Supplemental Digital Content is available in the text
Keywords: acute myocardial infarction, mortality, obesity, statins
Abstract
The phenomenon of obesity paradox after acute myocardial infarction (AMI) has been reported under strong recommendation of statin therapy. However, the impact of statin therapy on this paradox has not been investigated. This study investigated the impact of statin therapy on 1-year mortality according to obesity after AMI. A total of 2745 AMI patients were included from the Korea Acute Myocardial Infarction Registry after 1:4 propensity score matching analysis (n = 549 for nonstatin group and n = 2196 for statin group). Primary and secondary outcomes were all-cause and cardiac death, respectively. During 1-year follow-up, the incidence of all-cause (8.4% vs 3.7%) and cardiac (6.2% vs 2.3%) death was higher in nonstatin group than in statin (P < .001, respectively). In nonstatin group, the incidence of all-cause (7.2% vs 9.0%) and cardiac (5.5% vs 6.5%) death did not differ significantly between obese and nonobese patients. However, in statin group, obese patients had lower 1-year rate of all-cause (1.7% vs 4.8%) and cardiac (1.2% vs 2.9%) death (P < .05, respectively), and lower cumulative rates by Kaplan–Meier analysis of all-cause and cardiac death compared with nonobese patients (log-rank P < .05, respectively). The overall risk of all-cause death was significantly lower in obese than in nonobese patients only in statin group (hazard ratio: 0.35; P = .001). After adjusting for confounding factors, obesity was independently associated with decreased risk of all-cause death in statin group. In conclusion, the greater benefit of statin therapy for survival in obese patients is further confirmation of the obesity paradox after AMI.
1. Introduction
Obesity increases the risk of numerous comorbidities, including type 2 diabetes mellitus, hypertension, dyslipidemia, and cardiovascular (CV) disease.[1,2] High body mass index (BMI) is strongly associated with an increased mortality in the general population.[3] However, the phenomenon known as the “obesity paradox” in which obesity appears to improve survival after acute myocardial infarction (AMI) has been replicated in several studies.[4–9] Although a number of theories have been proposed, the mechanism by which obesity appears to improve survival after AMI remains uncertain.
The use of statins, 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors, reduces mortality and recurrent adverse cardiac events across a wide range of cholesterol levels in patients at high risk of having an atherosclerotic CV event.[10–12] Thus, statin therapy for secondary prevention is recommended in all high-risk patients.[13–14] A previous study found that intensive lipid lowering with a statin had a greater impact on the progression of atherosclerotic plaque burden in obese than in nonobese patients with coronary artery disease.[15] However, the effect of statin therapy on mortality according to obesity status has not been evaluated in patients with AMI. Considering that most studies documenting the phenomenon of obesity paradox were performed under the strong recommendation of statin therapy after AMI, this may be a substantial issue in clinical practice. Therefore, this study aimed to evaluate the association between obesity and 1-year mortality according to statin therapy in patients with AMI.
2. Methods
2.1. Subjects and study design
In the present study, we analyzed the baseline data from the Korea AMI Registry (KAMIR), a nationwide, prospective, observational registry in South Korea. Data were collected by a trained study coordinator using a standardized web-based case report form at each site. Of the 12,115 patients from the KAMIR registry between November 2011 and June 2015, 3395 patients were initially excluded from the analysis for the following reasons: BMI was not identified or was <18.5 kg/m2 (n = 968), statin therapy was not identified (n = 19), follow-up was not performed (n = 2068), and mortality was not identified or occurred in-hospital (n = 340). After this process, we performed 1:4 propensity score matching between patients on or not on statin therapy using prespecified clinical variables, including age, sex, hypertension, diabetes mellitus, dyslipidemia, smoking, and the use of antiplatelet agents, beta-blockers, and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers. High-intensity statin therapy (HIST) was defined as atorvastatin 40 or 80 mg/d or rosuvastatin 20 mg/d. Rosuvastatin 40 mg/d was not included in HIST because this dosage was not available in South Korea. Finally, 549 patients without statin therapy and 2196 patients with statin therapy were enrolled in the present study.
