Abstract
There are many difficulties distinguishing bacterial from viral meningitis that could be reasonably solved using biomarkers. The aim of this study was to evaluate lactate, procalcitonin (PCT), ferritin, serum-CRP (C-reactive protein), and other known biomarkers in differentiating bacterial meningitis from viral meningitis in children.
All children aged 28 days to 14 years with suspected meningitis who were admitted to Mofid Children's Hospital, Tehran, between October 2012 and November 2013, were enrolled in this prospective cross-sectional study. Children were divided into 2 groups of bacterial and viral meningitis, based on the results of cerebrospinal fluid (CSF) culture, polymerase chain reaction, and cytochemical profile. Diagnostic values of CSF parameters (ferritin, PCT, absolute neutrophil count [ANC], white blood cell count, and lactate) and serum parameters (PCT, ferritin, CRP, and erythrocyte sedimentation rate [ESR]) were evaluated.
Among 50 patients with meningitis, 12 were diagnosed with bacterial meningitis. Concentrations of all markers were significantly different between bacterial and viral meningitis, except for serum (P = .389) and CSF (P = .136) PCT. The best rates of area under the receiver operating characteristic (ROC) curve (AUC) were achieved by lactate (AUC = 0.923) and serum-CRP (AUC = 0.889). The best negative predictive values (NPV) for bacterial meningitis were attained by ANC (100%) and lactate (97.1%).
The results of our study suggest that ferritin and PCT are not strong predictive biomarkers. A combination of low CSF lactate, ANC, ESR, and serum-CRP could reasonably rule out the bacterial meningitis.
Keywords: aseptic meningitis, bacterial meningitis, biomarkers, ferritin, lactate, procalcitonin, viral meningitis
1. Introduction
Bacterial meningitis is one of the most serious and fatal diseases of childhood worldwide. Prevalence of meningococcal meningitis is approximately 1.2 per 100,000 people which increase to 100 cases per 100,000 people in the African meningitis belt.[1] Most of the countries of Africa and Southeast Asia have case fatality rates of higher than 8%, according to the WHO report. Generally, a case fatality rate of 4% to 27% is predicted for meningitis in developing countries.[1] Likewise, in developed countries, it is among the 10 chief causes of childhood death.[2] The average incidence of meningitis for all children under the age of 15 years was 12.8 and 6.6 cases per 100,000 population for men and women, respectively, in Tehran metropolis (1999–2005 inclusive).[3]
Although bacterial meningitis has a considerably lower incidence rate than viral/aseptic meningitis,[4,5] prompt diagnosis and treatment are necessary due to its hazardous nature.[6] Bacterial meningitis remains a source of substantial morbidity and mortality despite new and potent antibiotics use while aseptic meningitis generally has a benign course requiring only supportive care.[6] So, rapid diagnosis of bacterial meningitis and its differentiation from aseptic meningitis should be done to allow early initiation of therapy for children at risk of having the disease without over treating low-risk children.
Till now, the gold standard for the diagnosis of bacterial meningitis and distinguishing it from aseptic meningitis has been cerebrospinal fluid (CSF) which requires considerable time to prepare its result.[7] Consequently, most children with CSF pleocytosis are admitted to hospital to receive broad-spectrum antibiotics while awaiting culture test results.[8] Another drawback of CSF culture is its low yield in some centers. In a study in sub-Sahara Africa, Gram staining and CSF culture were reported positive in only a few cases of bacterial meningitis[9] and according to a study from Iran, CSF culture was positive in only 3.97% (11/277) of patients with bacterial meningitis.[10]
Moreover, the clinical symptoms and laboratory tests including CSF parameters could be overlapping in bacterial and viral/aseptic meningitis. The constellation of symptoms of fever, nuchal rigidity, and changes in mental status, a well-known triad of meningitis, was observed in only 44% of patients with bacterial meningitis.[11] Thus, relying on the clinical history and physical examination of patients suspected of having bacterial meningitis could be misleading.[12]
Besides the atypical history, nondistinctive physical examination and hesitant and low yield CSF cultures on some occasions, CSF parameters are occasionally indecisive and cannot discriminate between bacterial and aseptic meningitis.[13–15] Moreover, even insufficient doses of antibiotics can lead to atypical CSF results in patients.[16]
Due to the aforementioned practical problems in discriminating bacterial and viral/aseptic meningitis, new and strong biomarkers should be identified to help clinicians to start early and appropriate management. Such markers should logically have near 100% sensitivity to prevent any error in diagnosis and management.[5]
Anaerobic metabolism of microbes in CSF and/or ischemia of brain tissue lead to local lactate production, a process that is not affected by levels of lactate in the blood. Comparing with CSF glucose, this is a point of strength for lactate for differentiating bacterial and aseptic meningitis.[17–19]
Procalcitonin (PCT) is secreted in trivial amounts by C cells of the thyroid in response to increased blood calcium levels.[20–22] After this, bacterial infection is produced in the liver[23] and peripheral blood mononuclear cells and increases up to several thousand-fold.[24,25]
Ferritin is another acute phase reactant that is incapable of penetrating the blood brain barrier.[26] So, CSF ferritin levels are not influenced by its blood levels.[9] The CSF ferritin level increases during inflammation, a usual process in bacterial meningitis.[27]
This study was designed to evaluate the ability of serum and CSF levels of PCT, ferritin, and lactate in discrimination of bacterial and viral/aseptic meningitis. Also, other known biomarkers including erythrocyte sedimentation rate (ESR), serum-CRP (C-reactive protein), and white blood cell (WBC) values were measured and compared with each other.
2. Materials and methods
2.1. Study design
This prospective cross-sectional study was conducted on children admitted to Mofid children's hospital, between October 2012 and November 2013. This hospital is a multidisciplinary academic and referral one, exclusively devoted to children. With over 250 beds and above 20 clinical subspecialty wards and laboratory sections, it provides inpatient and outpatient services to Tehran city and surrounding provinces.
