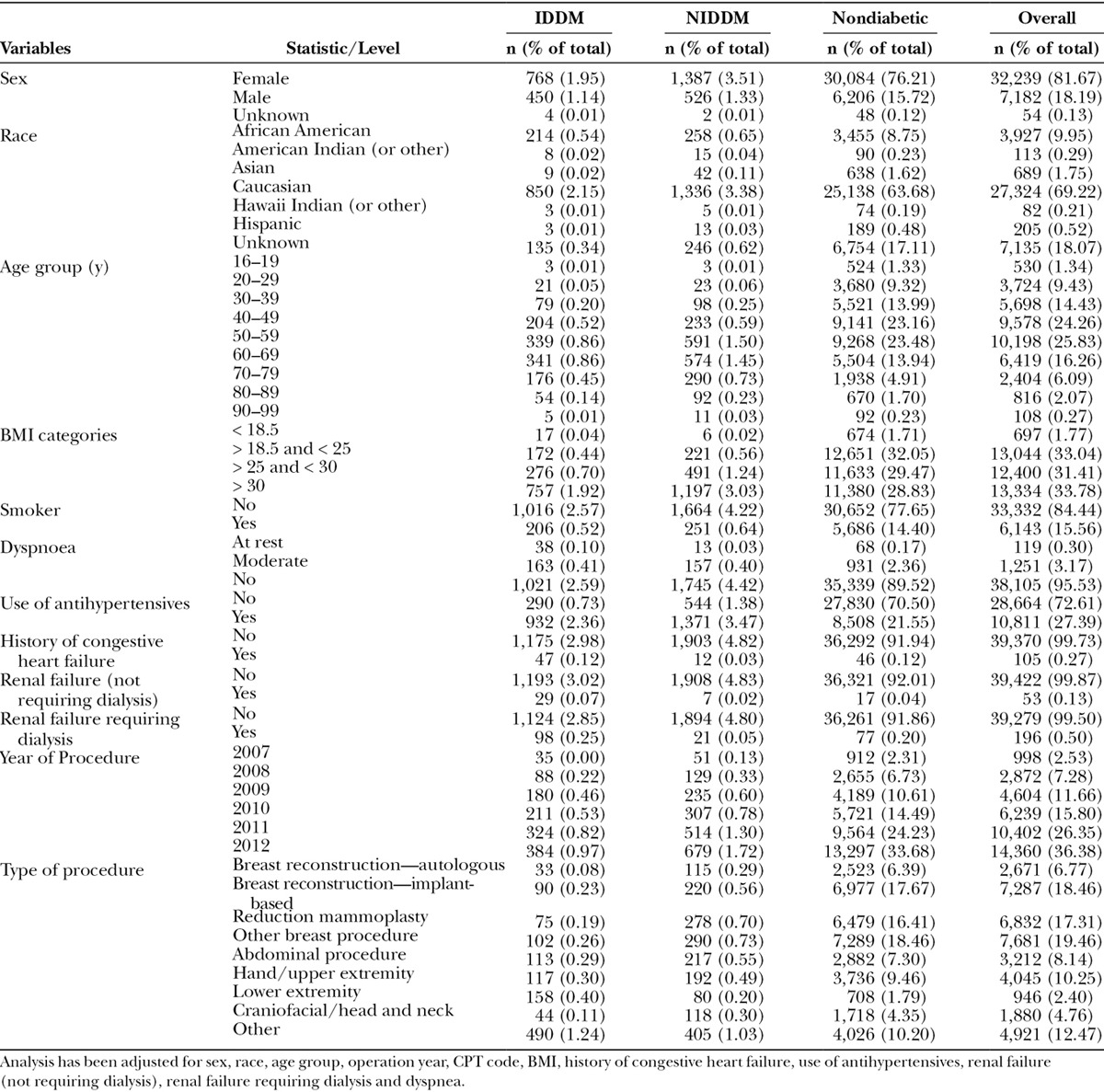
The majority of the procedures performed were breast procedures (61.84%). However, there was a representation of a wide range of plastic surgery procedures represented with 6.77% (2,671) autologous breast reconstruction, 18.46% (7,287) implant-based reconstruction, 17.31% (6,832) reduction mammoplasty, 19.46% (7,681) other breast procedures, 10.25% (4,045) hand/upper extremity procedures, 8.14% (3,212) abdominal procedures, 4.76% (1,880) craniofacial/head and neck procedures, 2.40% (946) lower extremity procedures, and 12.47% (4,921) other procedures. “Other” procedures included plastic surgery procedures such as aesthetic procedures; incision and drainage of hematoma; incision and drainage of seroma; foreign body removal; anastomosis of common sensory nerve in hand or foot; full-thickness skin graft in face, perineum, hand, or foot; free muscle or myocutaneous flap with microvascular anastomosis without defined location in the database; and all other procedures without defined location.