The overall design of the KAMIR has been previously described.[16] Briefly, AMI was diagnosed by characteristic clinical presentation, serial changes on the electrocardiogram suggesting infarction or injury, and increase in levels of cardiac enzymes.[17] All patients received a 300-mg loading dose of aspirin, 300 to 600 mg loading dose of clopidogrel, and heparin. The maintenance dose was 100 mg/day for aspirin and 75 mg/day for clopidogrel. Aspirin and clopidogrel were administered to all patients for ≥6 months.[18] Coronary angiography was performed via either the radial or the femoral artery by a standard technique. The use of stent type was at the individual operators’ discretion. During the in-hospital period, patients received essential medical treatment that included beta-blockers, angiotensin-converting enzyme inhibitors (ACEI), or angiotensin receptor blockers (ARB), if not contraindicated. After discharge, the patients continued to receive the same medications regimen that they had received at the hospital, except for some temporary medications. Clinical follow-up was performed at 1, 6, and 12 months and upon the occurrence of angina-like symptoms.
Coronary lesion morphology was classified using modified American College of Cardiology/ American Heart Association criteria.[19] Multivessel disease was defined as the presence of lesions with ≥50% stenosis in the noninfarct-related coronary artery. Thrombolysis in Myocardial Infarction score was used to determine the degree of coronary flow.[20] Transthoracic echocardiography was performed to assess the left ventricular ejection fraction (LVEF) using the modified Simpson's biplanar method. Chronic kidney disease (CKD) was defined as an estimated glomerular filtration rate <60 mL/min/1.73 m2 calculated by means of the modification of diet in renal disease formula.[21] BMI was calculated as weight (kg)/height (m2). Obesity was defined as a BMI ≥25 kg/m2 based on the criteria for an Asian population. The primary outcome was the occurrence of all-cause death, and the secondary outcome was the occurrence of cardiac death during 1-year follow-up. All death was considered cardiac unless there was a clear noncardiac cause. The study protocol was approved by the Institutional Review Board/Ethical Committees of each clinical site, and written informed consent was obtained from all patients.
2.2. Statistical analysis