2.2. Eligible patients for lumbar puncture
Any child aged 28 days to 14 years who was diagnosed with suspected meningitis, defined as follows, underwent lumbar puncture:
-
1.
Fever with signs of meningeal irritation, bulging fontanels, unexplained irritability, persistent nausea, and vomiting or petechial rash or purpura
-
2.
Unexplained alteration of consciousness with or without fever
-
3.
Fever with no localizing signs in an ill or toxic child
-
4.
First febrile seizure in under 12 to 18 months of age
-
5.
Complex febrile seizure.
A lumbar puncture was done by the pediatric resident or fellow, for every child who presented with suspected meningitis, in the absence of any contraindications. All the samples were immediately sent to the Pediatric Infections Research Center, affiliated to Shahid Beheshti University of Medical Sciences, Mofid Children's Hospital, Tehran, Iran, which has one of the most advanced research laboratories in the country, to be assessed. CSF samples were evaluated for simple cell analysis and differential count, absolute neutrophil count (ANC), checking sugar and protein, ferritin, PCT, culture and polymerase chain reaction (PCR) for Streptococcus pneumoniae, Neisseria meningitidis, Haemophilus influenzae, Listeria monocytogenes, and Group B streptococcus. Furthermore, serum samples were collected for checking PCT, ferritin, serum-CRP, and ESR and a questionnaire was filled out.
2.3. Patients
2.3.1. Inclusion criteria
After exclusion of CSF specimens without pleocytosis and doing tests, enrolled samples were divided into 2 groups;
-
(1)Bacterial meningitis group consisting of 2 subgroups:
-
a)Definite bacterial meningitis: samples with positive Gram staining, culture, or PCR (for S pneumoniae, N meningitidis, H influenzae, L monocytogenes, and Group B streptococcus)
-
b)Presumed bacterial meningitis: The presence of clinical picture of meningitis with at least 2 of the following: protein ≥80, glucose ≤40, WBC ≥300, and or polymorph nuclear cell predominancy were considered as bacterial meningitis.
-
a)
-
(2)Viral/aseptic meningitis consisting of 2 subgroups:
-
a)Definite viral/aseptic meningitis: samples with positive PCR of Enterovirus, Human Herpesvirus, Varicella zoster virus, and Epstein-Barr virus.
-
b)Presumed viral/aseptic meningitis: Presence of clinical symptoms of meningitis with any CSF lacking the bacterial characteristics (previously mentioned in the group 1b meningitis).
-
a)
2.3.2. Exclusion criteria
Immunocompromised patients, those without CSF pleocytosis (CSF WBC ≥10 cell/mm3) as well as CSF shunt infections were excluded.
The study was approved by the ethical committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran. Informed consent was obtained from the children's parents before doing lumbar puncture.
2.4. PCR
Using AccuPrep Genomic DNA extraction kit (cat.no.k-3032 lot no.1008J, BIONEER, Seoul, Korea), DNA was extracted from all Group B streptococcus isolates. PCR amplification profile comprised a 300 nM concentration of each oligonucleotide primer (Eurofins MWG Operon, Huntsville, Alabama); 200 mM (each) deoxynucleoside triphosphates dCTP, dGTP, dATP, and dUTP; 0.125 U of Taq DNA polymerase; and 5.5 mM MgCl2 (from GENET BIO, Daejeon, Korea, prime Taq TM DNA polymerase, URL: www.genetbio.com)
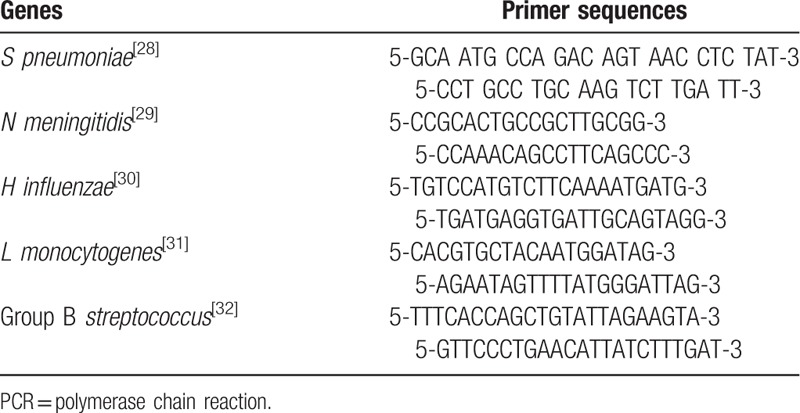
The PCR products were analyzed by gel electrophoresis on 1.5% BIONEER agarose gels in 1× TBE buffer (890 mM of boric acid, 890 mM Tris, 40 mL of 0.5 M EDTA, pH 8.0) at 100 V for 60 minutes. Green loading buffer with DNA stain (Jena Bioscience, Germany; Lot: 111.034) was used during loading of the samples and ladder. The sizes of the PCR products were determined by comparison with the molecular size standard (50 bp–1 Kb linear scale; low range DNA ladder, or 100 bp–3 Kb linear scale, and mid-range DNA ladder, Jena Bioscience). Primer sequences were presented in Table 1.
Table 1.
PCR primer sequences.
2.5. Assay of biomarkers
Serum and CSF ferritin concentrations were measured using the Ferritin AccuBind ELISA assay (Monobind Inc., Lake Forest). PCT levels in serum and CSF were determined by double-antibody sandwich enzyme-linked immunosorbent assay (ELISA) (Human PCT ELISA, BT Laboratory, Shanghai, China). Serum-CRP concentration was measured using immunoturbidimetric method with the lowest detected level of 2 mg/L (PARS AZMUN, Karaj, Iran). CSF Lactate level was measured using standard enzymatic test (Randox Laboratories, London, UK).