Major Complications by Diabetic Status
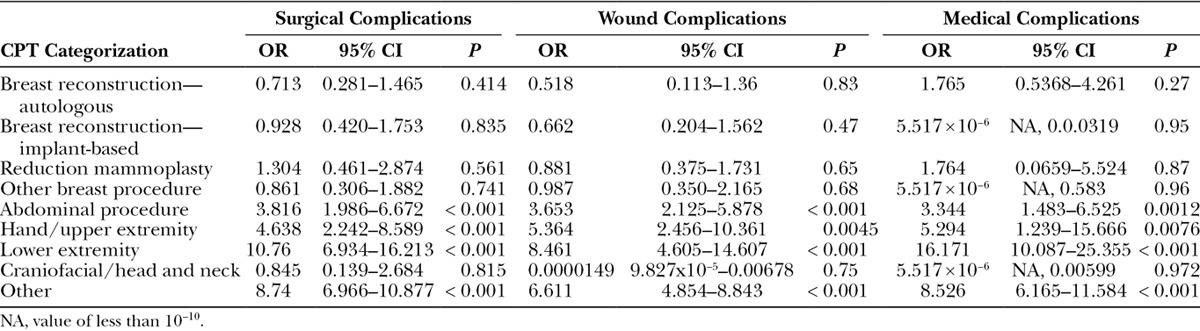
Overall, 8.34% (3,291) of patients experienced postoperative complications. The most common complications were wound-related, impacting 3.97% (1,566) of patients in the cohort (Table 2). Logistic regression analyses showed that only insulin-dependent diabetics had a higher likelihood of surgical complications [IDDM: odds ratio (OR), 1.78; P value < 0.0001; NIDDM: P value < 0.103], whereas both insulin-dependent and non–insulin-dependent diabetics had increased likelihoods of medical complications (IDDM: OR, 1.63; P value < 0.001; NIDDM: OR, 1.44; P value = 0.0093) compared with nondiabetics. The odds of an insulin-dependent diabetic having a medical complication were 1.63 times (95% CI, 1.22–2.14; P value < 0.001) and a non–insulin-dependent diabetic was 1.44 times (95% CI, 1.09–1.89; P value = 0.0093) compared with a nondiabetic. The odds of insulin-dependent diabetics having a major surgical complication were 1.78 times (95% CI, 1.45–2.16; P value < 0.0001) that of nondiabetics. Having non–insulin-dependent diabetes was not significantly associated with major surgical complications (P value = 0.104; Tables 3–5).
Table 2.
Logistic Regression Analysis of Major Complication Categories between Diabetics and Nondiabetics
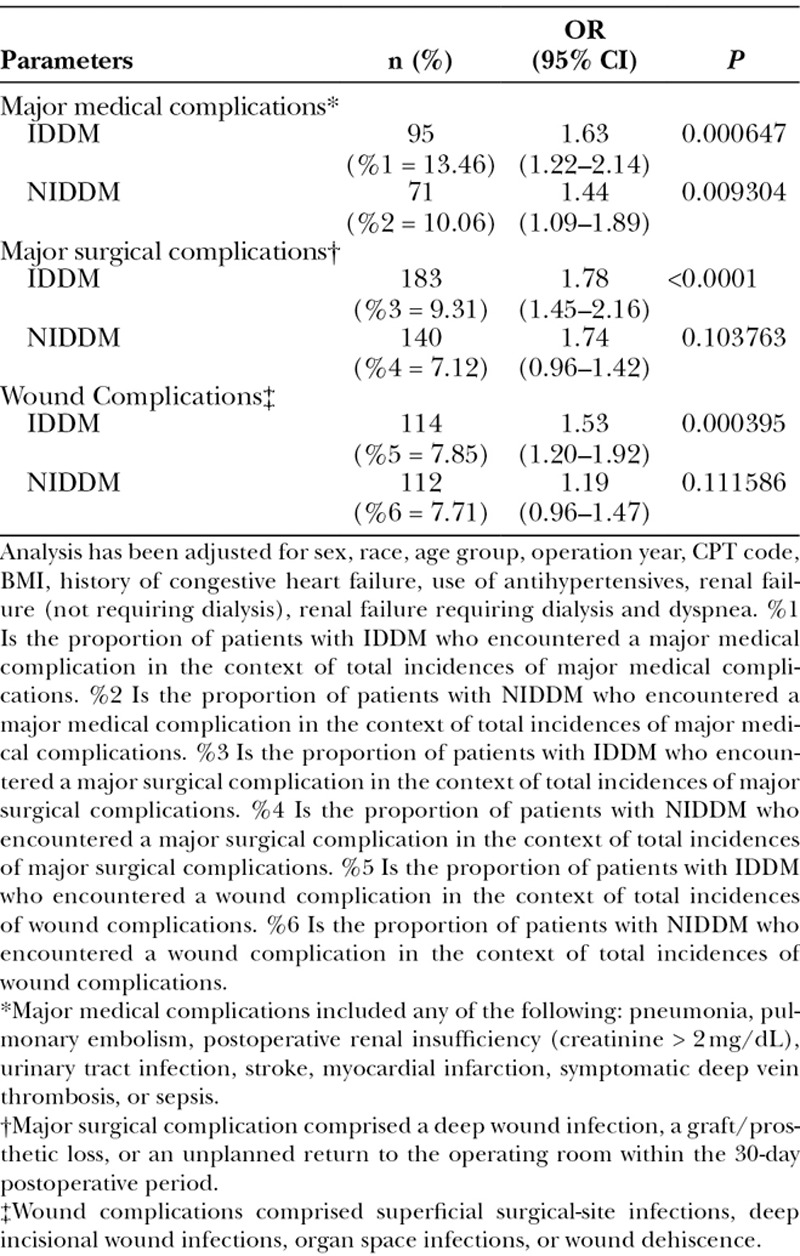
Table 3.