Clinical, biochemical, and procedural characteristics are shown according to statin therapy and obesity status. Values are expressed as mean ± SD for continuous variables and numbers and percentages for categorical variables. Continuous variables were compared using Student t test or Mann–Whitney rank-sum test, and categorical variables were compared using the χ2-test or Fisher exact test, as appropriate. Kaplan–Meier survival analysis was performed for the cumulative incidence of all-cause and cardiac death, and comparisons between groups were performed with the log-rank test. Subgroup analyses were performed to identify the hazard ratios (HR) of obesity as estimates for clinical outcomes according to statin therapy. Multiple Cox hazards regression models with consecutive adjustment of confounding risk factors were used to identify the effect of statin therapy on 1-year mortality in all participants and to identify the association between obesity and 1-year mortality in participants on statin therapy. The forced entry method was used to enter independent variables into the multiple regression models. MatchIt R-package was used in matching propensity scores. SPSS statistical software version 20.0 (SPSS, Inc., Chicago, IL) was used for statistical analyses. Values of P < .05 were considered statistically significant.
3. Results
3.1. Baseline clinical and procedural characteristics
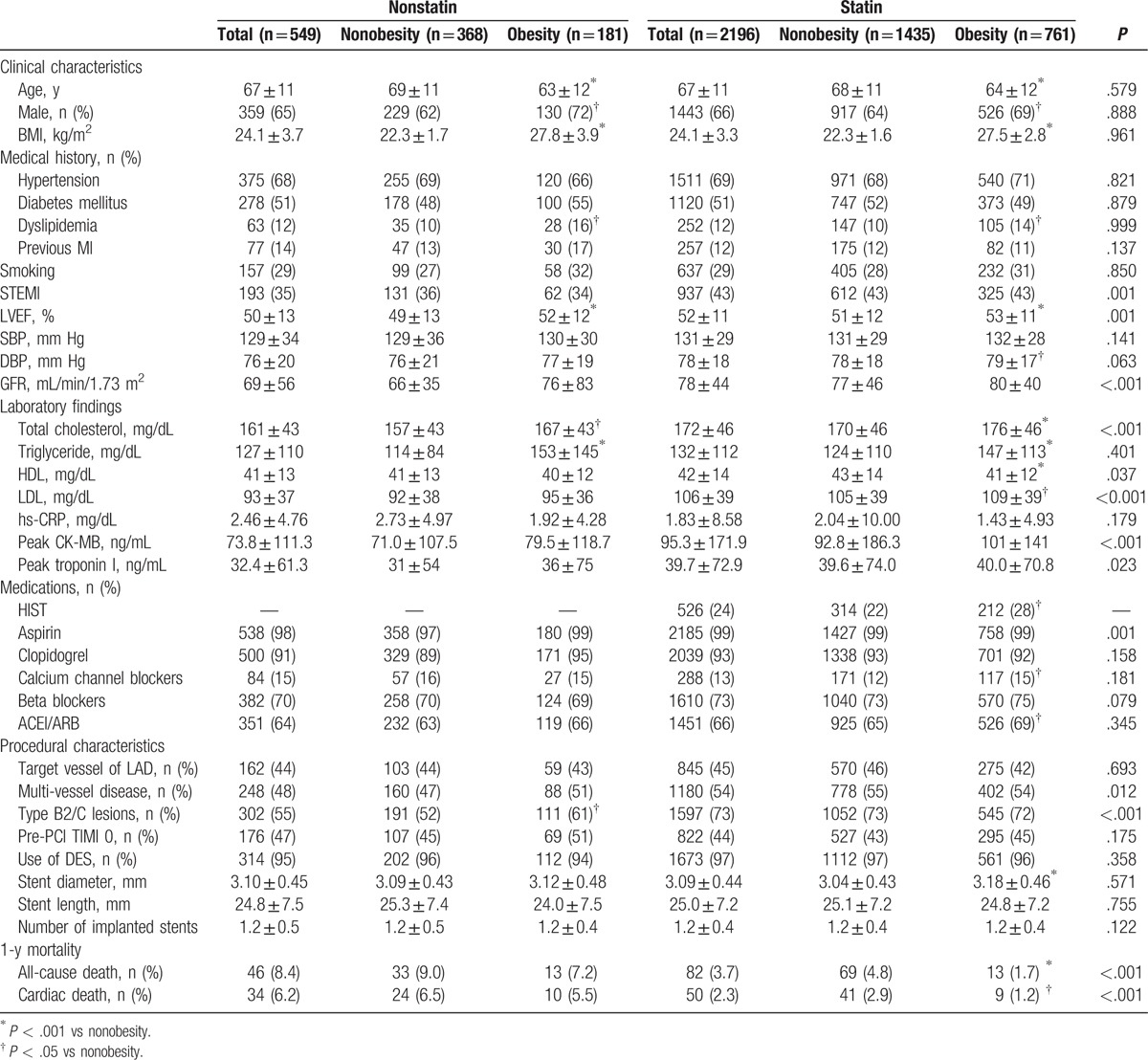
Clinical and procedural characteristics of the 2745 participants (age, 67 ± 11 years; 66% men) in this study are presented in Table 1. Initially, participants were divided into 2 groups according to statin therapy (nonstatin group: n = 549; statin: n = 2196). There were significant differences in clinical characteristics between nonstatin and statin groups, with a higher incidence of ST-elevation MI (STEMI), and higher levels of total cholesterol, low-density lipoproteins (LDL), peak creatine kinase-myocardial band (CK-MB), and peak troponin I in statin group. Among procedural characteristics, the incidence of multivessel disease and type B2/C lesions was significantly higher in statin group than in nonstatin group. Among clinical characteristics according to obesity status in statin and nonstatin group, the prevalence of male sex and dyslipidemia, LVEF, and the levels of total cholesterol and triglyceride were all significantly higher in obese patients than in nonobese patients in both groups. There were no significant differences in procedural characteristics according to obesity status in nonstatin and statin groups except for incidence of type B2/C lesions being significantly higher in obese patients than in nonobese patients in the nonstatin group, and stent diameter being significantly larger in obese patients than in nonobese patients in the statin group. In the statin group, the overall incidence of HIST was 24%, and was significantly higher in obese patients than in nonobese patients (28% vs 22%, P = .002).
Table 1.
Clinical and procedural characteristics.
