Abstract
Background:
In recent years, neuroendoscopy has been used as a method for treating intracerebral hemorrhages (ICHs). However, the efficacy and safety of neuroendoscopic surgery is still controversial compared with that of craniotomy. Our aim was to compare the outcomes of neuroendoscopic surgery and craniotomy in patients with supratentorial hypertensive ICH using a meta-analysis.
Methods:
We searched on PubMed, EMBASE, and Cochrane Central Register of Controlled Trials to identify relevant studies in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. Quality of eligible studies was evaluated and the related data were extracted by 2 reviewers independently. This study assessed clinical outcomes, evacuation rates, complications, operation time, and hospital stay for patients who underwent neuroendoscopic surgery (NE group) or craniotomy (craniotomy group).
Results:
Meta-analysis included 1327 subjects from verified studies of acceptable quality. There was no significant heterogeneity between the included studies based on clinical outcomes. Compared with craniotomy, neuroendoscopic surgery significantly improved clinical outcomes in both randomized controlled studies (RCTs) group (relative risk: 0.62; 95% confidence interval [CI], 0.47–0.81, P < .001) and non-RCTs group (relative risk: 0.84; 95% CI: 0.75–0.95, P = .005); decreased the rate of death (relative risk: 0.53; 95% CI, 0.37–0.76, P < .001) in non-RCTs group but not in RCTs group (relative risk: 0.58; 95% CI, 0.26–1.29, P = .18); increased evacuation rates in non-RCTs group (standard mean differences: 0.75; 95% CI, 0.24–1.26, P = .004) and had a tendency of higher evacuation rates in RCTs group (standard mean differences: 1.34; 95% CI, 0.01–2.68, P = .05); reduced the total risk of complications in non-RCTs group (relative risk: 0.45; 95% CI, 0.25–0.83, P = .01) and RCTs group (relative risk: 0.37; 95% CI, 0.28–0.49, P < .001); reduced the operation time in non-RCTs group (standard mean differences: 3.26; 95% CI: 1.20–5.33, P < .001) and RCTs group (standard mean differences: 4.37; 95% CI: 3.32–5.41, P < .001).
Conclusions:
Our results suggested that the NE group showed better clinical outcomes than the craniotomy group for patients with supratentorial hypertensive ICH. Moreover, the patients who underwent neuroendoscopy had a higher evacuation rate, lower risk of complications, and shorter operation time compared with those that underwent a craniotomy.
Keywords: craniotomy, hypertensive intracerebral hemorrhage, meta-analysis, neuroendoscopic surgery
1. Introduction
Hypertensive intracerebral hemorrhages (HICHs), which accounts for 70% of all strokes, are associated with a high mortality rate,[1] and 61% to 88% of survivors suffer from severe disabilities.[2,3] Despite the well-known harmful effects of HICH, there have been no breakthrough in treatment options.[1,4] Currently, conservative treatment and surgical evacuation are the only options for HICH management.[4] A review showed that surgery, rather than conservative treatment, was associated with better outcomes (P < .001).[5] However, the effect was weak for heterogeneity between the studies. The studies included different types of surgical procedures, such as endoscopic surgery, craniotomy, and stereotactic aspiration. However, which type of surgical procedures is most beneficial for HICH management still need to be elucidated.
Endoscopic surgery is a minimally invasive surgery that has been applied for the treatment of HICH in recent years. However, the application of neuroendoscopy (NE) for HICH is still controversial. Many studies suggested that endoscopic evacuation had some advantages, when compared with traditional craniotomy, in reducing damage to brain tissue.[6–10] In addition, endoscopic evacuation was considered a better procedure for improving outcomes than stereotactic evacuation.[11] However, from the retrospective research or limited sample size, no conclusion could be drawn about the effects of NE on outcomes in HICH patients. In contrast, some studies[7,12,13] showed that endoscopic evacuation did not reduce the rate of death when compared with medical treatment or craniotomy, and minimally invasive surgical procedures did not improve the long-term outcomes.[14,15]
Therefore, due to these controversial conclusions, we conducted a meta-analysis to identify which type of surgical procedure is the safest and most effective in promoting outcomes and reducing complications in patients with HICHs.