Logistic Regression Analysis of Wound-Related Complications between Diabetics and Nondiabetics
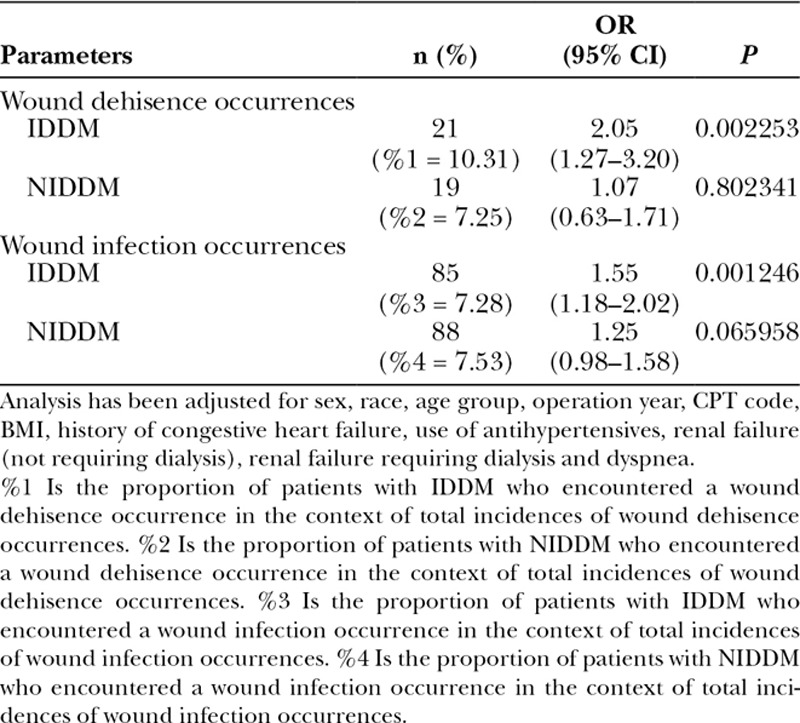
Table 5.
Logistic Regression Analysis of the Effect of NIDDM on Major Complications following Plastic Surgery Procedures, Categorized According to CPT Grouping Classifications
Analysis of Wound-Related Complications between Diabetics and Nondiabetics
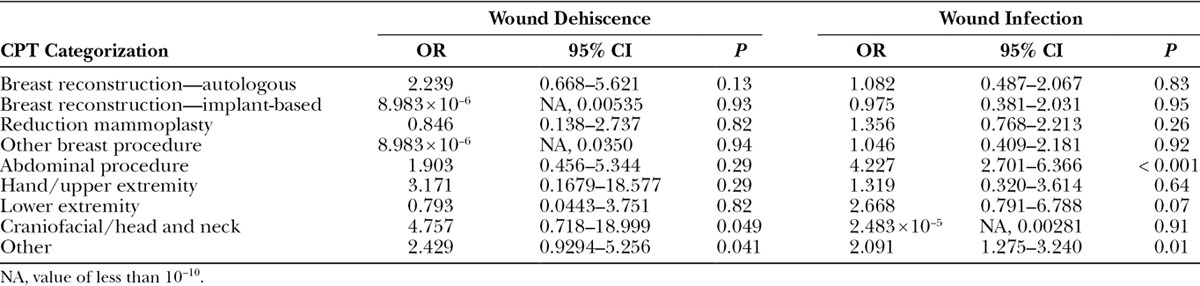
Insulin-dependent diabetic patients had a higher likelihood of wound complications (OR, 1.53; P < 0.001) than nondiabetic patients (Table 2). Having non–insulin-dependent diabetes was not associated with wound complications (P value = 0.112). The odds of patients with insulin-dependent diabetes having a wound complication was 1.53 times (95% CI, 1.20–1.92; P value < 0.001) that of nondiabetics.