3.2. Clinical outcomes according to statin therapy
During 1 year of follow-up, a total of 128 all-cause deaths and 84 cardiac deaths occurred. The occurrence of all-cause and cardiac death was significantly higher in nonstatin group than in statin group (all-cause death: 8.4% vs 3.7%, P < 0.001; cardiac death: 6.2% vs 2.3%, P < .001) (Table 1). Kaplan–Meier survival analysis showed that the cumulative incidence of all-cause and cardiac death was higher in nonstatin group than in statin group (log-rank P < .001, respectively) (Fig. 1). Statin therapy was associated with decreased risk of all-cause death (HR: 0.44; 95% confidence interval [CI]: 0.31–0.63; P < .001). After adjusting consecutive variables including age, sex, LVEF, previous MI, multivessel disease, STEMI, CKD, stent diameter, stent length, and traditional CV risk factors including hypertension, diabetes mellitus, dyslipidemia, obesity, and smoking, statin therapy was independently associated with decreased risk of all-cause death in all participants (Supplementary file: Table S1).
Figure 1.
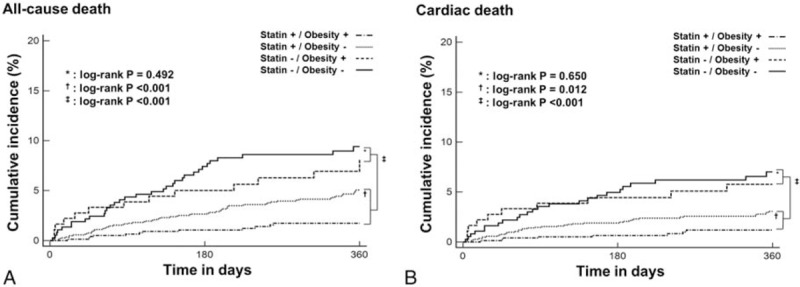
Estimates of the cumulative rate of (A) all-cause and (B) cardiac death according to statin therapy and obesity.
3.3. Clinical outcomes according to obesity in subjects with and without statin therapy
Nonstatin group. In non-statin group, the occurrence of all-cause and cardiac death was not different between obese and nonobese patients (all-cause death: 7.2% vs 9.0%, P = .478; cardiac death: 5.5% vs 6.5%, P = .649) (Table 1). The cumulative incidence of all-cause and cardiac death did not differ significantly according to obesity status in nonstatin group (Fig. 1). The risk of all-cause (HR: 0.80; 95% CI: 0.42–1.52; P = .492) and cardiac death (HR: 0.84; 95% CI: 0.40–1.76; P = .650) did not differ significantly according to obesity status in nonstatin group (Fig. 2).
Figure 2.
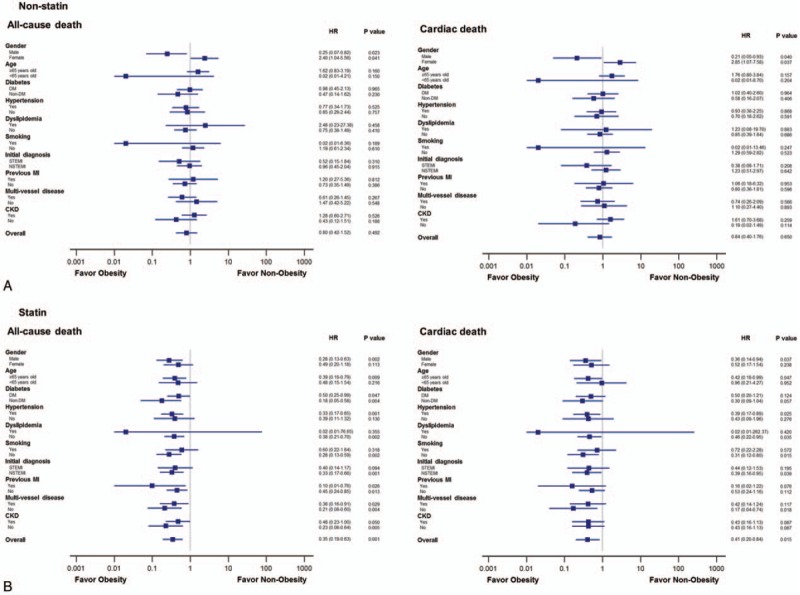
The estimated hazard ratios of obesity for all-cause and cardiac death in (A) nonstatin group and (B) statin group.
Statin group. In contrast to the results in nonstatin group, the occurrence of all-cause and cardiac death was significantly lower in obese patients than in nonobese patients in statin group (all-cause death: 1.7% vs 4.8%, P < .001; cardiac death: 1.2% vs 2.9%, P < .05) (Table 1). The cumulative incidence of all-cause (log-rank P < .001) and cardiac death (log-rank P = .012) was significantly lower in obese patients than in nonobese patients (Fig. 1). The cumulative incidence of all-cause and cardiac death did not differ significantly according to HIST (Supplementary file: Fig. S1). Obese patients had decreased risk of all-cause (HR: 0.35; 95% CI: 0.19–0.63; P = .001) and cardiac (HR: 0.41; 95% CI: 0.20–0.84; P = .015) death compared with nonobese patients in statin group (Fig. 2).