2. Materials and methods
2.1. Collecting the eligible articles
Since this study was a meta-analysis, ethical approval was not necessary. We searched for eligible articles in PubMed, Cochrane Central Register of Controlled Trials, and EMBASE, from 1911 to 2017, using the keywords “Endoscope OR endoscopic surgery OR neuroendoscopic surgery OR endoscopic evacuation” and “intracerebral hemorrhage (ICH) OR intracranial haemorrhage.” The references of the eligible articles were checked and relevant research articles were retrieved.
2.2. Inclusion criteria and exclusion criteria
Titles and abstracts were reviewed to find relevant articles, of which, full-text articles were reviewed by 2 reviewers independently. The eligible studies were included based on the following criteria: patients were diagnosed with supratentorial hypertensive ICH; treatment methods included endoscopic surgery and craniotomy; and randomized controlled studies (RCTs), cohort or case-control studies. We excluded studies that included case reports, systematic reviews, animal research, not in English language or had insufficient data.
2.3. Data extraction
Two reviewers extracted the data from the eligible articles independently, and all inconsistencies were discussed. They used a standard form to determine eligibility and to extract data, including major characteristics of the patients. The following factors were taken into consideration: author, publication year, number of cases and controls, Glass coma scale (GCS), hematoma volume, sex and mean age, mortality or morbidity, evacuation rate of hematoma, complications, operation time, and hospital stay.
The primary outcomes were death or dependency (efficacy outcome) and mortality (safety outcome). For the present study, dependence in activities of daily living (ADL) was defined using the Barthel Index (BI) ≤60, Glasgow Outcome Scale (GOS) ≤3, and modified Rankin Scale (mRS) from 3 to 6.[16,17] Second outcomes included evacuation rate of hematoma, complications, operation time, and hospital stay.
2.4. Quality assessment of the selected articles
Two reviewers assessed the quality of eligible articles independently using the Newcastle-Ottawa scale criteria for cohort and case-control studies, and the modified Jadad grading scale for randomized controlled trials.[18,19] The quality of the study is moderate to high based on Newcastle-Ottawa scale (>5) and modified Jadad grading scale (>3).
2.5. Data analysis
We pooled the data using the Review Manager (Version 5.3; Cochrane collaboration, Oxford, UK) and assessed the publication bias using STATA 13.0 (STATA Corporation, College Station, TX) to analyze the data. The data of RCTs would be pooled separately from the observational studies. Forest plots were used to evaluate the difference in clinical outcomes for the NE and craniotomy cases. The dichotomous variables were expressed as relative risk (RR) with a 95% confidence interval (CI).[20] The continuous variables were assessed using standard mean differences (SMD). All included articles were tested for statistical heterogeneity using the Q statistic and I2 statistic.[21] The I2 < 50% or P > .10 suggested there was no significant heterogeneity among the included articles, and data would be pooled using the fixed-effects model; otherwise the random-effects models would be applied.[22] The pooled RR and SMD were considered to be statistically significant if P < .05. Funnel plots, using Begg rank correlation, assessed for publication bias. If P > .05, there was no publication bias.
3. Results
3.1. Literature research
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow diagram described the procedure used to search for the eligible studies (Fig. 1).[23] A total of 843 articles were retrieved from the initial search. After removing duplicate articles, 769 articles remained. Of these, 737 articles were excluded based on the following reasons: unrelated studies (675), case reports (43), systematic reviews (14), and animal research (5). We reviewed the full-text of the 32 studies included, and 24 articles were excluded based on the following reasons: intervention not meet criteria (13), not in English (5), full text not provided (3), inappropriate data (2), and 1 article[24] was removed for including infratentorial hematoma.
Figure 1.