More specifically, the odds of an insulin-dependent diabetic having wound dehiscence was 2.05 times (95% CI, 1.27–3.20; P value < 0.0023) that of a nondiabetic, and the odds of an insulin-dependent diabetic having a wound infection (which includes superficial wound occurrences as well as deep incisional surgical site infection occurrences) was 1.55 times (95% CI, 1.18–2.02; P value < 0.0012) that of a nondiabetic. Wound subgroup analyses further identified that having non–insulin-dependent diabetes was not significantly associated with wound dehiscence (P value = 0.8023) and wound infection (P value = 0.066; Tables 6, 7).
Table 6.
Logistic Regression Analysis of the Effect of IDDM on Wounds following Plastic Surgery Procedures, Categorized According to CPT Grouping Classifications
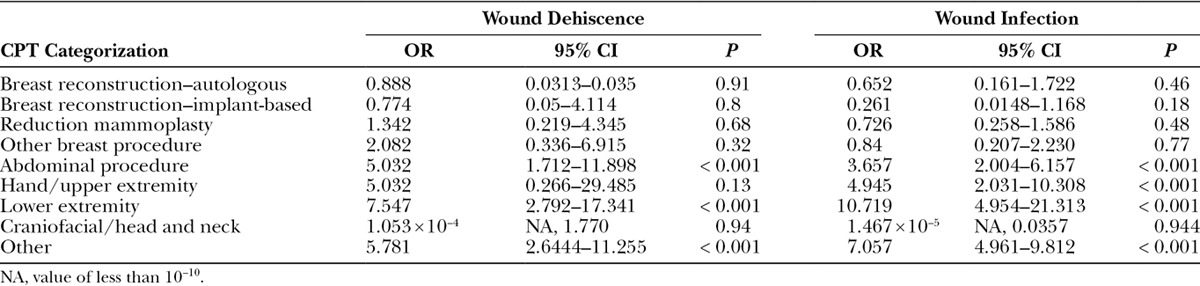
Table 7.
Logistic Regression Analysis of the Effect of NIDDM on Wounds following Plastic Surgery Procedures, Categorized According to CPT Grouping Classifications
Analysis of Hospital LOS between Diabetics and Nondiabetics
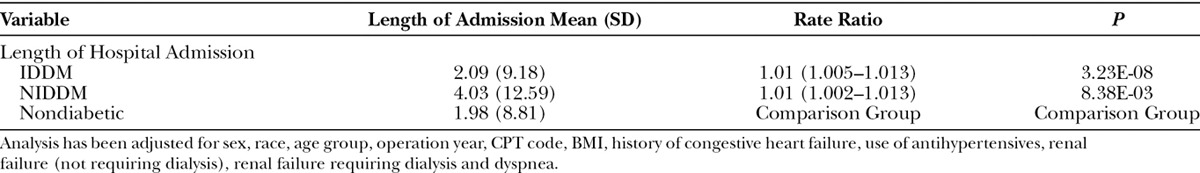
Negative binomial model analyses revealed that the average LOS for insulin-dependent diabetics was 1.06 days longer [relative risk (RR), 1.01 (95% CI, 1.005–1.013)] and for non–insulin-dependent diabetics was 2.04 days longer compared with nondiabetic patients (Table 8). More specifically, the average hospital stay of an insulin-dependent diabetic was 2.09 days (SD, 9.18), a non–insulin-dependent diabetic was 4.03 days (SD, 12.59), and for a nondiabetic it was 1.98 days (SD, 8.81).
Table 8.
Negative Binomial Model of Length of Hospital Admission
DISCUSSION
The aim of this study was to objectively quantify the complications associated with diabetes mellitus in plastic surgery procedures. With the inclusion of 39,475 plastic surgery patients as well as a national representation of patients by using the American College of Surgeons NSQIP (ACS-NSQIP) database, this study is the largest known study systematically examining the link between diabetes mellitus and plastic surgery outcomes. We found that only insulin-dependent diabetics had a higher likelihood of surgical complications (IDDM: OR, 1.78; P value < 0.0001; NIDDM: P value < 0.103), whereas both insulin-dependent and non–insulin-dependent diabetics had increased likelihoods of medical complications (IDDM: OR, 1.63; P value < 0.001; NIDDM: OR, 1.44; P value = 0.0093) compared with nondiabetics. Our data corroborate the findings by Bamba et al.,24 who studied diabetes as an independent risk factor for major complications following aesthetic surgery. In a prospective cohort of 129,007 patients, Bamba et al.24 found diabetics had a higher rate of complications (3.1%) compared with nondiabetics (1.9%; P < 0.01). Very few studies to date have examined the effects of diabetes on surgical outcomes, irrespective of surgical subspecialty. Therefore, our data provide a useful reference point for future studies investigating complications associated with surgery in diabetic patients.
