Supplemental Digital Content is available in the text
Keywords: glucocorticoids, meta-analysis, pain control, total knee replacement
Abstract
Background:
The ability of preoperative intravenous glucocorticoids to control pain after total knee arthroplasty (TKA) has been examined in many studies, but it remains controversial. Therefore, we undertook a meta-analysis of randomized controlled trials (RCTs) to evaluate the efficacy and safety of preoperative intravenous glucocorticoids for postoperative pain management after TKA.
Methods:
We systematically searched RCTs from electronic databases, including PubMed, Embase, Web of Science, the Cochrane Library, the Chinese Wanfang Database, and the China National Knowledge Infrastructure database. The outcomes included visual analogue scale (VAS) scores at 6, 12, 24, 48, and 72 hours after TKA; the occurrence of postoperative nausea and vomiting (PONV); blood glucose at 6 and 24 hours after TKA; and the occurrence of infection.
Results:
Of the identified studies, a total of 11 RCTs involving 1000 patients (glucocorticoids = 501, control = 499) were included in this meta-analysis. Compared with a placebo, preoperative intravenous glucocorticoids significantly reduced VAS scores at 6, 12, 24, and 48 hours, with decreases of 3.63 points, 6.81 points, 10.40 points, and 3.15 points, respectively, on a 110-point VAS. Moreover, intravenous glucocorticoids were associated with significant decreases of 19.4% and 16.8% in the occurrence of nausea and vomiting, respectively. However, intravenous glucocorticoids were also associated with increased blood glucose with no clinical importance at 6 hours after TKA. No significant difference was found in the occurrence of infection or in blood glucose at 24 hours after TKA.
Conclusion:
Preoperative intravenous glucocorticoids are an effective and safe method to reduce postoperative pain and PONV in patients following TKA. More studies are necessary to identify the optimal dose and type of glucocorticoids for maximal pain control.
1. Introduction
Appropriately 700,000 total knee arthroplasty (TKA) surgeries are performed annually in the United States.[1] The number of primary TKA procedures is expected to reach 3.48 million in the United States in 2030, demonstrating an 8-fold increase from 2005.[2] TKA is a painful surgery, and most patients will experience moderate to extreme pain in the early period after TKA. Inadequate postoperative analgesia impairs rehabilitation, prolongs hospitalization, and increases the risk of adverse events.[1,3–5] Many anesthesia modalities and medications have been used in various combinations to reduce the amount of pain experienced by patients postoperatively. The standard treatment for pain relief after TKA involves patient-controlled anesthesia (PCA) with morphine. Single-dose morphine usage is limited in its application due to morphine-related complications such as postoperative nausea and vomiting (PONV).[6–8]
Chang and Cho[9] noted that perioperative analgesia protocols for TKA vary greatly, as does postoperative pain intensity. Intravenous glucocorticoids manage pain by reducing inflammatory factors such as interleukin-6 (IL-6) and C-reactive protein (CRP).[10] Preoperative intravenous glucocorticoids have been used for pain control since 2011, many studies have compared the efficacy of preoperative intravenous glucocorticoids for pain management after surgery.[11,12] However, it should be noted that the sample size of these studies was limited (ranging from 11 to 134 patients), which may affect the accuracy of relevant conclusions. The purpose of this systematic review and meta-analysis of randomized controlled trials (RCTs) was to collect relevant studies to identify whether preoperative intravenous glucocorticoids are associated with lesser acute pain scores and PONV without increasing the occurrence of infection after TKA.
2. Materials and methods
This meta-analysis was conducted in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions[13] and was written in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses checklist.[14]
2.1. Search strategy
For this meta-analysis, PubMed, Embase, Web of Science, the Cochrane Library, the Chinese Wanfang database, and the China National Knowledge Infrastructure (CNKI) database were systematically searched from inception through March 2017 without language restrictions. The search strategies in the PubMed database were as follows: (((((((steroids) OR glucocorticoids) OR hydrocortisone) OR prednisolone) OR methylprednisolone) OR dexamethasone)) AND (((((“Arthroplasty, Replacement, Knee”[Mesh]) OR TKR) OR TKA) OR TKA) OR total knee replacement). The search strategies can be accessed in Supplement S1. In addition, the references of relevant reviews or meta-analyses were searched to identify any omitted studies. No animal experiments or direct human trials were involved in this meta-analysis; consequently, neither a special ethics review nor ethical approval was necessary.
2.2. Inclusion criteria
-
1.
Participants: patients diagnosed with degenerative or rheumatoid arthritis undergoing primary TKA.
-
2.
Intervention: preoperative intravenous glucocorticoids for pain control.
-
3.
Comparison: placebo or intravenous saline.
-
4.
Outcomes: visual analogue scale (VAS) at 6, 12, 24, 48, and 72 hours; total morphine consumption at 24 and 48 hours; blood glucose at 6 and 24 hours after TKA; the occurrence of nausea, vomiting, pruritus, and infection.
-
5.
Study design: only RCTs were included in this meta-analysis.
2.3. Data extraction
A standard data extraction form was designed using Microsoft Excel, and data were independently extracted from the included studies by 2 reviewers (L.-X.C. and L.-J.L.). The following data were extracted: author, publication year, country, language, publication status, number, age and ratio of males of the glucocorticoids group and control group, type of anesthesia, equivalency to dexamethasone dose, and interval and postoperative anesthesia. The outcomes included the following: (1) VAS score at 6, 12, 24, 48, and 72 hours on a 110-point VAS (0 indicated “no pain,” and 100 indicated the “worst imaginable pain”); (2) the occurrence of nausea, vomiting, pruritus, and infection; (3) total morphine consumption at 24 and 48 hours after TKA; (4) blood glucose at 6 and 24 hours after TKA.
Different types of glucocorticoids were converted to the equivalent doses of dexamethasone, as follows: 0.75 mg dexamethasone = 4 mg methylprednisolone = 5 mg prednisolone = 20 mg hydrocortisone.[15] The subgroup analysis was conducted based on the dose of glucocorticoids [≥10 mg (high dose) or <10 mg (low dose)]. If there was a disagreement, a consensus was reached by consulting a senior reviewer (S.-G.H.) or through discussion.
2.4. Quality assessment
The quality assessment of the included RCTs was performed by 2 reviewers according to the Cochrane Handbook for Systematic Reviews of Interventions.[13] A total of 7 domains were assessed: random sequence generation (selection bias), allocation concealment (selection bias), blinding to participants and personnel (performance bias), blinding of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other potential bias. These domains were categorized as having a low risk of bias, a high risk of bias, or an unclear risk of bias. Studies with a high risk of bias in 1 or more domains were categorized as having a high risk of bias. Studies with a low risk of bias in all domains were categorized as having a low risk of bias. If the assessment was neither high risk of bias nor low risk of bias, the studies were categorized as having an unclear risk of bias.