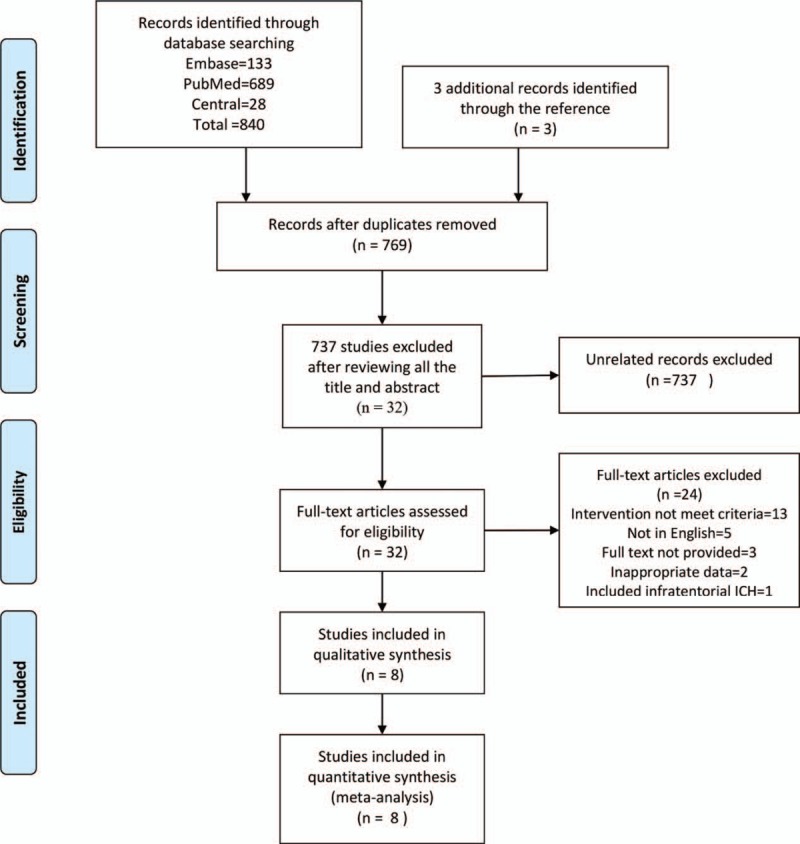
The PRISMA flow diagram of procedure to search the eligible studies. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
3.2. Characteristics of included studies
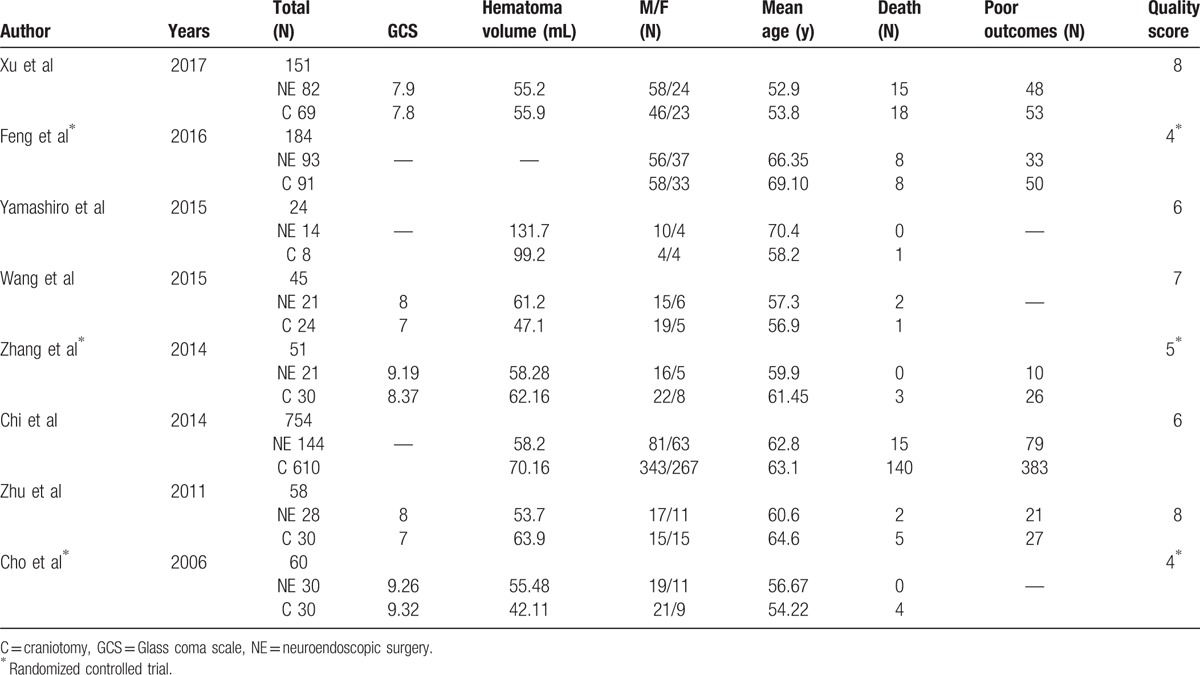
Eight studies were included in the analysis, consisting of a total of 1327 patients.[6–9,13–15,25] Among these studies, 5 articles reported poor outcomes in 1189 patients (BI ≤60, GOS ≤3, or mRS ≥3); 8 articles reported rate of mortality in 1327 patients; 5 articles assessed the evacuation rate of hematoma in 342 patients; 6 articles evaluated the risk of total complications in 549 patients (including rebleeding, infection, digestive tract ulcers, hypoproteinemia, epilepsy); 5 articles assessed the risk of rebleeding in 365 patients; 3 articles on infection reported on the hospital stay of 293 patients; 2 articles evaluated the hospital stay of 211 patients; and 4 articles reported the operation time in 282 patients. All included articles were of moderate to high quality based on scores from the Newcastle-Ottawa scale (>5) or modified Jadad grading scale (>3) (Table 1).