Given the increasing prevalence of diabetes mellitus in the Unites States, impacting almost 10% of the population, it is important to consider the social determinants of health on endocrine care for diabetics in this type of analysis.25–30 Recently, Walker et al.25 published a holistic review of the social determinants of health on the endocrine care that diabetic patients are provided, emphasizing the pervasive gap in care seen especially in the rural south. Walker et al.25 discussed strong evidence that race, ethnicity, and social determinants of health differences continue to persist in the clinical outcomes for diabetes, including glycemic, blood pressure, and lipid control, and yet these disparities are largely ignored.25 Interestingly, glycemic control and quality of life for diabetics were shown to be consistently associated with psychosocial factors, namely depression, social support, perceived stress, and neighborhood factors.25 Hence, the socioeconomic and psychological inequalities related to diabetes care are crucial considerations in preoperative surgical decision making and patient counseling on expectations.
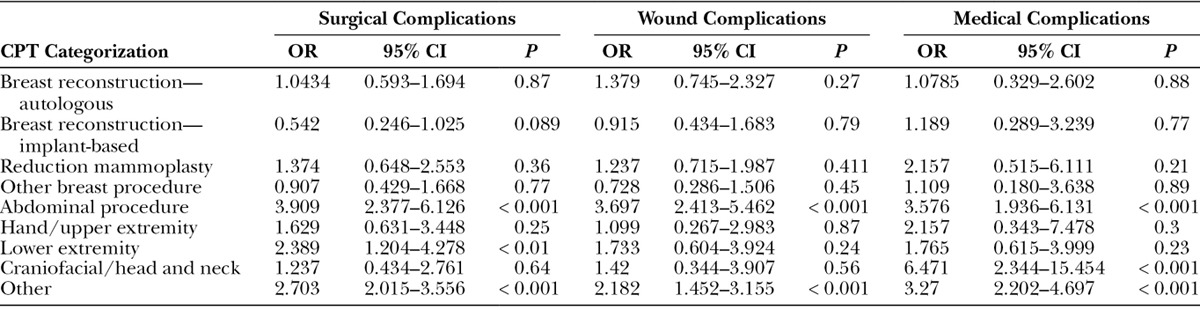
As a comorbidity in surgical patients, diabetes mellitus had a profound impact on plastic surgery outcomes in our cohort. In particular, insulin-dependent diabetic patients had a higher likelihood of wound complications (OR, 1.53; P < 0.001) than nondiabetic patients. However, having non–insulin-dependent diabetes was not associated with wound complications (P value = 0.112). More specifically, the odds of an insulin-dependent diabetic having wound dehiscence was 2.05 times (95% CI, 1.27–3.20; P value < 0.0023) that of a nondiabetic, and the odds of an insulin-dependent diabetic having a wound infection (which includes superficial wound occurrences as well as deep incisional surgical site infection occurrences) was 1.55 times (95% CI, 1.18–2.02; P value < 0.0012) that of a nondiabetic. Moreover, wound subgroup analyses further identified that having non–insulin-dependent diabetes was not significantly associated with wound dehiscence (P value = 0.8023) or wound infection (P value = 0.066). With regard to breast reconstruction, Hart et al.31 found that diabetic patients had a significantly higher incidence of delayed wound healing following implant-based reconstruction (OR, 2.52). However, this significant association was not seen with autologous reconstruction (OR, 0.97) in the cohort of 1,371 breast reconstructions performed in 1,035 patients as described by Hart et al.31 This difference in outcomes from our ACS-NSQIP data could potentially be attributed to their smaller patient cohort reflecting a single institutional experience, and thus, further highlights the importance of a large national study to examine diabetes across a number of plastic surgery procedures. Moreover, with respect to large studies quantifying the risk of infections in diabetic patients, Shah and Hux32 determined that the risk of nearly every type of infection studied was higher among patients with diabetes, with the highest risk ratios seen for osteomyelitis (RR = 4.39; 95% CI, 3.80–5.06), sepsis (RR = 2.45; 95% CI, 2.23–2.68), postoperative infections (RR = 2.02; 95% CI, 1.80–2.27), and cellulitis (RR = 1.81; 95% CI, 1.76–1.86). Strikingly, in our cohort, IDDM was significantly associated with highly increased surgical, medical, and wound complications in abdominal procedures, hand/upper extremity, lower extremity, and other namely aesthetic procedures and those classified in the aforementioned “other” category compared with nondiabetics Table 4 (all OR > 3.0). Overall, our large data set provides compelling data to support the significant difference in risks associated with plastic surgery procedures for different diabetic patients, namely insulin-dependent diabetic patients compared with non-insulin diabetic patients. Our analyses demonstrate objective data to support that preoperative counseling should be different for insulin-dependent diabetic patients versus non-insulin diabetic patients, as all diabetes mellitus is not to be generalized as one risk factor.