2.6. Statistical analysis
Statistical analyses of obtained data were performed using SPSS software, version 21.0 (SPSS, Chicago, IL). Data were analyzed using chi-square test, Fisher exact test, and independent t test. We used receiver operating characteristic (ROC) curve to determine the optimal cutoff point for each biomarker to distinguish bacterial meningitis from aseptic/viral meningitis. Also, sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) were calculated for biomarkers in chosen cutoff point. ROC curve analysis was done by SPSS software. Binary logistic regression was done and the obtained values presented as AUC to compare the biomarkers combination. P value of <.05 was considered statistically significant.
3. Results
In this study, lumbar punctures were done for 370 patients with suspected meningitis. Of 62 patients diagnosed with meningitis, 50 immunocompetent patients with CSF pleocytosis were enrolled in the study (Fig. 1). Among them, 20 patients (40%) were women and 30 (60%) were men, with viral/aseptic meningitis being more common.
Figure 1.

Flow diagram of study selection process.
The average age was 42.75 months with the minimum of 21 days and maximum of 144 months. The highest rate of meningitis was among children aged 13 months.
CSF smear was positive in 7 patients including 3 gram-positive cocci and 4 gram-negative diplococci. There were 2 positive CSF cultures (S pneumoniae and S aureus).
From 50 cases of meningitis, 38 (76%) patients had viral/aseptic meningitis and 12 (24%) had bacterial. Of bacterial meningitis: 10 were definite bacterial meningitis (5 cases of S pneumoniae, 4 cases of H influenzae, and 1 case S aureus) and 2 cases were presumed bacterial meningitis (overall defined as bacterial meningitis). Among 38 viral/aseptic cases, 2 cases were definite viral/aseptic (herpes simplex virus type 1 and Enterovirus), and 36 cases were presumed viral/aseptic meningitis (overall defined as viral/ aseptic meningitis).
S pneumoniae was the most commonly isolated pathogen in 5 cases (41.6%) of patients with bacterial meningitis whose age varied from 1 month to 8 years.