Table 1.
Major characteristics of all included articles.
3.3. Pooled results
3.3.1. Poor outcomes between NE and craniotomy groups
Data from 5 articles were pooled to evaluate the poor outcomes between the NE group and the craniotomy group. There was no significant heterogeneity between these articles, with I2 = 0%, P = .62, and I2 = 0%, P = .58 in the non-RCT group and the RCT group, respectively. Three non-RCTs showed that patients with poor outcomes accounted for 58% (148/254) in the NE group and 65% (463/709) in the craniotomy group, with RR of 0.84 (95% CI, 0.75–0.95, P = .005; Fig. 2), evaluated using a fixed-effect model. Two RCTs showed the patients with poor outcomes accounted for 38% (43/114) in the NE group and 63% (76/121) in the craniotomy group, with RR of 0.62 (95% CI, 0.47–0.81, P < .001), evaluated using a fixed-effect model.
Figure 2.
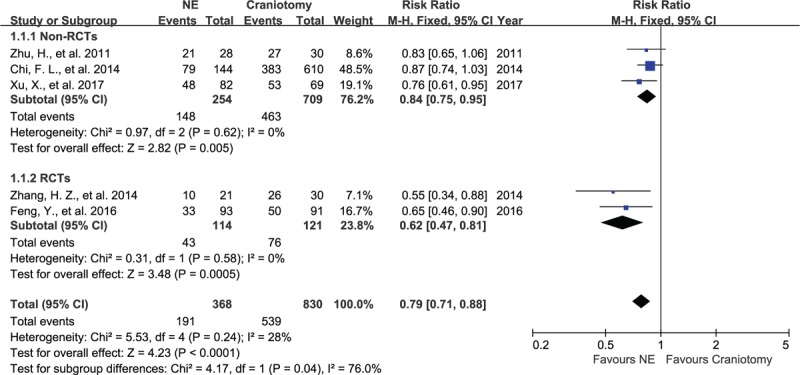
Forest plots for poor outcomes between NE and craniotomy groups. The patients undergoing NE had a better outcomes than craniotomy in RCT group and non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.2. Mortality in NE and craniotomy groups
Eight articles, with data on 1327 patients, assessed the mortality after NE or craniotomy. Three studies were RCTs and the remaining 5 were case-control studies. There was no significant heterogeneity between these articles, with I2 = 0%, P = .53 and I2 = 33%, P = .23 in the non-RCT group and the RCT group, respectively. Mortality in 5 non-RCTs was 12% (34/289) in the NE group and 22% (165/741) in the craniotomy group, with RR of 0.53 (95% CI, 0.37–0.76, P < .001; Fig. 3), evaluated using a fixed-effect model. Mortality in the 3 RCTs was 6% (8/144) in the NE group and 10% (15/151) in the craniotomy group, and there was no significant difference with RR of 0.58 (95% CI, 0.26–1.29, P = .18), evaluated using a fixed-effect model.
Figure 3.

Forest plots for death between NE and craniotomy groups. The patients undergoing NE had lower mortality than craniotomy in non-RCTs group while there was no significant difference in RCT group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.3. Evacuation rate of hematoma in NE and craniotomy groups
In 5 studies, containing data on 342 patients, the evacuation rates for the NE group (175) and the craniotomy group (167) were pooled. The SMD were 89.8% for the NE group and 82.5% for the craniotomy group. Three non-RCTs showed that the SMD of the evacuation rate were higher in the NE group than in the craniotomy group (SMD, 0.75; 95% CI, 0.24–1.26, P = .004, Fig. 4), evaluated using a random-effect model. Two RCTs showed that NE tended to increase the evacuation rate (SMD, 1.34; 95% CI, 0.01–2.68, P = .05), evaluated using a random-effect model. However, the heterogeneity was significant between these articles, with I2 = 62%, P = .07 and I2 = 90%, P = .002 in the non-RCT group and the RCT group, respectively.