2.5. Statistical analysis
Stata 12.0 (Stata Corp, College Station, TX) was used to perform the meta-analysis. The weighted mean difference (WMD) and its corresponding 95% confidence interval (CI) were used to assess the following continuous variable outcomes: VAS at 6, 12, 24, 48, and 72 hours and blood glucose at 6 and 24 hours after TKA. The dichotomous outcomes (the incidence of nausea, vomiting, pruritus, and infection) are presented as risk ratios (RRs) with corresponding 95% CIs. Heterogeneity across studies was assessed based on I2 values. An I2 value <50% indicated a low level of heterogeneity across studies, and a fixed-effects model was used for these variables. Otherwise, a random-effects model was used for the purposes of this meta-analysis. To further analyze the level of heterogeneity across studies, sensitivity and subgroup analyses were performed to determine the source of heterogeneity. Egger linear regression test and funnel plots were used to evaluate publication bias. The relationship between steroid dosage and incidence of nausea was explored using GraphPad Prism software (Version 6.0; GraphPad Software, San Diego, CA). A correlation coefficient (r) was generated to evaluate the relationship between glucocorticoid dosage and VAS at 6, 12, 24, and 48 hours.
3. Results
3.1. Search results
The literature search and selection process are shown in Figure 1. A total of 816 studies were identified during the initial search (PubMed = 207, Embase = 312, Web of Science = 90, Cochrane Library = 66, Chinese Wanfang database = 100, CNKI = 41), of which 554 were excluded due to duplication and 250 were excluded because they did not meet the eligibility criteria based on a review of their titles and abstracts. After the full texts of the remaining 12 studies were reviewed, 1 study[11] was excluded because it assessed the application of glucocorticoids for joint arthroplasty. Finally, 11 clinical trials involving 1000 patients (glucocorticoids = 501, control = 499) were included in this meta-analysis.[15–25]
Figure 1.
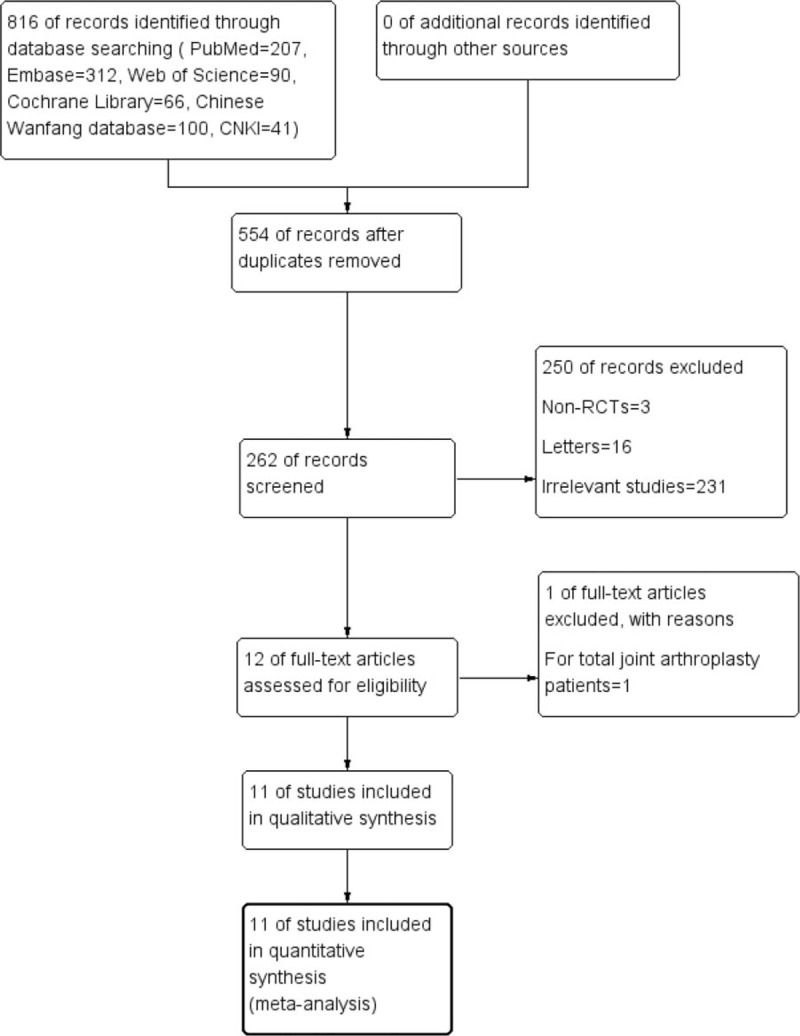
Study selection flowchart.
3.2. General characteristic
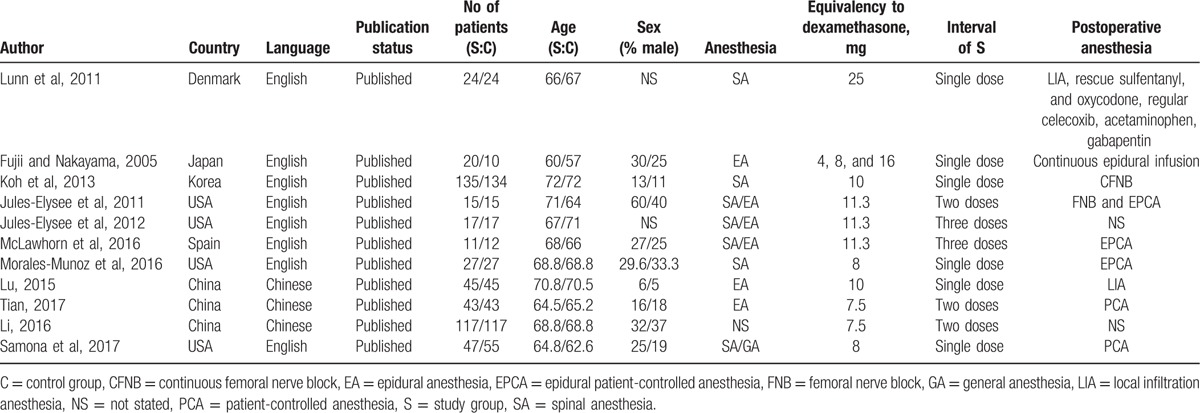
The general characteristics of the included RCTs are shown in Table 1. The publication years of the included studies ranged from 2011 to 2017. Three articles were in Chinese, and the rest were in English. The equivalent dexamethasone doses ranged from 4 to 25 mg. The postoperative anesthetics including PCA, femoral nerve block, and patient-controlled epidural anesthesia. The anesthesia methods for surgery included general anesthesia, epidural anesthesia, and spinal anesthesia.
Table 1.
The general characteristic of the included studies.
3.3. Quality assessment
The risk of bias summary and risk of bias graph are shown in Figures 2 and 3, respectively. Only 4 studies did not describe random sequence generation; these studies were assessed as having an unclear risk of bias.[18,19,22,24] Only 1 study was assessed as having an unclear risk of bias due to allocation concealment, blinding of participants, and blinding of outcomes assessment.[24] The rest studies all carried low risk of bias. That is, that the overall quality of the included studies was high.
Figure 2.