Fever was the most frequent initial symptoms on arrival in all patients. The second most prevalent symptom in bacterial group was poor feeding/loss of appetite (83%) and in viral group was nausea and vomiting (78%) (Table 2). Clinical features did not differ significantly between the 2 groups. Poor feeding and headache were the main reasons for referring to hospital in children under 14 months of age and over 3 years, respectively (Table 3).
Table 2.
Prevalence of clinical symptoms between bacterial and viral/aseptic meningitis patients at time of admission.
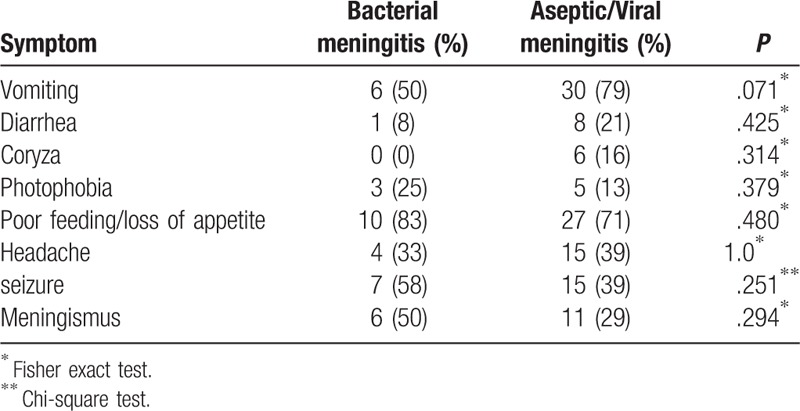
Table 3.
Prevalence of clinical symptoms in different age groups.

There were significant differences in biomarker distributions between bacterial and viral/aseptic meningitis, except for PCT levels in serum and CSF (Table 4).
Table 4.
Distribution of biomarkers between 2 groups.
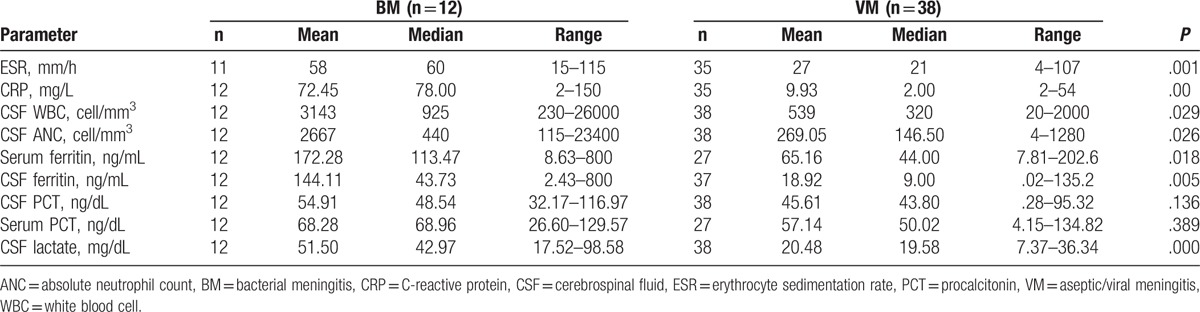
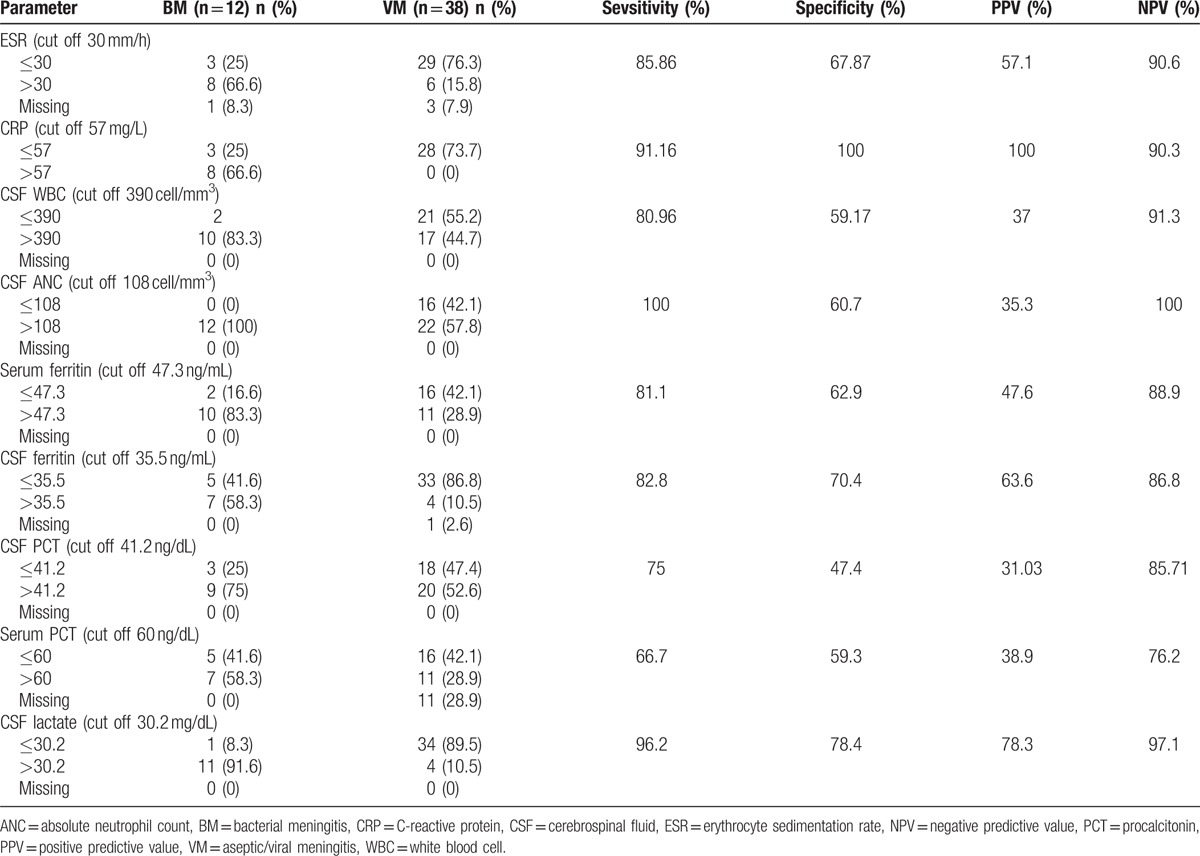
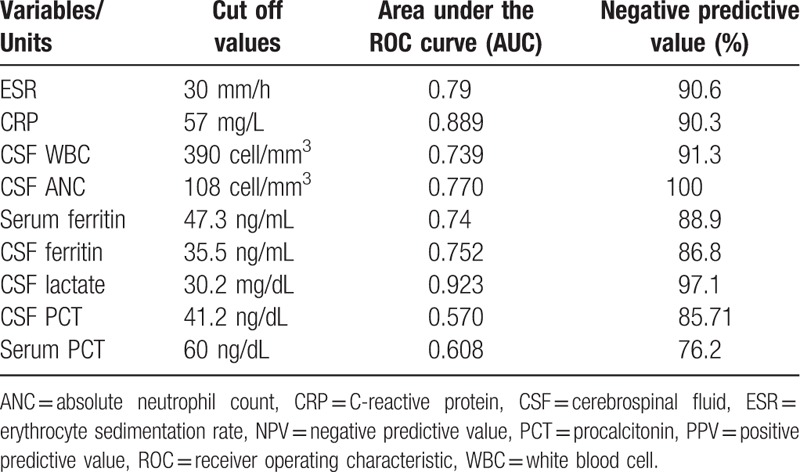
Sensitivity and specificity of each biomarker with PPV and NPV are presented in Table 5. Among biomarkers, serum-CRP at a cutoff value of 57 mg/L had the highest specificity (100%) and lactate had the best sensitivity (96.2%). Two cases of S pneumoniae and H influenzae meningitis had a truly negative serum-CRP. The mean CSF-PCT levels in patients with bacterial and viral/aseptic meningitis were 59.4 and 45.6 ng/dL, respectively (P = .136) (Tables 4 and 5). The mean serum PCT levels were 68.27 and 57.14 ng/dL in bacterial and viral/aseptic groups, respectively (P = .389) (Tables 4 and 5).
Table 5.
Evaluation of diagnostic value of CSF and serum parameter betweeen 2 group.
The area under the ROC curves (AUC), shown in Table 6, indicated that lactate was a much better biomarker for distinguishing bacterial meningitis from viral meningitis (AUC = 0.923). Also, PCT levels in serum and CSF had the lowest AUC, compared with other factors (Table 6). It is important to mention, ANC (100%) and lactate (97.1%) were identified as the best markers to rule out bacterial meningitis with the highest NPV (Table 6).
Table 6.
Comparison of markers according to AUC, NPV.
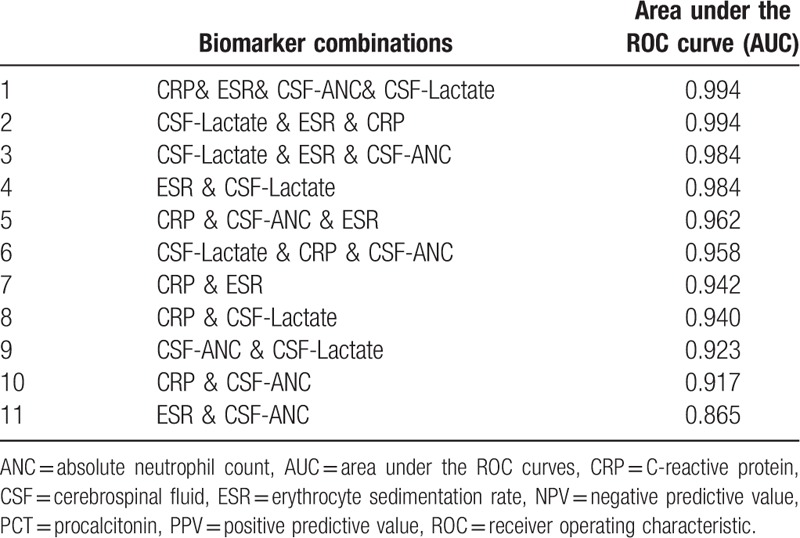
Diagnostic values of combination of biomarkers with good NPV and AUC are presented in Table 7.