Figure 4.
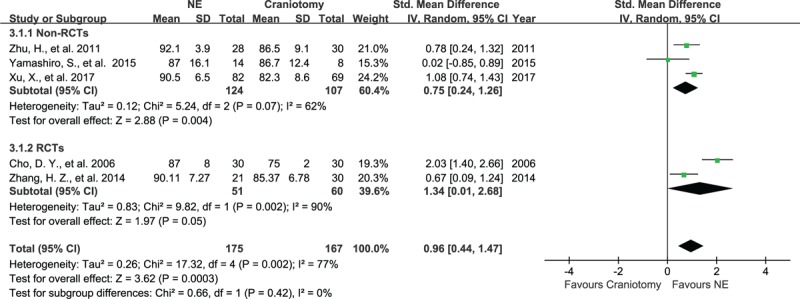
Forest plots for evacuation rate between NE and craniotomy groups. The evacuation rate of hematoma with NE was higher than craniotomy in non-RCTs group and had a tendency of higher evacuation rate in RCT group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.4. Total risk of complications in the NE and craniotomy groups
Six articles, containing data on 549 subjects, assessed all complications after NE or craniotomy, including rebleeding, infection, digestive tract ulcers, hypoproteinemia, and epilepsy. There was no significant heterogeneity between these articles, with I2 = 12%, P = .32 and I2 = 0%, P = .76 in non-RCT group and RCT group, respectively. In the NE group, 17% (46/275) of patients had complications, while in the craniotomy group, 45% (122/274) of patients had complications. The 3 non-RCTs and 3 RCTs showed that patients in the NE group showed a lower rate of complications, with RR of 0.45 (95% CI, 0.25–0.83, P = .01, Fig. 5) and RR of 0.37 (95% CI, 0.28–0.49, P < .001) respectively, evaluated using a fixed-effect model.
Figure 5.
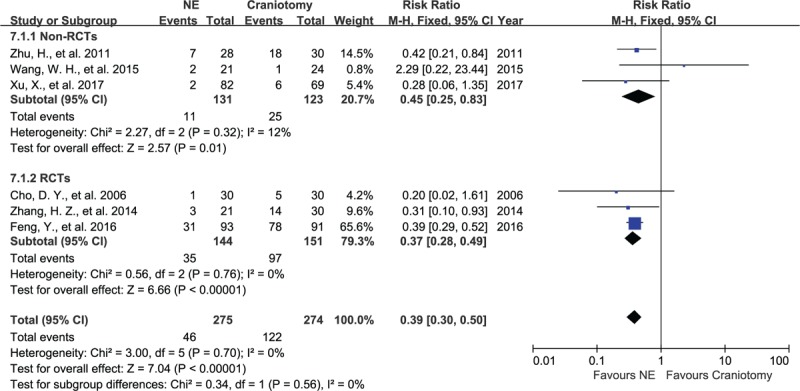
Forest plots for total complications between NE and craniotomy groups. The total risk of complications in patients undergoing NE was lower than craniotomy in RCT group and non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.5. Rebleeding in NE and craniotomy groups
In 5 studies containing 365 subjects, the data of rebleeding for the 2 groups were pooled. There was no significant heterogeneity between these articles, with I2 = 11%, P = .33 and I2 = 0%, P = .98 in the non-RCT group and the RCT group, respectively. The 5 studies showed that rebleeding occurred in 4% (7/182) of patients in the NE group and in 8% (15/183) of patients in the craniotomy group. No significant difference was seen in the occurrence of rebleeding in the non-RCTs group and the RCTs group, with RR of 0.48 (95% CI, 0.17–1.35, P = .17, Fig. 6) and RR of 0.49 (95% CI, 0.10–2.42, P = .38), respectively, evaluated using a fixed-effect model.
Figure 6.