3.4. Impact of obesity on the primary outcome in patients on statin therapy
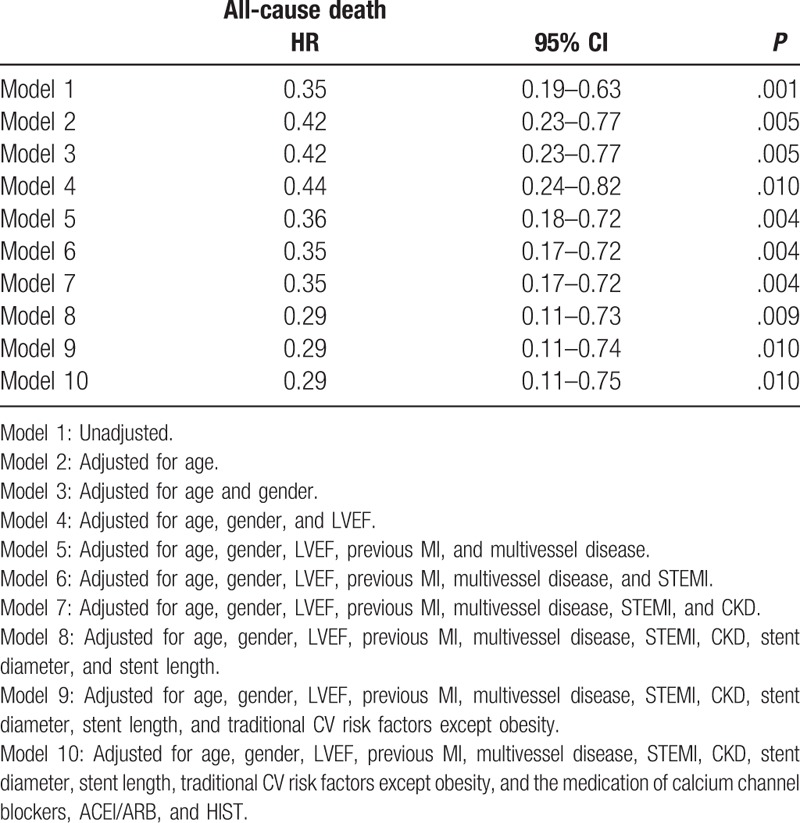
Multiple Cox hazard regression models were analyzed to identify the association between obesity and 1-year mortality in patients on statin therapy. After adjusting consecutive variables including age, sex, LVEF, previous MI, multivessel disease, STEMI, CKD, stent diameter, stent length, traditional CV risk factors except obesity, and the medication of calcium channel blockers, ACEI or ARB, and HIST, obesity remained independently associated with decreased risk of all-cause death in participants taking a statin (Table 2).
Table 2.
Multiple Cox hazard regression models to identify the impact of obesity on 1-year mortality in patients on statin therapy.
4. Discussion
This study confirmed the beneficial effect of statin therapy on mortality in patients with AMI. Despite more complex lesion characteristics in statin group than in nonstatin group, statin group had significantly lower mortality compared with nonstatin group. The major new findings were that 1-year mortality did not differ significantly according to obesity status in nonstatin group, but was significantly lower in obese patients than in nonobese patients in statin group; and this relationship between obesity and 1-year mortality in statin group persisted after adjustment for numerous factors that might impact mortality. The results of this study suggest that the obesity paradox after AMI may be confined to those taking a statin.
The use of statins has reduced the risk of death or CV events in patients with coronary heart disease.[22–24] In this nationwide study, we could identify that statin therapy was more emphasized in cases of complex coronary and procedural characteristics in current clinical practice after performing propensity score matching using numerous clinical factors. Despite adverse lesional and procedural features in statin group, the cumulative incidence of all-cause and cardiac death was significantly lower in statin group than in nonstatin group in the present study. Moreover, statin therapy was independently associated with the decreased risk of all-cause mortality in patients with AMI.