Table 7.
Characteristics of ANC, lactate, CRP, and ESR (the best markers based on AUC and NPV) combination.
4. Discussion
Differentiating bacterial from viral meningitis could be done through clinical signs and symptoms, CSF analysis, Gram staining, antigen assays, peripheral WBC, and neutrophil count with some uncertainty and ideally via CSF culture.[6,14,15,33–35] However, this diagnostic gold standard of bacterial meningitis is time-consuming and has a modest sensitivity (70–85%) and is even lower due to factors related to applied materials and techniques and receiving antibiotic(s) before lumbar puncture, making it unsuitable for timely decision-making.[15] According to our study, the yield of CSF culture was 83.3%. In this study, the rate of pneumococcal meningitis in patients with bacterial meningitis was 41.6%. S pneumoniae was the most commonly isolated agent of bacterial meningitis among children under 24 months in the United States in 1995.[36] In a multicenter investigation of children with invasive pneumococcal infections in the United States over an observation time of 6 years, about 15% of pneumococcal infections were bacterial meningitis.[37] Our findings in a previous study revealed that more than 40% of young children in Tehran carry S pneumoniae in their nasopharynx.[38]
Using biomarkers in CSF or serum could contribute to early diagnosis and prevention of unnecessary antibiotic usage. The most critical question facing a patient with suspected meningitis, who has CSF pleocytosis, before withholding antibiotics, is to rule out bacterial meningitis. This task could be accomplished best through a test with a high negative predictive value; a diagnostic characteristic of a test which can exclude a disease. Considering the results in the current study, CSF-ANC with the highest NPV (100%) is a valuable indicator for discrimination of different types of meningitis. So, all children with an ANC lower than 108 cell/μL (cutoff point) were not in the bacterial meningitis group, in our study. In a multicenter case cohort study, at a 100 cell/μL cutoff point, sensitivity of ANC in CSF was 82% and specificity was 73%.[5]
While neutrophil predominance may be seen in the CSF, in the acute phase of aseptic meningitis it is usually a transient phenomenon and neutrophil dominance in the established phase of meningitis is generally in favor of a bacterial process.[39–42] This fact is demonstrated by a research study that showed a significant reduction in the rate of ANC in CSF after 24 hours in patients with aseptic meningitis and a stable or increased rate of ANC after 24 hours in bacterial meningitis patients.[43]
Inclusion of CSF-ANC as a component of a clinical decision rule, named “bacterial meningitis score” (BMS) shows its position in the diagnosis of bacterial meningitis. The other elements of the score are: positive CSF Gram stain, CSF-ANC of 1000/μL or higher, CSF protein level of 80 mg/dL or higher, peripheral blood ANC of 10,000/μL or higher, and patients presenting with seizure.[34] BMS application in 900 children with clinically diagnosed meningitis accurately detected 889 children with bacterial meningitis.[44]
After ANC, CSF lactate with the NPV of 97.1% was the second powerful marker to rule out bacterial meningitis in our study. According to previous reports, lactate could be a helpful biomarker for differentiation of viral and bacterial meningitis in children and adults. Though the sensitivity may decrease in antibiotic-pretreated patients,[19] it is assumed that CSF lactate could be a helpful marker to discriminate bacterial meningitis (>6 mmol/L) from partially treated meningitis (4–6 mmol/L) and aseptic meningitis (<2 mmol/L).[45] A 15-year retrospective study of children with meningitis showed that in bacterial group CSF lactate has a sensitivity of 95% and specificity of 94%, at the cutoff point of 3.0 mmol/L.[46]
Substantial elevation of serum-CRP levels was seen in patients with bacterial meningitis, compared with aseptic meningitis, with sensitivity and specificity ranging from 70% to 100% to 90% to 95%, respectively.[47–49] In our study, this acute phase reactant had a NPV, PPV, sensitivity, and specificity of 90.3%, 100%, 85.86%, and 100%, respectively, indicating it is a good biomarker.
WBC count in CSF rises as a result of inflammatory cytokines released during meningitis. The CSF WBC count was significantly higher in bacterial meningitis (mean = 4839 cell/μL), compared with viral meningitis (mean = 159 cell/μL) (P < .001) in a research and at the cutoff value of 321 WBC cell/μL the sensitivity and specificity for bacterial meningitis were 80.6% and 81.4%, respectively.[50] In the present study, the mean values of WBC count of CSF in bacterial meningitis and viral/aseptic meningitis patients were 3143 and 539 cell/μL, respectively and with NPV of 91.3% and PPV of 37%, this showed a high sensitivity and low specificity (Table 4).
CSF-PCT has been reported as a sensitive marker in a few studies,[51–53] and less sensitivity in the others.[54,55] A retrospective analysis of published data from European countries[5] showed the serum PCT level as a very sensitive and specific marker to distinguish between bacterial and viral/aseptic meningitis with a sensitivity and specificity of 99% and 83% at the cutoff value of 0.5 ng/mL. In 2 studies by Makoo et al[56] and Kepa et al,[57] the diagnostic productivity of serum and CSF-PCT levels were evaluated. According to the results, serum PCT level was a powerful biomarker for differentiation of bacterial meningitis from viral meningitis but the diagnostic value of CSF-PCT was slightly weaker.[57]
According to the current study, CSF-PCT had modest sensitivity, specificity, NPV, and PPV of 75%, 47.4%, 85.71, and 31.03, respectively, and serum PCT level had a sensitivity of 66.7% and specificity of 59.3%, in a cutoff point of 6 ng/dL. These findings represent CSF or serum PCT as a weak marker for differentiating bacterial and viral meningitis.