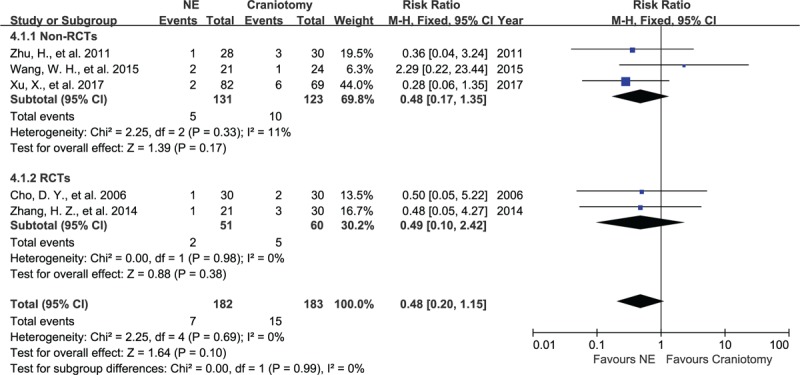
Forest plots for rebleeding between NE and craniotomy groups. The occurrence of rebleeding between NE and craniotomy had no significant difference in RCT group and non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.6. Infections in the NE and craniotomy groups
Three studies, consisting of 293 subjects, assessed infections in patients after NE or craniotomy procedure. There was no significant heterogeneity between these articles, with I2 = 0%, P = .67 in RCT group. The 3 studies showed that infections occurred in 15% (22/142) of patients from the NE group and in 42% (64/151) of patients from the craniotomy group. The craniotomy group had a higher occurrence of infection in non-RCTs group and RCTs group, with RR of 0.43 (95% CI, 0.19–0.95, P = .04; Fig. 7) and RR of 0.34 (95% CI, 0.21–0.57, P < .001) respectively, evaluated using a fixed-effect model.
Figure 7.
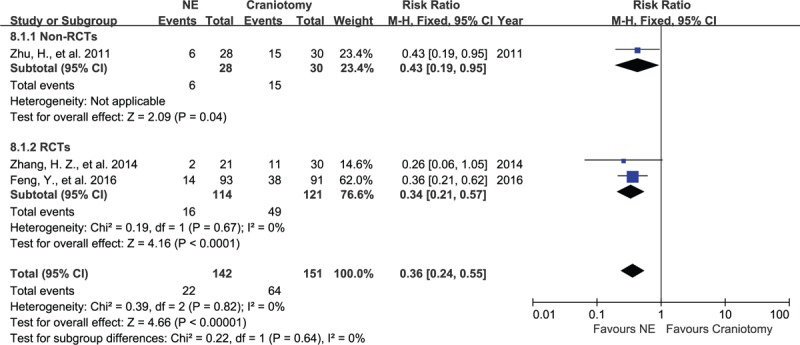
Forest plots for infections between NE and craniotomy groups. The patients undergoing NE had a lower risk of infection than those undergoing craniotomy, regardless of RCT group or non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.7. Duration of operation for NE and craniotomy groups
In 4 studies, consisting of 282 patients, the operation time for the 2 groups was pooled. There was a significance in heterogeneity in the non-RCTs group (I2 = 94%, P < .001). The SMD for the operation time were 104 minutes for the NE group and 257 minutes for the craniotomy group. The NE group had a shorter operation time than the craniotomy group in the non-RCTs group and the RCTs group with SMD of 3.26 (95% CI, 1.20–5.33, P < .001; Fig. 8) and SMD of 4.37 (95% CI, 3.32–5.41, P < .001), evaluated using a random-effect model.
Figure 8.

Forest plots for operation time between NE and craniotomy groups. The operation time of NE was shorter than operation time of craniotomy in RCT group and non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.3.8. Duration of hospital stay for NE and craniotomy groups
Two articles, consisting of 211 subjects, assessed the duration of the hospital stay for patients in the NE and the craniotomy groups. The SMD for the hospital stay were 18.5 days in the NE group and 23.8 days for the craniotomy group. There was no significant difference in hospital stay for the non-RCTs and the RCTs groups with SMD of 0.22 (95% CI, −0.11–0.54, P = .19; Fig. 9) and SMD of 0.43 (95% CI, −0.09–0.94, P = .10), respectively, evaluated using a random-effect model.
Figure 9.