It is well known that obesity increases CV risk through risk factors such as increased levels of fasting plasma triglycerides, LDL cholesterol, blood glucose, and insulin, elevated blood pressure, and decreased levels of high-density lipoproteins (HDL) cholesterol and accelerates the progression of coronary atherosclerosis in the general population.[25,26] However, the phenomenon of obesity paradox has been replicated, especially after the event of AMI. Although several hypotheses have been proposed to explain it in patients with AMI,[27–32] the exact mechanism of this phenomenon remains unknown.
Recently, Nicholls et al[15] reported that an intensive lipid-lowering strategy had greater impact on atherosclerotic burden in patients with higher BMI. According to this study, obese patients had significantly lower plaque progression rates compared with nonobese patients under the intensive lipid-lowering therapy. This result highlighted the potential benefits of statin therapy in obese patients with coronary artery disease. However, there is a paucity of data on the effect of statin therapy on mortality according to obesity status in patients with AMI. In the present study, we focused on the fact that statin is considered the primary lipid-lowering drug for the treatment of dyslipidemia irrespective of obesity status in clinical practice; and the phenomenon of obesity paradox has been observed in AMI patients under the strong recommendation of statin therapy after the event of AMI. Thus, we formulated the hypothesis that statin therapy could be significantly associated with the phenomenon of obesity paradox after the event of AMI. Interestingly, we found that obesity paradox was observed only in patients treated with statin after AMI and that obesity was independently associated with decreased all-cause mortality after adjusting confounding risk factors in these patients. These results might imply that statin therapy significantly contributed to the phenomenon of obesity paradox after AMI in clinical practice.
Considering that intensive pharmacotherapy could play a substantial role in the potential obesity paradox after AMI, it is possible that the use of HIST could influence on 1-year mortality. In the present study, we found that the incidence of HIST was relatively low, about 24%, and that the incidence of HIST was significantly higher in obese patients than in nonobese patients in the present study (28% vs 22%, P < .05). It is noteworthy that the 1-year cumulative incidence of all-cause and cardiac death was not different according to HIST in the present study. Although the baseline levels of LDL cholesterol were significantly higher in patients with HIST than in those without HIST, participants either with or without HIST achieved acceptable mean LDL cholesterol levels at 1-year follow-up according to the recent guidelines of statin therapy for secondary prevention after AMI (Supplementary file: Table S2).[33] This might imply that statin therapy has a greater beneficial effect on mortality in AMI patients with obesity than in those without obesity regardless of HIST if the acceptable levels of LDL cholesterol are achieved. However, further prospective studies with larger sample sizes are warranted considering the paucity of data on the definition and efficacy of HIST in patients with AMI in Asian populations.
Although a number of studies have reported the phenomenon of obesity paradox, there remains much debate and uncertainties regarding its validity as a true paradox.[34] Recently, several studies have emphasized the quality or function of adipose tissue compared with its amount with respect to CV disease and the optimal treatment for atherogenic dyslipidemia in association with obesity.[35,36] In addition, the paradoxical preservation of endothelial function in obese patients also has been reported.[37] Thus, it should be necessary to evaluate the phenomenon of obesity paradox focusing on these novel issues.
Several limitations should be acknowledged in the present study. First, statin therapy was not randomly administered to patients during hospitalization and follow-up because the KAMIR is an observational study. Second, we could not examine the effects of specific types of statins or dosages on clinical outcomes. Our data seem to be limited only to a standard low dose of statins and this might be influenced by our national medical insurance system. Third, we only used 2 categories (nonobese vs obese) based on the criteria of BMI 25.0 kg/m2 for Asian populations because the prevalence of BMI ≥30.0 kg/m2 was only about 4% in the present study, which is typical of Asian patients with AMI. Fourth, our study did not have adequate sample size or randomization to prove the benefits based on statin dose. Finally, although we performed propensity score matching analysis using a number of confounding factors, the latter analysis based on a registry study is not able to adjust for all confounders. Despite the limitations of the present study, it was unique in that our results contribute to better understand the phenomenon of “obesity paradox” related to the benefit of statin therapy in obese patients after the event of AMI.
In conclusion, the phenomenon of obesity paradox appears to be confined to only patients treated with statin after AMI. This may be associated with the greater benefit of statin on mortality in AMI patients with obesity than in those without obesity. Statin therapy may be strongly associated with the phenomenon of obesity paradox after the event of AMI in recent clinical practice.