High concentration of ferritin in the CSF is the consequence of its passage through dissipated blood brain barrier and local synthesis in CNS as well, resulting in increased concentration of ferritin in patients with bacterial meningitis.[27,58] The present study showed a significantly higher CSF ferritin in bacterial meningitis, denoting more intense systemic inflammation in bacterial meningitis.[27] At the cutoff point of 35.5 ng/dL, the sensitivity and specificity were 82.8% and 70.55%, respectively. Similarly, Jebamalar et al[27] found a significant correlation between the ferritin levels in CSF and serum of patients with bacterial meningitis, compared with those with viral meningitis. Another study on 42 children with meningitis in Iran showed that CSF ferritin level was about 10-fold higher in bacterial meningitis than in viral form.[59]
Total CSF WBC mean count and ESR levels were significantly higher in patients with bacterial meningitis. However, due to relatively lower specificity of these biomarkers, they should be used along with other tests. The prognostic power of WBC count in distinguishing bacterial meningitis may be constrained by some agents; a high rate of variability is present in CSF WBC counts and lower cell counts may be observed in children, immunocompromised patients, and/or even in immunocompetent patients with bacterial meningitis due to meningococcus and pneumococcus. Also, increased WBC count in CSF of viral/aseptic patients can be observed, similar to our results.
ROC curve analysis of biomarkers (Table 6) indicated that the highest value of AUC was related to CSF lactate (0.92), serum-CRP (0.88), ESR (0.79), and CSF-ANC (0.77), respectively.
A comparison between area under ROC curve as well as NPV (Table 6) showed that lactate, ANC, ESR, and serum-CRP could be considered as valuable biomarkers in distinguishing bacterial from viral/aseptic meningitis. As is emphasized in other studies, due to the critical nature of bacterial meningitis, using a biomarker alone is not recommended to establish or exclude the diagnosis.[5,15,60] We combined 4 biomarkers (CRP, ESR, CSF-lactate, and CSF-ANC) together to find the best compositions. According to Table 7, combinations of biomarkers increase the AUC (Table 7). When considering the AUC values in numbers 2, 4, and 9 it can be concluded that low value added to the AUC in the presence of ANC.
5. Conclusion
According to our study, CSF lactate, CSF ANC, serum ESR, and serum CRP are useful biomarkers in distinguishing bacterial from viral meningitis, based on their AUC values. In addition, combination of these potent biomarkers strengthens the predictive value.
Acknowledgments
This article is based on the Dr. Shekoofan Alizadeh thesis (number: 1393/141). The authors would like to thank the Pediatric Infection Research Center, affiliated to Shahid Beheshti University of Medical Sciences and Center for Development of Clinical Research of Nemazee Hospital affiliated to Shiraz University of Medical Sciences, Shiraz, Iran and Hassan Khajehei for copy-editing the manuscript.
Footnotes
Abbreviations: ANC = absolute neutrophil count, AUC = area under the ROC curves, CRP = C-reactive protein, CSF = cerebrospinal fluid, ELISA = enzyme-linked immunosorbent assay, ESR = erythrocyte sedimentation rate, NPV = negative predictive value, PCT = procalcitonin, PPV = positive predictive value, ROC = receiver operating characteristic.
Financial support: The financial support of this project was supported by the “Vice- Presidency for Science and Technology” affiliated to “Presidency of the I.R. Iran”.
Authors have no conflicts of interest to declare.
References
- [1].World Health Organization. Control of Epidemic Meningococcal Disease: WHO practical guidelines. 2nd ed.1998;Geneva: World Health Organization, 1–84. [Google Scholar]
- [2].Won H, Yang S, Gaydos C, et al. A broad range assay for rapid detection and etiologic characterization of bacterial meningitis: performance testing in samples from sub-Sahara. Diagn Microbiol Infect Dis 2012;74:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mosavi-Jarrahi A, Esteghamati A, Asgari F, et al. Temporal analysis of the incidence of meningitis in the Tehran metropolitan area, 1999-2005. Popul Health Metr 2009;7:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Nigrovic LE, Kuppermann N, Macias CG, et al. Clinical prediction rule for identifying children with cerebrospinal fluid pleocytosis at very low risk of bacterial meningitis. JAMA 2007;297:52–60. [DOI] [PubMed] [Google Scholar]
- [5].Dubos F, Korczowski B, Aygun DA, et al. Serum procalcitonin level and other biological markers to distinguish between bacterial and aseptic meningitis in children: a European multicenter case cohort study. Arch Pediatr Adolesc Med 2008;162:1157–63. [DOI] [PubMed] [Google Scholar]
- [6].Sáez-Llorens X, McCracken GH. Bacterial meningitis in children. The Lancet 2003;361:2139–48. [DOI] [PubMed] [Google Scholar]
- [7].Jansen G, Mooibroek M, Idema J, et al. Rapid identification of bacteria in blood cultures by using fluorescently labeled oligonucleotide probes. J Clin Microbiol 2000;38:814–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Poppert S, Essig A, Stoehr B, et al. Rapid diagnosis of bacterial meningitis by real-time PCR and fluorescence in situ hybridization. J Clin Microbiol 2005;43:3390–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Makoo ZB, Ahadi N, Hasani A, et al. Cerebrospinal fluid (CSF) ferritin for differentiation of aseptic and bacterial meningitis in adults. Am J Infect Dis 2010;6:98. [Google Scholar]