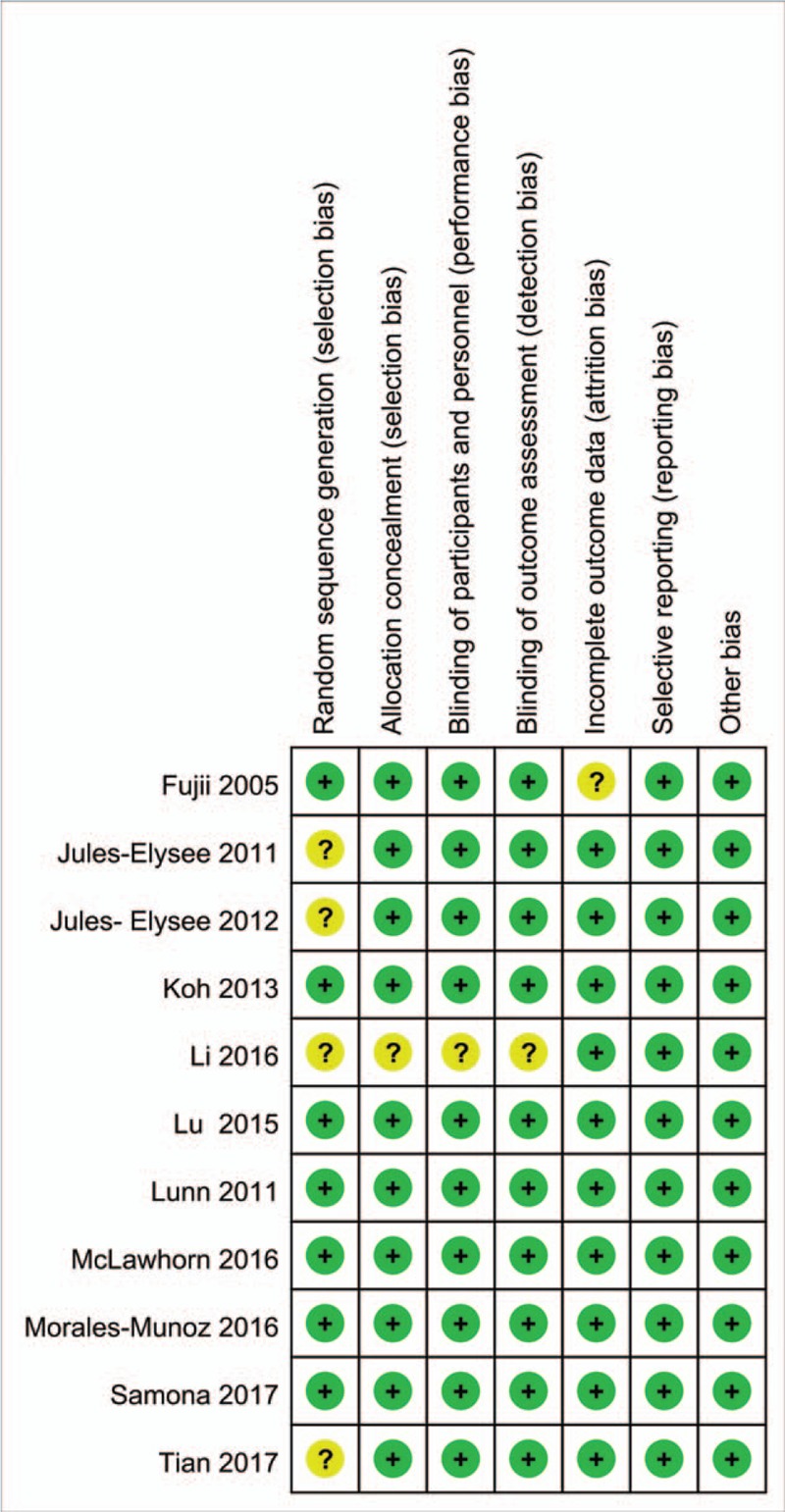
Summary of the risk of bias.
Figure 3.
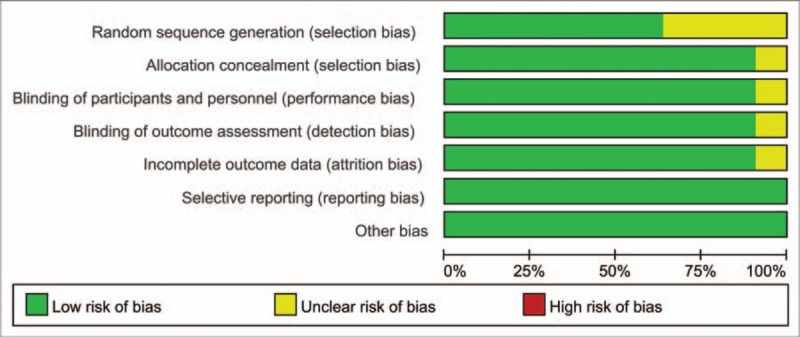
Graph of the risk of bias.
4. Results of meta-analysis
4.1. VAS at 6, 12, 24, 48, and 72 hours
Four studies involved patient-reported VAS scores at 6 hours, and there was great heterogeneity among the included studies (I2 = 84.4%, P = .000). Compared with the control group, preoperative intravenous glucocorticoids were associated with a significantly reduction in VAS scores at 6 hours (WMD = −3.63, 95% CI = −5.53 to −1.73, P = .000, Fig. 4).
Figure 4.
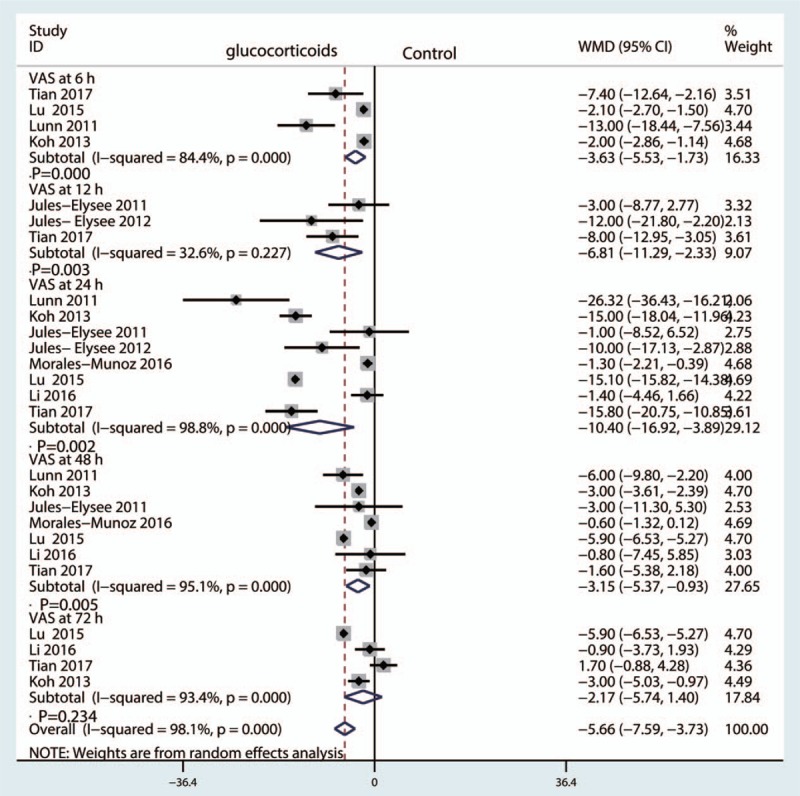
Forest plot comparing VAS at 6, 12, 24, 48, and 72 hours after TKA between the glucocorticoid group and the control group.