Acknowledgments
We thank all the KAMIR investigators and Ji Min Sung for their critical contribution to this study. These persons gave permission to be named in the Acknowledgments of the present study.
Supplementary Material
Footnotes
Abbreviations: ACEI = angiotensin converting enzyme inhibitors, ARB = angiotensin receptor blockers, BMI = body mass index, CKD = chronic kidney disease, CK-MB = creatine kinase-myocardial band, CV = cardiovascular, DBP = diastolic blood pressure, DES = drug-eluting stent, DM = diabetes mellitus, GFR = estimated glomerular filtration rate, HDLC = high-density lipoprotein cholesterol, HIST = high-intensity statin therapy, hs-CRP = high sensitivity C-reactive protein, LAD = left anterior descending coronary artery, LDLC = low-density lipoprotein cholesterol, LVEF = left ventricular ejection fraction, MI = myocardial infarction, NT-proBNP = N-terminal pro-B-type natriuretic peptide, PCI = percutaneous coronary intervention, SBP = systolic blood pressure, STEMI = ST-elevation MI, TIMI = thrombolysis in myocardial infarction.
This study was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korea Government (MSIP) (No. 2014R1A5A2010008).
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Han TS, Leer EMV, Seidell JC, et al. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 1995;311:1401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wilson PF, D’Agostino RB, Sullivan L, et al. Overweight and obesity as determinants of cardiovascular risk: the Framingham experience. Arch Intern Med 2002;162:1867–72. [DOI] [PubMed] [Google Scholar]
- [3].Calle EE, Thun MJ, Petrelli JM, et al. Body mass index and mortality in a prospective cohort of US adults. N Engl J Med 1999;341:1097–105. [DOI] [PubMed] [Google Scholar]
- [4].Kragelund C, Hassager C, Hildebrandt P, et al. Impact of obesity on long-term prognosis following acute myocardial infarction. Int J Cardiol 2005;98:123–31. [DOI] [PubMed] [Google Scholar]
- [5].Buettner HJ, Mueller C, Gick M, et al. The impact of obesity on mortality in UA/non-ST-segment elevation myocardial infarction. Eur Heart J 2007;28:1694–701. [DOI] [PubMed] [Google Scholar]
- [6].Bucholz EM, Rathore SS, Reid KJ, et al. Body mass index and mortality in acute myocardial infarction patients. Am J Med 2012;125:796–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wienbergen H, Gitt AK, Juenger C, et al. Impact of the body mass index on occurrence and outcome of acute ST-elevation myocardial infarction. Clin Res Cardiol 2008;97:83–8. [DOI] [PubMed] [Google Scholar]
- [8].Won KB, Kim BK, Chang HJ, et al. Metabolic syndrome does not impact long-term survival in patients with acute myocardial infarction after successful percutaneous coronary intervention with drug-eluting stents. Catheter Cardiovasc Interv 2014;83:713–20. [DOI] [PubMed] [Google Scholar]
- [9].Won KB, Hur SH, Cho YK, et al. Comparison of 2-year mortality according to obesity in stabilized patients with type 2 diabetes mellitus after acute myocardial infarction: results from the DIAMOND prospective cohort registry. Cardiovasc Diabetol 2015;14:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schwartz GG, Olsson AG, Ezekowitz MD, et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–8. [DOI] [PubMed] [Google Scholar]
- [11].Cannon CP, Braunwald E, McCabe CH, et al. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004;350:1495–504. [DOI] [PubMed] [Google Scholar]
- [12].de Lemos JA, Blazing MA, Wiviott SD, et al. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA 2004;292:1307–16. [DOI] [PubMed] [Google Scholar]
- [13].Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421. [PubMed] [Google Scholar]
- [14].Grundy SM, Cleeman JI, Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation 2004;110:227–39. [DOI] [PubMed] [Google Scholar]
- [15].Nicholls SJ, Tuzcu EM, Sipahi I, et al. Effects of obesity on lipid-lowering, anti-inflammatory, and antiatherosclerotic benefits of atorvastatin or pravastatin in patients with coronary artery disease (from the reversal study). Am J Cardiol 2006;97:1553–7. [DOI] [PubMed] [Google Scholar]