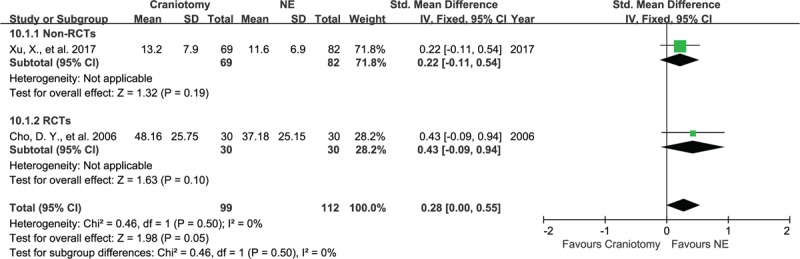
Forest plots for hospital stays between NE and craniotomy groups. The hospital stays in NE and craniotomy groups had no significant difference, regardless of RCTs group and non-RCTs group. NE = neuroendoscopy, RCTs = randomized controlled studies.
3.4. Publication bias
The publication bias was assessed using STATA 13.0 and no publication bias was found in this meta-analysis (P = .806; Fig. 10).
Figure 10.
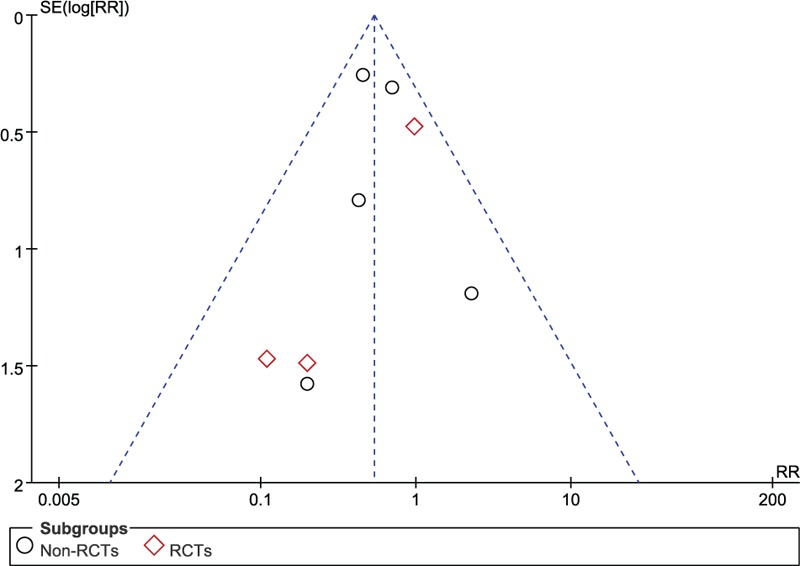
The Begg publication bias plot of 5 studies that reported clinical outcomes between NE and craniotomy, and no publication bias was found in these studies with P = .806. NE = neuroendoscopy.
4. Discussion
4.1. Implications
This study pooled the data from the articles concerning the effects of NE and craniotomy on suprotentorial HICH using a meta-analysis. The main results showed that NE was associated with better outcomes (efficacy) and lower risk of death (safety) in patients with HICH. Compared with craniotomy, NE decreased the risk of complications during treatment and increased the evacuation rate of hematomas in the non-RCTs group. Although the incidence of rebleeding and hospital stay showed no significant difference between the 2 groups, NE reduced the rate of infections and the operation time.
The role of craniotomy in HICH can still be debated upon because of the lack of large multicentric RCTs,[1] however, a recent RCT of 601 patients demonstrated that early surgery might show a small benefit in reducing mortality in patients with ICH.[5] The surgery could remove hematomas and neurotoxic chemicals,[26] and, therefore, help eliminate a local mass effect and reduce secondary injury to brain tissue. Adversely, the surgical procedure might cause additional trauma and increase the risk of rebleeding with a tamponade hematoma.[13]
In general, surgical procedures for hematoma evacuation in HICH patients include traditional craniotomy, endoscopic surgery, and stereotactic aspiration. Among these, endoscopic surgery and stereotactic aspiration are minimally invasive surgeries. A meta-analysis of 12 RCTs suggested that minimally invasive surgeries benefit more than other treatments in appropriate patients with HICH.[27] However, most articles of this review compared NE with conservative medical treatments and only 1 study compared NE with craniotomy. Another review compared stereotactic aspiration with craniotomy and suggested that aspiration reduced death or dependence in primary ICH.[28] Theoretically, brain tissue is visualized through NE to identify the site of bleeding and evaluate the region with the hematoma, while stereotactic aspiration is a slow and inadequate method for evacuation of a hematoma, without visualization.[11] Thus, the efficacy and safety of NE might be higher than that for stereotactic aspiration and craniotomy in patients with HICH. Recently, with the development of NE, there is an increasing amount of enthusiasm in using NE for the treatment of HICH and several studies are concerned with the application of NE in the HICH. NE has many advantages when compared with craniotomy, including less injury to brain tissue, higher evacuation rates, shorter operation time, lower mortality, and lower rate of complications.[6–10] Therefore, we pooled these articles to identify the efficacy and safety of NE in HICH.