Three studies reported the VAS at 12 hours in the glucocorticoid group and control group, and there was little heterogeneity among the included studies (I2 = 32.6%, P = .227). Compared with the control groups, preoperative intravenous glucocorticoids were associated with a reduction in VAS scores at 12 hours (WMD = −6.81, 95% CI = −11.29 to −2.33, P = .003, Fig. 4).
Eight studies involving 435 patients reported VAS scores at 24 hours,[15,17–19,21] with substantial heterogeneity among the included studies (I2 = 98.8%, P = .000). A random-effect model was used to pool the data. Compared with the control groups, preoperative intravenous glucocorticoids were associated with a reduction in VAS scores at 24 hours (WMD = −10.40, 95% CI = −16.92 to −3.89, P = .000, Fig. 4).
Seven studies[15,17,18,21] assessed VAS at 48 hours, and there was great heterogeneity among the included studies (I2 = 95.1%, P = .000). Compared with the control groups, preoperative intravenous glucocorticoids were associated with a reduction in VAS scores at 48 hours (WMD = −3.15, 95% CI = −5.37 to −0.93, P = .005, Fig. 4).
Four studies involving 435 patients reported VAS at 72 hours,[15,17–19,21] with substantial heterogeneity among the included studies (I2 = 93.4%, P = .000). A random-effect model was used to pool the data. There was no significant difference in VAS scores at 72 hours between the glucocorticoid group and the control groups (WMD = −2.17, 95% CI = −5.74 to −3.89, P = .234, Fig. 4).
We plotted the equivalent dexamethasone doses as the abscissa against the VAS at 6, 12, 24, and 48 hours as the ordinate to generate a scatterplot. The results indicated that the VAS tended to drop as the glucocorticoid dose increased. In addition, the linear correlation coefficient (r) was calculated by Spearman method. A negative correlation was observed between glucocorticoids dosage and the VAS at 6 hours (r = −0.316, P = .011, Fig. 5A), 12 hours (r = −0.866, P < .001, Fig. 5B), 24 hours (r = −0.546, P = .048, Fig. 5C) and 48 hours (r = −0.789, P = .038, Fig. 5D).
Figure 5.
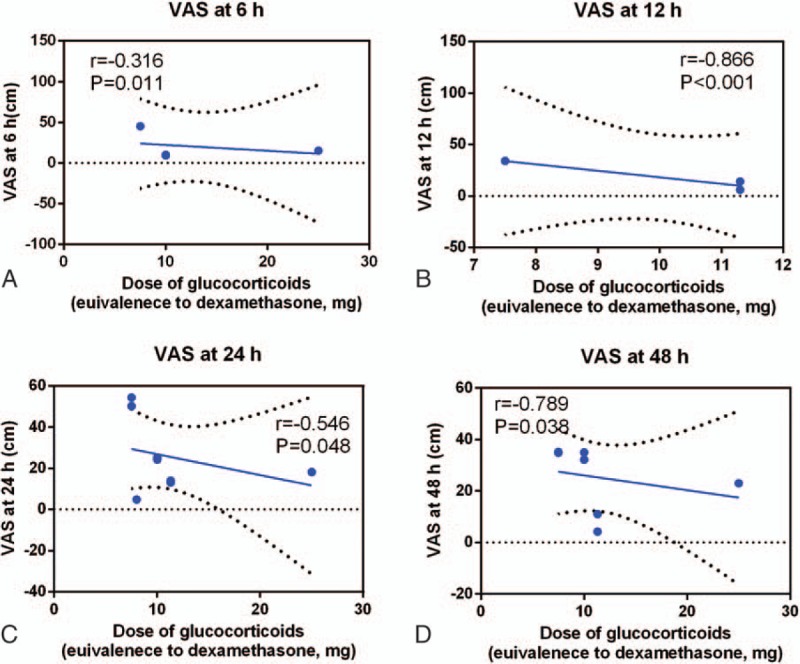
Scatterplot comparing the dose of glucocorticoids with the VAS at (A) 6 hours, (B) 12 hours, (C) 24 hours, and (D) 72 hours.
4.2. Total morphine consumption at 24 and 48 hours After TKA
Two studies involving 435 patients reported total morphine consumption at 24 hours[15,17–19,21] with substantial heterogeneity between the included studies (I2 = 87.6%, P = .004). Three studies involving 526 patients reported total morphine consumption at 48 hours with great heterogeneity (I2 = 63.1%, P = .067). A random-effect model was used to pool the data. There was no significant difference in total morphine at 24 hours (WMD = −60.70, 95% CI = −128.78–7.38, P = .081, Fig. 6) or 48 hours (WMD = −24.59, 95% CI = −49.43–0.52, P = .0521, Fig. 6) between the glucocorticoid and control groups.
Figure 6.
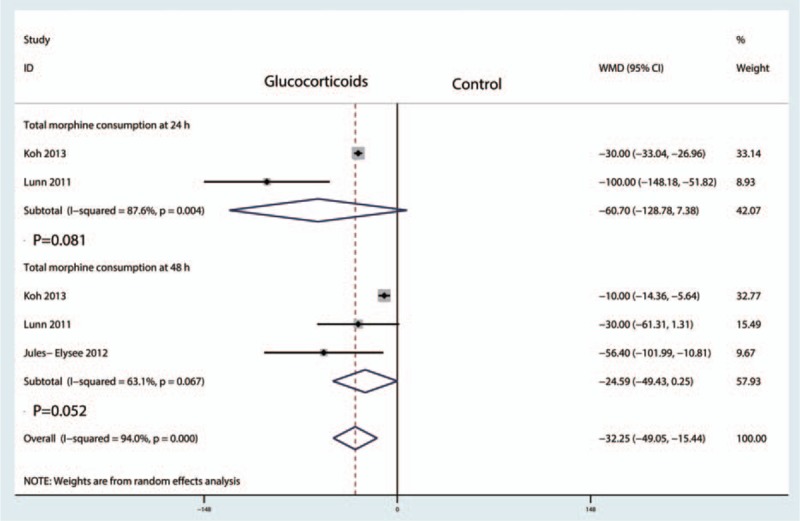
Forest plot comparing total morphine consumption at 24 and 48 hours between the glucocorticoid group and the control group.
4.3. The occurrence of nausea and vomiting
The occurrence of nausea was reported in 10 studies[15,17–19,21] with little heterogeneity (I2 = 15.3%, P = .303), and thus a fixed-effect model was applied. Compared with the control groups, preoperative intravenous glucocorticoids were associated with a 19.4% reduction in the occurrence of nausea (25.2% vs 44.6%, RR = 0.57, 95% CI = 0.47–0.68, P = .000, Fig. 7).
Figure 7.