- [16].Lee KH, Jeong MH, Kim HM, et al. Benefit of early statin therapy in patients with acute myocardial infarction who have extremely low low-density lipoprotein cholesterol. J Am Coll Cardiol 2011;58:1664–71. [DOI] [PubMed] [Google Scholar]
- [17].The Joint European Society of Cardiology/American College of Cardiology Committee. Myocardial infarction redefined—a consensus document of the Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Eur Heart J 2000;21:1502–1513. [DOI] [PubMed] [Google Scholar]
- [18].Levine GN, Bates ER, Bittl JA, et al. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Thorac Cardiovasc Surg 2016;152:1243–75. [DOI] [PubMed] [Google Scholar]
- [19].Ellis SG, Vandormael MG, Cowley MJ, et al. Coronary morphologic and clinical determinants of procedural outcome with angioplasty for multivessel coronary disease. Implications for patient selection. Multivessel Angioplasty Prognosis Study Group. Circulation 1990;82:1193–202. [DOI] [PubMed] [Google Scholar]
- [20].Manginas A, Gatzov P, Chasikidis C, et al. Estimation of coronary flow reserve using the Thrombolysis In Myocardial Infarction (TIMI) frame count method. Am J Cardiol 1999;83:1562–5. [DOI] [PubMed] [Google Scholar]
- [21].Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med 2003;139:137–47. [DOI] [PubMed] [Google Scholar]
- [22].Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- [23].Sacks RM, Pfeffer MA, Moye LA, et al. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels. N Engl J Med 1996;335:1001–9. [DOI] [PubMed] [Google Scholar]
- [24].The Long-Term Intervention with Pravastatin in Ischaemic Disease (LIPID) Study Group. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med 1998;339:1349–57. [DOI] [PubMed] [Google Scholar]
- [25].Boden G, Salehi S. Why does obesity increase the risk for cardiovascular disease? Curr Pharm Des 2013;19:5678–83. [DOI] [PubMed] [Google Scholar]
- [26].McGill HC, Jr, McMahan CA, Herderick EE, et al. Obesity accelerates the progression of coronary atherosclerosis in young men. Circulation 2002;105:2712–8. [DOI] [PubMed] [Google Scholar]
- [27].Salie R, Huisamen B, Lochner A. High carbohydrate and high fat diets protect the heart against ischaemia/reperfusion injury. Cardiovasc Diabetol 2014;13:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Poncelas M, Inserte J, Vilardosa Ú, et al. Obesity induced by high fat diet attenuates postinfarct myocardial remodeling and dysfunction in adult B6D2F1 mice. J Mol Cell Cardiol 2015;84:154–61. [DOI] [PubMed] [Google Scholar]
- [29].Lundergan CF, Ross AM, McCarthy WF, et al. Predictors of left ventricular function after acute myocardial infarction: Effects of time to treatment, patency, and body mass index. Am Heart J 2001;142:43–50. [DOI] [PubMed] [Google Scholar]
- [30].Sohn GH, Kim EK, Hahn JY, et al. Impact of overweight on myocardial infarct size in patients undergoing primary percutaneous coronary intervention: a magnetic resonance imaging study. Atherosclerosis 2014;235:570–5. [DOI] [PubMed] [Google Scholar]
- [31].Habbu A, Lakkis NM, Dokainish H. The obesity paradox: fact or fiction? Am J Cardiol 2006;98:944–8. [DOI] [PubMed] [Google Scholar]
- [32].Knudtson MD, Klein BE, Klein R, et al. Association with weight loss and subsequent mortality risk. Ann Epidemiol 2005;15:483–91. [DOI] [PubMed] [Google Scholar]
- [33].Grundy SM, Arai H, Barter P, et al. An International Atherosclerosis Society position paper: global recommendations for the management of dyslipidemia: executive summary. Atherosclerosis 2014;232:410–3. [DOI] [PubMed] [Google Scholar]
- [34].Banack HR, Kaufman JS. Does selection bias explain the obesity paradox among individuals with cardiovascular disease? Ann Epidemiol 2015;25:342–9. [DOI] [PubMed] [Google Scholar]
- [35].Bastien M, Poirier P, Lemieux I, et al. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 2014;56:369–81. [DOI] [PubMed] [Google Scholar]
- [36].Fruchart JC, Davignon J, Hermans MP, et al. Residual macrovascular risk in 2013: what have we learned? Cardiovasc Diabetol 2014;13:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Biasucci LM, Graziani F, Rizzello V, et al. Paradoxical preservation of vascular function in severe obesity. Am J Med 2010;123:727–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