Table 4.
Logistic Regression Analysis of the Effect of IDDM on Major Complications following Plastic Surgery Procedures, Categorized According to CPT Grouping Classifications
Furthermore, our study revealed that the average hospital LOS for insulin-dependent diabetics was 0.11 days longer [RR, 1.01 (95% CI, 1.005–1.013)] and for non–insulin-dependent diabetics was 2.05 RR 1.01 (95% CI, 1.002–1.013) days longer compared with nondiabetic patients. Yang et al.33 in a collaborative effort published a study quantifying the economic burden of diagnosed diabetes, increased health resource use, and lost productivity associated with diabetes in 2012. Interestingly, Yang et al.33 reported that of the projected 168 million hospital inpatient days in the United States in 2012, an estimated 43.1 million days (25.7%) are incurred by people with diabetes, of which 26.4 million days are attributed to diabetes. In fact, diabetes contributed to longer hospital LOS regardless of the reason for admission, even while controlling for other factors that affect hospital LOS.33,34
Comparatively, with respect to the effect of diabetes on surgical subspecialty outcomes and LOS, Maloney et al.35 found that mean LOS was significantly longer in diabetic patients (1.9 versus 1.4 days; P < 0.0001) undergoing neurosurgery, namely open lumbar microdiscectomy procedures. Likewise, in the otolaryngology literature, Raikundalia et al.36 demonstrated that diabetic patients had longer LOS following facial fracture repair in a large cohort of 45,509 inpatients using the Nationwide Inpatient Sample database. Additionally, Bur et al.,37 using the ACS-NSQIP dataset, reported that diabetes was a clinical factor independently associated with unplanned readmission within the first 30 days postoperatively following head and neck surgery for malignant neoplasms. Thus, our results substantiate national diabetes data as well as data reported by surgical subspecialties on hospital LOS. Future studies conducting cost analyses on hospitalization of diabetic patients undergoing a range of plastic surgery procedures are warranted.
Finally, given these results examining the effect of diabetes mellitus on outcomes in a wide spectrum of plastic surgery procedures, it is crucial to acknowledge that multiple approaches exist for preoperative and perioperative glucose control. Recently, Dortch et al.38 published an example of a practical approach to perioperative blood glucose management based on current guidelines set by the Endocrine Society and Mayo Clinic Institutional Protocol. These guidelines are based on preoperative hemoglobin A1C levels and vary depending on whether surgery is in-patient or out-patient, and elective or urgent. Ultimately, our data provide a compelling impetus and foundation upon which to consider national glucose management guidelines for plastic surgeons for this high risk patient population. In fact, a future study focusing on perioperative glucose management and the development of a widely applicable algorithm would be a great resource for clinicians, as the plastic surgery literature is lacking on this topic.
Study Limitations
Although this study has systematically presented a quantified association between diabetes and postoperative outcomes for a wide range of plastic surgery procedures using the largest cohort to date, the study has certain limitations that merit discussion. First, this study cohort is compiled from the NSQIP database, which is predicated upon all procedures being entered and available in the database. Second, a disadvantage to the use of this NSQIP database is that the resultant study is retrospective in nature, and the database does not provide robust clinical information, namely specifics of the nature of each procedure collated by CPT code or hemoglobin A1c values for diabetic patients. Finally, our study lacks the ability to extrapolate long-term complication data for diabetic patients undergoing plastic surgery procedures, as the follow-up period for NSQIP is limited to 30 days postoperatively from each procedure. For example, by limiting postoperative data collection to 30 days, the database does not capture certain complications that are important to plastic surgeons, such as capsular contracture rates, keloid and hypertrophic scar formation, and long-term pain.