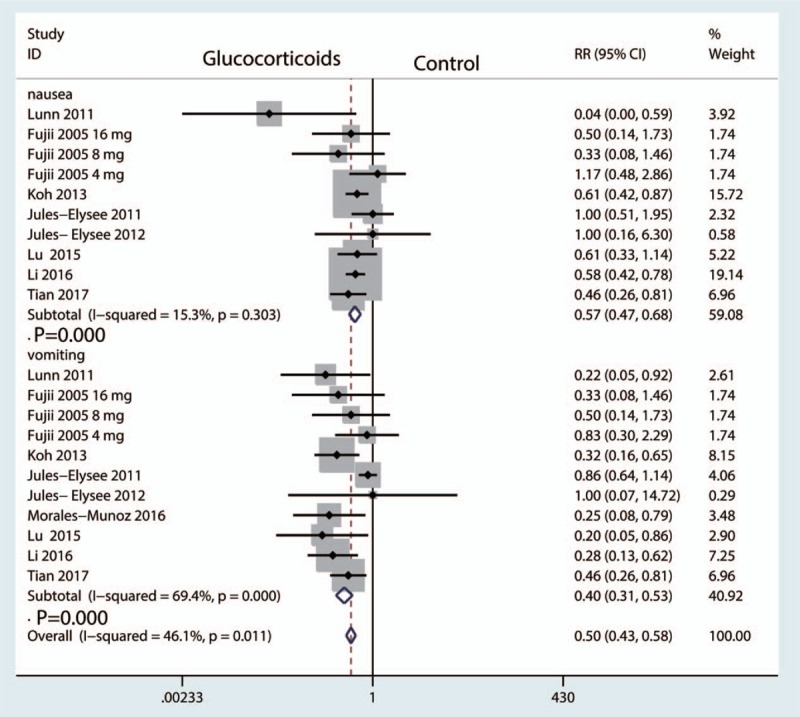
Forest plot comparing the occurrence of postoperative nausea and vomiting between glucocorticoids group and control group.
Eleven studies with patients reported the occurrence of vomiting, and there was high heterogeneity among the included studies (I2 = 69.4%, P = .000). Therefore, we used a random-effect model to pool the data. Compared with the control groups, preoperative intravenous glucocorticoids were associated with a 16.8% reduction in the occurrence of vomiting (12.0% vs 28.8% RR = 0.40, 95% CI = 0.31–0.53, P = .000, Fig. 7).
We plotted the equivalent dexamethasone doses as the abscissa against the incidence of nausea as the ordinate to generate a scatterplot. The results indicated that the incidence of nausea tended to drop as the glucocorticoid dose increased. In addition, the linear correlation coefficient (r) was calculated by the Spearman method. A negative correlation was observed between glucocorticoid dosage and the incidence of nausea (r = −0.540, P = .015, Fig. 8). There was no correlation between glucocorticoids dosage and the incidence of vomiting (r = −0.266, P = .410, Fig. 9).
Figure 8.
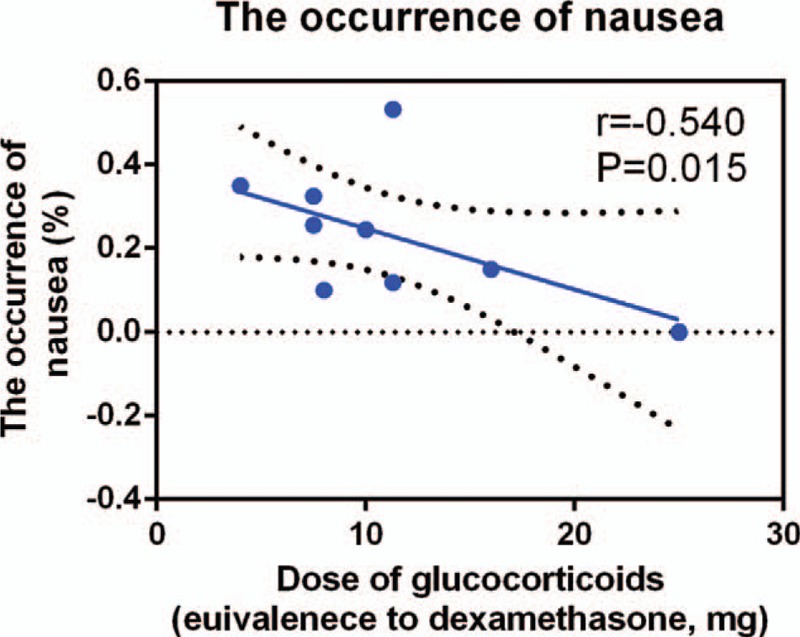
Scatterplot comparing glucocorticoids dose with the incidence of nausea.
Figure 9.
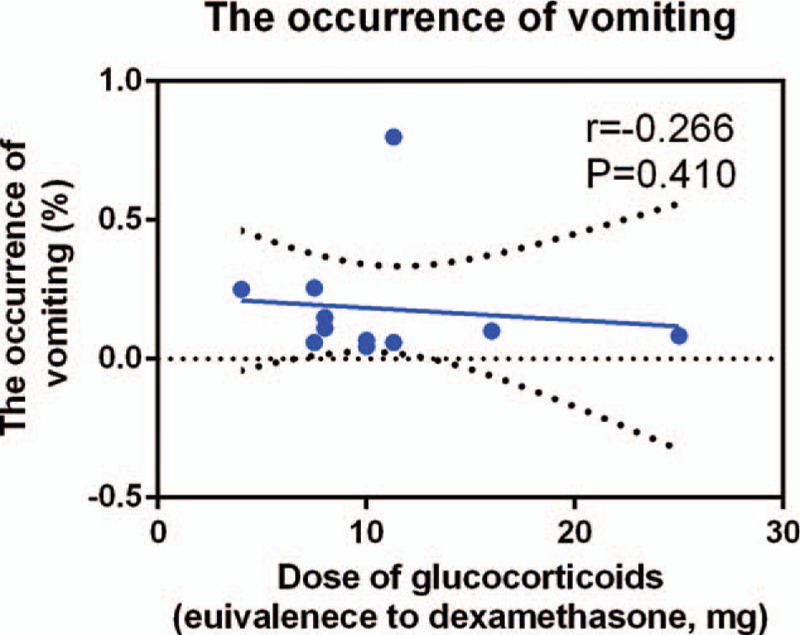
Scatterplot comparing glucocorticoids dose with the incidence of vomiting.
4.4. The occurrence of pruritus
Eleven studies with patients reported the occurrence of pruritus, and there was moderate heterogeneity among the included studies (I2 = 40.7%, P = .108). Therefore, we used a fixed-effect model to pool the data. Compared with the control groups, preoperative intravenous glucocorticoids were associated with a 13.0% reduction in the occurrence of pruritus (28.9% vs 41.9%, RR = 0.62, 95% CI = 0.49–0.78, P = .000, Fig. 10).
Figure 10.