- [10].Ghotaslou R, Farajnia S, Yeganeh F, et al. Detection of acute childhood meningitis by PCR, culture and agglutination tests in Tabriz, Iran. Acta Med Iran 2012;50:192. [PubMed] [Google Scholar]
- [11].Van de Beek D, de Gans J, Spanjaard L, et al. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med 2004;351:1849–59. [DOI] [PubMed] [Google Scholar]
- [12].Waghdhare S, Kalantri A, Joshi R, et al. Accuracy of physical signs for detecting meningitis: a hospital-based diagnostic accuracy study. Clin Neurol Neurosurg 2010;112:752–7. [DOI] [PubMed] [Google Scholar]
- [13].Aronin SI, Peduzzi P, Quagliarello VJ. Community-acquired bacterial meningitis: risk stratification for adverse clinical outcome and effect of antibiotic timing. Ann Intern Med 1998;129:862–9. [DOI] [PubMed] [Google Scholar]
- [14].Hegen H, Deisenhammer F. Cerebrospinal fluid biomarkers in bacterial meningitis/biomarker im liquor cerebrospinalis bei bakterieller meningitis. Laboratoriums Medizin 2009;33:321–31. [Google Scholar]
- [15].Tunkel AR, Hartman BJ, Kaplan SL, et al. Practice guidelines for the management of bacterial meningitis. Clin Infect Dis 2004;39:1267–84. [DOI] [PubMed] [Google Scholar]
- [16].Swartz M. Acute Bacterial Meningitis. Infectious Diseases 2nd ed.1998;Philadelphia, PA: WB Saunders, 1382–1415. [Google Scholar]
- [17].Guerra-Romero L, Täauber MG, Fournier MA, et al. Lactate and glucose concentrations in brain interstitial fluid, cerebrospinal fluid, and serum during experimental pneumococcal meningitis. J Infect Dis 1992;166:546–50. [DOI] [PubMed] [Google Scholar]
- [18].Posner JB, Plum F. Independence of blood and cerebrospinal fluid lactate. Arch Neurol 1967;16:492. [DOI] [PubMed] [Google Scholar]
- [19].Sakushima K, Hayashino Y, Kawaguchi T, et al. Diagnostic accuracy of cerebrospinal fluid lactate for differentiating bacterial meningitis from aseptic meningitis: a meta-analysis. J Infect 2011;62:255–62. [DOI] [PubMed] [Google Scholar]
- [20].Abramson JS, Hampton KD, Babu S, et al. The use of C-reactive protein from cerebrospinal fluid for differentiating meningitis from other central nervous system diseases. J Infect Dis 1985;151:854–8. [DOI] [PubMed] [Google Scholar]
- [21].Meisner M. Pathobiochemistry and clinical use of procalcitonin. Clin Chim Acta 2002;323:17–29. [DOI] [PubMed] [Google Scholar]
- [22].Simon L, Gauvin F, Amre DK, et al. Serum procalcitonin and C-reactive protein levels as markers of bacterial infection: a systematic review and meta-analysis. Clin Infect Dis 2004;39:206–17. [DOI] [PubMed] [Google Scholar]
- [23].Groothuis G, Limburg P, ten Duis H, et al. Procalcitonin behaves as a fast responding acute phase protein in vivo and in vitro. Crit Care Med 2000;28:458–61. [DOI] [PubMed] [Google Scholar]
- [24].Müller B, Becker KL, Schächinger H, et al. Calcitonin precursors are reliable markers of sepsis in a medical intensive care unit. Crit Care Med 2000;28:977–83. [DOI] [PubMed] [Google Scholar]
- [25].Taskın E, Turgut M, Kılıc M, et al. Serum procalcitonin and cerebrospinal fluid cytokines level in children with meningitis. Mediators Inflamm 2004;13:269–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sharma S, Dabla PK. Evaluation of CSF ferritin as an early marker for differentiating meningitis in pediatric patients. Bali Med J 2014;3:2. [Google Scholar]
- [27].Jebamalar AA, Prabhat, Balakrishnapillai AK, et al. Cerebrospinal fluid ferritin and albumin index: potential candidates for scoring system to differentiate between bacterial and viral meningitis in children. Biomarkers 2016;21:424–8. [DOI] [PubMed] [Google Scholar]
- [28].Leung MH, Bryson K, Freystatter K, et al. Sequetyping: serotyping Streptococcus pneumoniae by a single PCR sequencing strategy. J Clin Microbiol 2012;50:2419–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Jordens JZ, Heckels JE. A novel porA-based real-time PCR for detection of meningococcal carriage. J Med Microbiol 2005;54:463–6. [DOI] [PubMed] [Google Scholar]
- [30].Ohkusu K, Nash K, Inderlied C. Molecular characterisation of Haemophilus influenzae type a and untypeable strains isolated simultaneously from cerebrospinal fluid and blood: novel use of quantitative real-time PCR based on the cap copy number to determine virulence. Clin Microbiol Infect 2005;11:637–43. [DOI] [PubMed] [Google Scholar]
- [31].Aznar R, Alarcón B. PCR detection of Listeria monocytogenes: a study of multiple factors affecting sensitivity. J Appl Microbiol 2003;95:958–66. [DOI] [PubMed] [Google Scholar]
- [32].Ke D, Ménard C, Picard FJ, et al. Development of conventional and real-time PCR assays for the rapid detection of group B streptococci. Clin Chem 2000;46:324–31. [PubMed] [Google Scholar]
- [33].Kuppermann N, Malley R, Inkelis SH, et al. Clinical and hematologic features do not reliably identify children with unsuspected meningococcal disease. Pediatrics 1999;103:e20–30. [DOI] [PubMed] [Google Scholar]
- [34].Nigrovic LE, Kuppermann N, Malley R. Development and validation of a multivariable predictive model to distinguish bacterial from aseptic meningitis in children in the post-Haemophilus influenzae era. Pediatrics 2002;110:712–9. [DOI] [PubMed] [Google Scholar]