CONCLUSIONS
Diabetes, especially IDDM, increases a multitude of postoperative complications, and the overall risk profile of patients undergoing plastic surgery, as demonstrated in this large analysis. The negative consequences of diabetes status on outcomes following plastic surgery can be used to counsel patients on the importance of optimizing preoperative diabetes management, particularly glycemic control, and to enhance perioperative decision making while risk stratifying based on type of plastic surgery procedure to be performed. Ultimately, given the full spectrum of plastic surgery procedures included in this study, these results have wide-scale applicability to the specialty and enable plastic surgeons to provide diabetic patients with tangible, quantifiable complication risks for the specific procedures they undergo.
ACKNOWLEDGMENT
We thank Dr. Mario D’Souza for assisting with components of the statistical analyses utilized in this study.
Footnotes
Disclosure: The authors have no financial interest to declare in relation to the content of this article. The Article Processing Charge was paid for by the authors.
REFERENCES
- 1.Centers for Disease Control and Prevention. National Diabetes Statistics Report: estimates of diabetes and its burden in the United States, 2014. 2014. Atlanta, GA: US Department of Health and Human Services; Available at https://www.cdc.gov/diabetes/pubs/statsreport14/national-diabetes-report-web.pdf. Accessed November 1, 2015. [Google Scholar]
- 2.Thorpe KE, Florence CS, Howard DH, et al. The impact of obesity on rising medical spending. Health Aff. 2004;23:W4-480–6.. [DOI] [PubMed] [Google Scholar]
- 3.Jaacks LM, Siegel KR, Gujral UP, et al. Type 2 diabetes: a 21st century epidemic. Best Pract Res Clin Endocrinol Metab. 2016;30:331–343.. [DOI] [PubMed] [Google Scholar]
- 4.Ali MK, Bullard KM, Saaddine JB, et al. Achievement of goals in U.S. diabetes care, 1999-2010. N Engl J Med. 2013;368:1613–1624.. [DOI] [PubMed] [Google Scholar]
- 5.Menke A, Casagrande S, Geiss L, et al. Prevalence of and trends in diabetes among adults in the United States, 1988-2012. JAMA. 2015;314:1021–1029.. [DOI] [PubMed] [Google Scholar]
- 6.Gregg EW, Cheng YJ, Saydah S, et al. Trends in death rates among U.S. adults with and without diabetes between 1997 and 2006: findings from the National Health Interview Survey. Diabetes Care. 2012;35:1252–1257.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kong AP, Luk AO, Chan JC. Detecting people at high risk of type 2 diabetes—how do we find them and who should be treated? Best Pract Res Clin Endocrinol Metab. 2016;30:345–355.. [DOI] [PubMed] [Google Scholar]
- 8.Haffner SM, Lehto S, Rönnemaa T, et al. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–234.. [DOI] [PubMed] [Google Scholar]
- 9.Lind M, Svensson AM, Kosiborod M, et al. Glycemic control and excess mortality in type 1 diabetes. N Engl J Med. 2014;371:1972–1982.. [DOI] [PubMed] [Google Scholar]
- 10.Patel A, MacMahon S, Chalmers J, et al. ; ADVANCE Collaborative Group. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med. 2008;358:2560–2572.. [DOI] [PubMed] [Google Scholar]
- 11.Wallaert JB, Nolan BW, Adams J, et al. The impact of diabetes on postoperative outcomes following lower-extremity bypass surgery. J Vasc Surg. 2012;56:1317–1323.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Armaghani SJ, Archer KR, Rolfe R, et al. Diabetes is related to worse patient-reported outcomes at two years following spine surgery. J Bone Joint Surg Am. 2016;98:15–22.. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura T, Toda K, Kuratani T, et al. Diabetes mellitus impairs left ventricular mass regression after surgical or transcatheter aortic valve replacement for severe aortic stenosis. Heart Lung Circ. 2016;25:68–74.. [DOI] [PubMed] [Google Scholar]
- 14.Lee H, Kwak C, Kim HH, et al. Diabetes mellitus as an independent predictor of survival of patients surgically treated for renal cell carcinoma: a propensity score matching study. J Urol. 2015;194:1554–1560.. [DOI] [PubMed] [Google Scholar]
- 15.Adams AL, Paxton EW, Wang JQ, et al. Surgical outcomes of total knee replacement according to diabetes status and glycemic control, 2001 to 2009. J Bone Joint Surg Am. 2013;95:481–487.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.King JT, Jr, Goulet JL, Perkal MF, et al. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg. 2011;253:158–165.. [DOI] [PubMed] [Google Scholar]