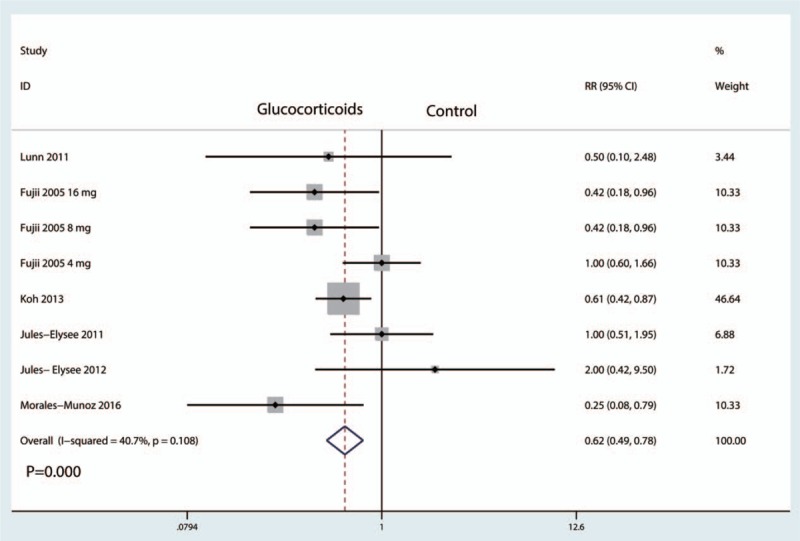
Forest plot comparing the occurrence of postoperative pruritus between glucocorticoids group and control group.
4.5. The occurrence of infection
Eight studies[15,17–19,21] with patients reported the incidence of infection with no heterogeneity (I2 = 0.0%, P = .735). There was no significant difference between the glucocorticoid group and control groups in terms of the occurrence of infection (RR = 0.71, 95% CI, 0.23–2.19, P = .552, Fig. 11).
Figure 11.
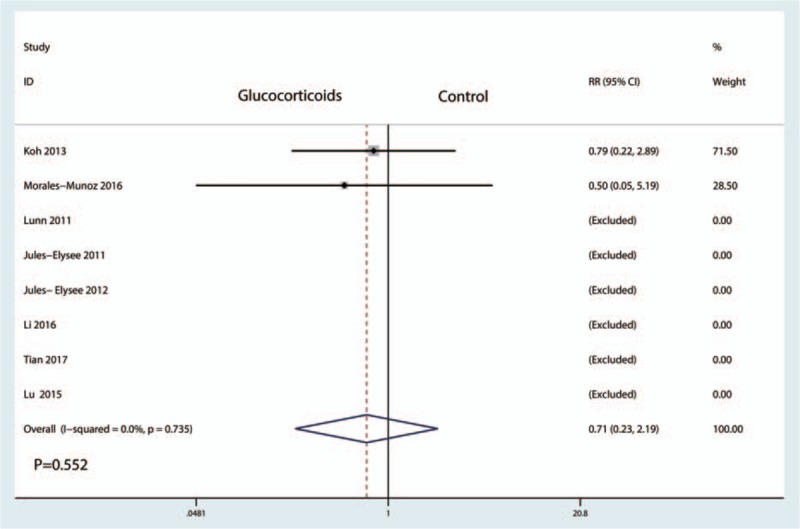
Forest plot comparing the incidence of infection between the between glucocorticoids group and control group.
4.6. Blood glucose at 6 and 24 hours After TKA
Five studies involving patients reported blood glucose at 6 hours after TKA, with great heterogeneity between the included studies (I2 = 91.5, P = .000). Compared with the control groups, preoperative intravenous glucocorticoids increased blood glucose by 14.13 mg/dL [mean difference (MD) = 14.13, 95% CI = 0.94–27.32, P = .036, Fig. 12].
Figure 12.
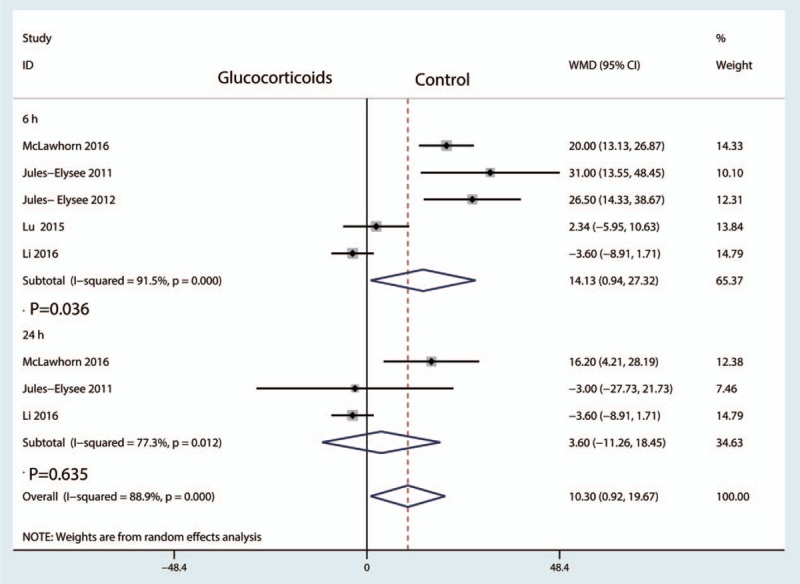
Forest plot comparing the blood glucose between the glucocorticoids group and control group.
Three studies involving patients reported blood glucose at 24 hours after TKA, with great heterogeneity (I2 = 77.3%, P = .012). The pooled results indicated that there was no significant difference in blood glucose at 24 hours after TKA (MD = 3.60, 95% CI = −11.26–18.45, P = .635, Fig. 12).
4.7. Publication bias and subgroup analysis
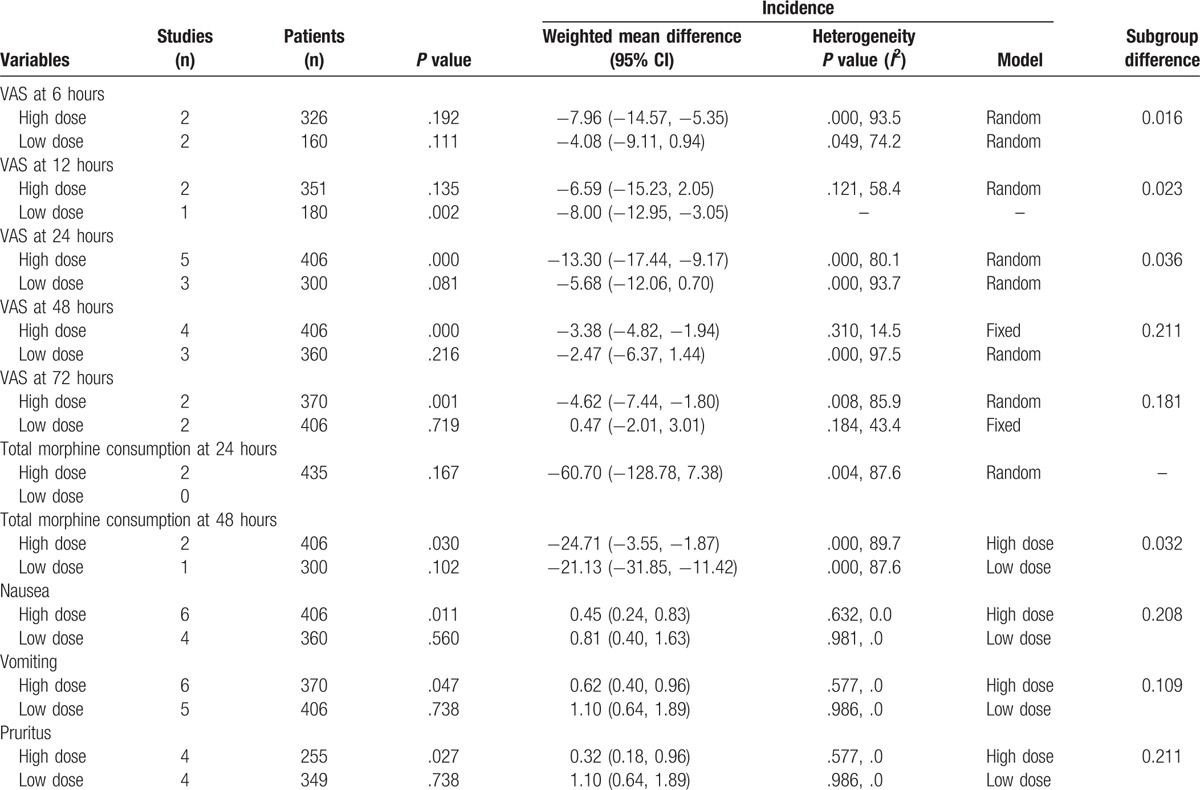
We applied a funnel plot and Begg test to VAS at 24 and 48 hours because to the included studies in these outcomes were large enough that we could assess publication bias. The results showed that there was no publication bias among the included studies. Subgroup analysis results are presented in Table 2. The results indicated that high doses of glucocorticoids were superior to low doses of glucocorticoids (P < .05).