- [35].Maxson S, Lewno MJ, Schutze GE. Clinical usefulness of cerebrospinal fluid bacterial antigen studies. J Pediatr 1994;125:235–8. [DOI] [PubMed] [Google Scholar]
- [36].Schuchat A, Robinson K, Wenger JD, et al. Bacterial meningitis in the United States in 1995. N Engl J Med 1997;337:970–6. [DOI] [PubMed] [Google Scholar]
- [37].Kaplan SL, Mason EO, Jr, Wald ER, et al. Six year multicenter surveillance of invasive pneumococcal infections in children. Pediatr Infect Dis 2002;21:141–7. [DOI] [PubMed] [Google Scholar]
- [38].Dashti AS, Abdinia B, Karimi A. Nasopharyngeal carrier rate of Streptococcus pneumoniae in children: serotype distribution and antimicrobial resistance. Arch Iran Med 2012;15:500. [PubMed] [Google Scholar]
- [39].Berkley JA, Mwangi I, Ngetsa CJ, et al. Diagnosis of acute bacterial meningitis in children at a district hospital in sub-Saharan Africa. The Lancet 2001;357:1753–7. [DOI] [PubMed] [Google Scholar]
- [40].Coll M-T, Uriz M-S, Pineda V, et al. Meningococcal meningitis with ‘normal’ cerebrospinal fluid. J Infect 1994;29:289–94. [DOI] [PubMed] [Google Scholar]
- [41].Negrini B, Kelleher KJ, Wald ER. Cerebrospinal fluid findings in aseptic versus bacterial meningitis. Pediatrics 2000;105:316–9. [DOI] [PubMed] [Google Scholar]
- [42].Schut ES, de Gans J, van de Beek D. Community-acquired bacterial meningitis in adults. Pract Neurol 2008;8:8–23. [DOI] [PubMed] [Google Scholar]
- [43].Straussberg R, Harel L, Nussinovitch M, et al. Absolute neutrophil count in aseptic and bacterial meningitis related to time of lumbar puncture. Pediatr Neurol 2003;28:365–9. [DOI] [PubMed] [Google Scholar]
- [44].Dubos F, De la Rocque F, Levy C, et al. Sensitivity of the bacterial meningitis score in 889 children with bacterial meningitis. J Pediatr 2008;152:378–82. [DOI] [PubMed] [Google Scholar]
- [45].Cunha BA. Distinguishing bacterial from viral meningitis: the critical importance of the CSF lactic acid levels. Intensive Care Med 2006;32:1272–3. [DOI] [PubMed] [Google Scholar]
- [46].Filho EM, Horita SM, Gilio AE, et al. Cerebrospinal fluid lactate level as a diagnostic biomarker for bacterial meningitis in children. Int J Emerg Med 2014;1:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shimetani KS, M. Mori N. Levels of three inflammation markers, C-reactive protein, serum amyloid A protein and procalcitonin, in the serum and cerebrospinal fluid of patients with meningitis. Scand J Clin Lab Invest 2001;61:567–74. [DOI] [PubMed] [Google Scholar]
- [48].Pemde HK, Harish K, Thawrani Y, et al. C-reactive protein in childhood meningitides. Indian J Pediatr 1996;63:73–7. [DOI] [PubMed] [Google Scholar]
- [49].Paradowski M, Łobos M, Kuydowicz J, et al. Acute phase proteins in serum and cerebrospinal fluid in the course of bacterial meningitis. Clin Biochem 1995;28:459–66. [DOI] [PubMed] [Google Scholar]
- [50].Águeda S, Campos T, Maia A. Prediction of bacterial meningitis based on cerebrospinal fluid pleocytosis in children. Braz J Infect Dis 2013;17:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dubos F, Moulin F, Gajdos V, et al. Serum procalcitonin and other biologic markers to distinguish between bacterial and aseptic meningitis. J Pediatr 2006;149:72–6. [DOI] [PubMed] [Google Scholar]
- [52].Korczowski B, Bijoś A, Rybak A. [Procalcitonin in diagnosis of purulent and aseptic meningitis in children]. Pol Merkur Lekarski 2000;9:755–7. [PubMed] [Google Scholar]
- [53].Viallon A, Zeni F, Lambert C, et al. High sensitivity and specificity of serum procalcitonin levels in adults with bacterial meningitis. Clin Infect Dis 1999;28:1310–6. [DOI] [PubMed] [Google Scholar]
- [54].Schwarz S, Bertram M, Schwab S, et al. Serum procalcitonin levels in bacterial and abacterial meningitis. Crit Care Med 2000;28:1828–32. [DOI] [PubMed] [Google Scholar]
- [55].Bygum Knudsen T, Larsen K, Birk Kristiansen T, et al. Diagnostic value of soluble CD163 serum levels in patients suspected of meningitis: comparison with CRP and procalcitonin. Scand J Infect Dis 2007;39:542–53. [DOI] [PubMed] [Google Scholar]
- [56].Makoo ZB, Soltani HR, Hasani A, et al. Diagnostic value of serum and cerebrospinal fluid procalcitonin in differentiation bacterial from aseptic meningitis. Am J Infect Dis 2010;6:93. [Google Scholar]
- [57].Kepa L, Oczko-Grzesik B, Błedowski D. [Procalcitonin (PCT) concentration in cerebrospinal fluid and plasma of patients with purulent and lymphocytic meningoencephalitis—own observations]. Przegl Epidemiol 2005;59:703–9. [PubMed] [Google Scholar]
- [58].Takahashi S, Oki J, Miyamoto A, et al. Beta-2-microglobulin and ferritin in cerebrospinal fluid for evaluation of patients with meningitis of different etiologies. Brain Dev 1999;21:192–9. [DOI] [PubMed] [Google Scholar]
- [59].Rezaei M, Mamishi S, Mahmoudi S, et al. Cerebrospinal fluid ferritin in children with viral and bacterial meningitis. Br J Biomed Sci 2013;70:101–3. [DOI] [PubMed] [Google Scholar]
- [60].Kanegaye JT, Soliemanzadeh P, Bradley JS. Lumbar puncture in pediatric bacterial meningitis: defining the time interval for recovery of cerebrospinal fluid pathogens after parenteral antibiotic pretreatment. Pediatrics 2001;108:1169–74. [PubMed] [Google Scholar]