- 17.Dryden M, Baguneid M, Eckmann C, et al. Pathophysiology and burden of infection in patients with diabetes mellitus and peripheral vascular disease: focus on skin and soft-tissue infections. Clin Microbiol Infect. 2015;21:S27–S32.. [DOI] [PubMed] [Google Scholar]
- 18.Browne JA, Cook C, Pietrobon R, et al. Diabetes and early postoperative outcomes following lumbar fusion. Spine (Phila Pa 1976). 2007;32:2214–2219.. [DOI] [PubMed] [Google Scholar]
- 19.Gallacher SJ, Thomson G, Fraser WD, et al. Neutrophil bactericidal function in diabetes mellitus: evidence for association with blood glucose control. Diabet Med. 1995;12:916–920.. [DOI] [PubMed] [Google Scholar]
- 20.Rajagopalan S. Serious infections in elderly patients with diabetes mellitus. Clin Infect Dis. 2005;40:990–996.. [DOI] [PubMed] [Google Scholar]
- 21.Nykiel M, Sayid Z, Wong R, et al. Management of mastectomy skin flap necrosis in autologous breast reconstruction. Ann Plast Surg. 2014;72:S31–S34.. [DOI] [PubMed] [Google Scholar]
- 22.Fischer JP, Tuggle CT, Au A, et al. A 30-day risk assessment of mastectomy alone compared to immediate breast reconstruction (IBR). J Plast Surg Hand Surg. 2014;48:209–215.. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim AM, Shuster M, Koolen PG, et al. Analysis of the National Surgical Quality Improvement Program database in 19,100 patients undergoing implant-based breast reconstruction: complication rates with acellular dermal matrix. Plast Reconstr Surg. 2013;132:1057–1066.. [DOI] [PubMed] [Google Scholar]
- 24.Bamba R, Gupta V, Shack RB, et al. Evaluation of diabetes mellitus as a risk factor for major complications in patients undergoing aesthetic surgery. Aesthet Surg J. 2016;36:598–608.. [DOI] [PubMed] [Google Scholar]
- 25.Walker RJ, Strom Williams J, Egede LE. Influence of race, ethnicity and social determinants of health on diabetes outcomes. Am J Med Sci. 2016;351:366–373.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell JA, Walker RJ, Smalls BL, et al. Glucose control in diabetes: the impact of racial differences on monitoring and outcomes. Endocrine. 2012;42:471–482.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirk JK, Passmore LV, Bell RA, et al. Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2008;31:240–246.. [DOI] [PubMed] [Google Scholar]
- 28.Barker LE, Kirtland KA, Gregg EW, et al. Geographic distribution of diagnosed diabetes in the U.S.: a diabetes belt. Am J Prev Med. 2011;40:434–439.. [DOI] [PubMed] [Google Scholar]
- 29.Williams DR, Costa MV, Odunlami AO, et al. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14:S8–17.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolucci A, Kovacs Burns K, Holt RI, et al. Correlates of psychological outcomes in people with diabetes: results from the second Diabetes Attitudes, Wishes and Needs (DAWN2(™)) study. Diabet Med. 2016;33:1194–1203.. [DOI] [PubMed] [Google Scholar]
- 31.Hart A, Funderburk CD, Chu CK, et al. The impact of diabetes mellitus on wound healing in breast reconstruction. Ann Plast Surg. 2017;78:260–263.. [DOI] [PubMed] [Google Scholar]
- 32.Shah BR, Hux JE. Quantifying the risk of infectious diseases for people with diabetes. Diabetes Care. 2003;26:510–513.. [DOI] [PubMed] [Google Scholar]
- 33.Yang W, Dall TM; American Diabetes Association. Economic costs of diabetes in the U.S. in 2012. Diabetes Care. 2013;36:1033–1046.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.American Diabetes Association. Economic costs of diabetes in the U.S. in 2007. Diabetes Care. 2008;31:596–615.. [DOI] [PubMed] [Google Scholar]
- 35.Maloney PR, Halasz SR, Mallory GW, et al. The effect of diabetes mellitus on 30-day outcomes following single-level open lumbar microdiscectomy: an aged-matched case-control study. J Neurosurg Sci. 2017;61:1–7.. [DOI] [PubMed] [Google Scholar]
- 36.Raikundalia M, Svider PF, Hanba C, et al. Facial fracture repair and diabetes mellitus: an examination of postoperative complications. Laryngoscope. 2017;127:809–814.. [DOI] [PubMed] [Google Scholar]
- 37.Bur AM, Brant JA, Mulvey CL, et al. Association of clinical risk factors and postoperative complications with unplanned hospital readmission after head and neck cancer surgery. JAMA Otolaryngol Head Neck Surg. 2016;142:1184–1190.. [DOI] [PubMed] [Google Scholar]
- 38.Dortch JD, Eck DL, Ladlie B, et al. Perioperative glycemic control in plastic surgery: review and discussion of an institutional protocol. Aesthet Surg J. 2016;36:821–830.. [DOI] [PubMed] [Google Scholar]