Table 2.
Subgroup analysis for the VAS at 6, 12, 24, 48, and 72 hours, total morphine consumption at 24 and 48 hours, the occurrence of vomiting, nausea, and pruritus.
5. Discussion
This is the first systematic review and meta-analysis to evaluate the efficacy and safety of glucocorticoids for reducing postoperative pain and PONV after TKA. The pooled results indicated that preoperative intravenous glucocorticoids were associated with a significant reduction of acute pain at 6, 12, 24, and 48 hours after TKA. There was no significant difference in the VAS at 72 hours after TKA. However, preoperative intravenous glucocorticoids were not associated with a reduction of total morphine consumption at 24 or 48 hours. The morphine-related complications (nausea, vomiting, and pruritus) were reduced in the glucocorticoids group. As for the safety of intravenous glucocorticoids, the major concerns were blood glucose and the occurrence of infection. Pooled results indicated that there was no significant difference between treatments in the occurrence of infection or in blood glucose at 24 hours after TKA. Preoperative intravenous glucocorticoids were associated with a statistically significant increase in blood glucose at 6 hours after TKA (P < .05). And preoperative intravenous glucocorticoids increased blood glucose by 14.13 mg/dL at 6 hours after TKA.
The overall quality of the included studies was high, and the level of evidence was moderate due to the large heterogeneity between the included studies. The main reason was because the dose and type of glucocorticoids differed among the included studies. Despite these shortcomings, many highlights can be seen in the current meta-analysis. First, we search both English- and Chinese-language databases to decrease the potential publication bias. Second, we used a random-effect model when the heterogeneity was large. Third, no previous meta-analysis has been performed on this subject; therefore, the present study is highly novel.
Pooled results indicated that preoperative intravenous glucocorticoids were associated with a significant reduction in VAS scores at 6, 12, 24, and 48 hours. At 72 hours after TKA, there was no significant difference between the glucocorticoid group and control group. Toner et al[26] conducted a meta-analysis to compare the effects of intravenous glucocorticoids in abdominal surgery, and pooled results indicated that perioperative intravenous glucocorticoids were an effective method to control postoperative pain. The most extreme pain in TKA patients occurs between 6 and 24 hours after the procedure.[27] Backes et al[11] revealed that preoperative intravenous dexamethasone can mitigate postoperative pain and nausea after total joint arthroplasty. We then supposed that the pain control effects would increase as the dose of glucocorticoids increased. Pooled results indicated that there was a negative correlation between the glucocorticoid dose and the VAS at 6, 12, 24, and 48 hours.
Glucocorticoids may also decrease postoperative complications such as nausea, vomiting, and pruritus after TKA. The most common adverse effects of morphine were nausea, vomiting, and pruritus. Feo et al[28] reported that preoperative dexamethasone can reduce the occurrence of nausea and vomiting after laparoscopic cholecystectomy. The reason was that glucocorticoids has potent anti-inflammatory effects and thus may prevent PONV. Study shown that glucocorticoids significantly decreased the postoperative level of CRP and IL-6.[29]
There was an elevation of blood glucose by 14.13 mg/dL at 6 hours after TKA. At 24 hours after TKA, there was no significant difference between the glucocorticoid group and the control group in terms of the blood glucose. Nurok et al[30] reported in a retrospective study that only 5.6% (95% CI: 3.8–7.5) of TKA patients had postoperative glucose levels >200 mg/dL, and thus the use of dexamethasone appears to be safe in the TKA setting. Toner et al[26] conducted a meta-analysis regarding the safety of preoperative intravenous glucocorticoids in elective noncardiac surgery, and the evidence did not highlight any safety concerns with respect to the use of perioperative glucocorticoids and subsequent infection, hyperglycemia, or other adverse outcomes. The current meta-analysis mainly compared the occurrence of infection and found that there was no significant difference between the glucocorticoid group and the control group. Richardson et al[31] reported that perioperative dexamethasone administration does not increase the incidence of postoperative infection in total hip and knee arthroplasty.
Our meta-analysis has a total of 5 limitations: the doses of glucocorticoids differed and thus may have caused the heterogeneity among the included studies; two studies included patients undergoing bilateral TKA, which may have caused the observed heterogeneity across studies; the perioperative anesthesia protocols were different and thus may have contributed to the great heterogeneity among the included studies; knee function and perioperative blood loss were not compared among the included studies due to insufficient data; and Potential bias may exist in the outcomes.
6. Conclusions
Preoperative intravenous glucocorticoids are an effective and safe method to reduce acute pain, PONV, and pruritus in patients after TKA. There was a transient blood glucose elevation at 6 hours after TKA, but the change was not clinically important. More studies are necessary to determine the optimal dose and type of glucocorticoids for TKA.
Supplementary Material
Footnotes
Abbreviations: CI = confidence interval, CNKI = China National Knowledge Infrastructure, CRP = C-reactive protein, IL-6 = interleukin-6, MD = mean difference, PCA = patient-controlled anesthesia, PONV = postoperative nausea and vomiting, RCT = randomized controlled trial, RR = risk ratio, TKA = total knee arthroplasty, VAS = visual analogue scale.
There was no funding for this research.
The authors report no conflicts of interest.
Supplemental Digital Content is available for this article.
References
- [1].Dong CC, Dong SL, He FC. Comparison of adductor canal block and femoral nerve block for postoperative pain in total knee arthroplasty: a systematic review and meta-analysis. Medicine (Baltimore) 2016;95:e2983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007;89:780–5. [DOI] [PubMed] [Google Scholar]
- [3].Sun XL, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhai L, Song Z, Liu K. The effect of gabapentin on acute postoperative pain in patients undergoing total knee arthroplasty: a meta-analysis. Medicine (Baltimore) 2016;95:e3673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Terkawi AS, Mavridis D, Sessler DI, et al. Pain management modalities after total knee arthroplasty: a network meta-analysis of 170 randomized controlled trials. Anesthesiology 2017;126:923–37. [DOI] [PubMed] [Google Scholar]
- [6].DiIorio TM, Sharkey PF, Hewitt AM, et al. Antiemesis after total joint arthroplasty: does a single preoperative dose of aprepitant reduce nausea and vomiting? Clin Orthop Relat Res 2010;468:2405–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Apfel CC, Laara E, Koivuranta M, et al. A simplified risk score for predicting postoperative nausea and vomiting: conclusions from cross-validations between two centers. Anesthesiology 1999;91:693–700. [DOI] [PubMed] [Google Scholar]
- [8].Fujii Y. Current review of ramosetron in the prevention of postoperative nausea and vomiting. Curr Drug Saf 2011;6:122–7. [DOI] [PubMed] [Google Scholar]
- [9].Chang CB, Cho WS. Pain management protocols, peri-operative pain and patient satisfaction after total knee replacement: a multicentre study. J Bone Joint Surg Br 2012;94:1511–6. [DOI] [PubMed] [Google Scholar]
- [10].Bjornsson GL, Thorsteinsson L, Gudmundsson KO, et al. Inflammatory cytokines in relation to adrenal response following total hip replacement. Scand J Immunol 2007;65:99–105. [DOI] [PubMed] [Google Scholar]
- [11].Backes JR, Bentley JC, Politi JR, et al. Dexamethasone reduces length of hospitalization and improves postoperative pain and nausea after total joint arthroplasty: a prospective, randomized controlled trial. J Arthroplasty 2013;28(8 suppl):11–7. [DOI] [PubMed] [Google Scholar]
- [12].Viriyaroj V, Boonsinsukh T, Rookkachart T, et al. The effects of single-dose preoperative intravenous dexamethasone on clinical outcome after laparoscopic cholecystectomy. J Med Assoc Thai 2015;98(suppl 10):S112–7. [PubMed] [Google Scholar]
- [13].Higgins JPT, Greens S. Cochrane handbook for systematic reviews of interventions version 5.1.0. 2011. http://www.cochrane-handbook.org. Acessed 2011. [Google Scholar]
- [14].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lunn TH, Kristensen BB, Andersen LO, et al. Effect of high-dose preoperative methylprednisolone on pain and recovery after total knee arthroplasty: a randomized, placebo-controlled trial. Br J Anaesth 2011;106:230–8. [DOI] [PubMed] [Google Scholar]
- [16].Fujii Y, Nakayama M. Effects of dexamethasone in preventing postoperative emetic symptoms after total knee replacement surgery: a prospective, randomized, double-blind, vehicle-controlled trial in adult Japanese patients. Clin Ther 2005;27:740–5. [DOI] [PubMed] [Google Scholar]
- [17].Koh IJ, Chang CB, Lee JH, et al. Preemptive low-dose dexamethasone reduces postoperative emesis and pain after TKA: a randomized controlled study. Clin Orthop Relat Res 2013;471:3010–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jules-Elysee KM, Lipnitsky JY, Patel N, et al. Use of low-dose steroids in decreasing cytokine release during bilateral total knee replacement. Reg Anesth Pain Med 2011;36:36–40. [DOI] [PubMed] [Google Scholar]
- [19].Jules- Elysee KM, Wilfred SE, Memtsoudis SG, et al. Steroid modulation of cytokine release and desmosine levels in bilateral total knee replacement: a prospective, double-blind, randomized controlled trial. J Bone Joint Surg Am 2012;94:2120–7. [DOI] [PubMed] [Google Scholar]
- [20].McLawhorn AS, Beathe J, YaDeau J, et al. Effects of steroids on thrombogenic markers in patients undergoing unilateral total knee arthroplasty: a prospective, double-blind, randomized controlled trial. J Orthop Res 2015;33:412–6. [DOI] [PubMed] [Google Scholar]
- [21].Morales-Munoz C, Sanchez-Ramos JL, Diaz-Lara MD, et al. Analgesic effect of a single-dose of perineural dexamethasone on ultrasound-guided femoral nerve block after total knee replacement. Rev Esp Anestesiol Reanim 2017;64:19–26. [DOI] [PubMed] [Google Scholar]
- [22].Haitao T, Yuanhe W, Shaoqi T, et al. Effects and safety assessment of methylprednisolone on postoperative nausea and vomiting and pain after total knee arthroplasty. Chin J Tissue Eng Res 2017;21:335–9. [Google Scholar]
- [23].Liang L, Xilong W, Xifu S. Preventive low-dose dexamethasone reduces postoperative emesis and pain after total knee arthroplasty. J Clin Orthop 2015;18:318–21. [Google Scholar]
- [24].Qicai L, Shaoqi T, Changxin J, et al. Methylprednisolone: its efficacy of postoperative nausea and vomiting in patients undergoing total knee arthroplasty. Med J Qilu 2016;31:535–7. [Google Scholar]
- [25].Samona J, Cook C, Krupa K, et al. Effect of intraoperative dexamethasone on pain scores and narcotic consumption in patients undergoing total knee arthroplasty. Orthop Surg 2017;9:110–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Toner AJ, Ganeshanathan V, Chan MT, et al. Safety of perioperative glucocorticoids in elective noncardiac surgery: a systematic review and meta-analysis. Anesthesiology 2017;126:234–48. [DOI] [PubMed] [Google Scholar]
- [27].Valeberg BT, Hovik LH, Gjeilo KH. Relationship between self-reported pain sensitivity and pain after total knee arthroplasty: a prospective study of 71 patients 8 weeks after a standardized fast-track program. J Pain Res 2016;9:625–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Feo CV, Sortini D, Ragazzi R, et al. Randomized clinical trial of the effect of preoperative dexamethasone on nausea and vomiting after laparoscopic cholecystectomy. Br J Surg 2006;93:295–9. [DOI] [PubMed] [Google Scholar]
- [29].Lunn TH, Kehlet H. Perioperative glucocorticoids in hip and knee surgery—benefit vs. harm? A review of randomized clinical trials. Acta Anaesthesiol Scand 2013;57:823–34. [DOI] [PubMed] [Google Scholar]
- [30].Nurok M, Cheng J, Romeo GR, et al. Dexamethasone and perioperative blood glucose in patients undergoing total joint arthroplasty: a retrospective study. J Clin Anesth 2017;37:116–22. [DOI] [PubMed] [Google Scholar]
- [31].Richardson AB, Bala A, Wellman SS, et al. Perioperative dexamethasone administration does not increase the incidence of postoperative infection in total hip and knee arthroplasty: a retrospective analysis. J Arthroplasty 2016;31:1784–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