In our study, data from 5 articles were pooled to assess clinical outcomes, which were considered as the primary outcome in many previous studies. We found that NE significantly improved clinical outcomes and decreased the mortality rate as compared with craniotomy. These results might be attributed to the less injury to brain tissue, higher evacuation rate,[7,13] shorter operation time,[9] and lower rate of complications.[8–10,29] NE is a minimally invasive procedure in which a 5 to 8-mm-diameter endoscope is inserted into the brain region with the hematoma through a small bur hole.[13] Traditional craniotomy, however, required a relatively large skin incision and bone flap,[9,13,30] which increases the operation time and the risk of infection. In addition, after suction and irrigation, adequate hemostasis must be achieved based on the visibility of the bleeding site and enough space to decrease the risk of rebleeding and avoid exceeding retraction of brain tissue. However, in traditional craniotomy, the effective hemostasis in the deep location need enough operation space after brain retraction, which can cause aggravated brain edema and increase the risk of brain ischemia. Three articles were not assessed for the followed reasons: Yamashiro et al[7] and Cho et al[25] defined poor outcomes as mRS ≥4 and Wang[6,9,13] defined poor outcomes as GOS ≤4, while patients with mRS ≥3 or GOS ≤3 presented poor outcomes in most of the previous studies. However, the mean BI or GCS at 1 week after surgery indicated that the patients that underwent NE had a tendency to be better, which was in accordance with the conclusion of this study.
Previous reviews suggested that minimally invasive surgeries were suitable for hematomas with a volume of 25 to 40 mL,[27] while hematomas >40 mL should undergo other forms of treatments, such as evacuation through a craniotomy. In this review, the mean volume of the hematomas in most of studies was between 40 and 70 mL. However, Yamashiro's research included patients with mean hematoma volume of 99 to 130 mL in the 2 groups, and still showed that NE was associated with lower rate of death.[7] Thus, the volume of a hematoma might not be a limitation on the use of NE, and further research will be required to identify the suitable volume for hematomas that can be evacuated using NE.
NE has shown a high evacuation rate in previous studies.[25,31–34] This rate ranges from 84% to 99%, while the evacuation rate for craniotomies is around 75%.[25] In our study, the means for the evacuation rates in the NE and craniotomy groups were 89.8% and 82.5% respectively. Cho et al[25] compared 3 methods for evacuation in HICH and found that NE had the highest evacuation rate compared with craniotomy and stereotactic aspiration. They suggested that the high evacuation rate was attributed to better visualization, allowing for a more accurate operation.[15,25]
Complications during treatments that were associated with the outcomes included rebleeding, infection, digestive tract ulcers, hypoproteinemia, and epilepsy. We found that patients that underwent NE had less complications than those that underwent a craniotomy. This is because NE attributed to reduced injury in the cortex and avoided compression of brain tissue.[6] Zhang et al[9] suggested that the lower rate of infections in the NE groups was attributed to the smaller skin incision and shorter operation time. Moreover, without brain retraction, NE could decrease the risk of brain edema by providing better visualizing to the bleeding site, while traditional craniotomy needs more space to operate, and therefore requires brain retraction, which can increase the risk of complications.
Stereotactic aspiration showed no significant difference in the total risk of complications when compared with craniotomy.[28] Nearly, 33% of patients that underwent stereotactic aspiration needed urokinase therapy to dissolve the residual hematoma, which increased the risk of rebleeding and infections.[25] In addition, the stereotactic aspiration procedure should not take longer than 6 hours as it could increase the risk of bleeding.[27] However, those limitations of stereotactic aspiration had no effect on the use of NE, which could be conducted as soon as possible and did not increase the rate of rebleeding, as compared with craniotomy (Fig. 6).
4.2. Limitations
Some limitations existed in this meta-analysis: First, only 3 articles were RCTs with limited number of patients, while the others were retrospective studies. Second, the number of included subjects was limited in this study, which may effect on the results. Then, there were some differences in baselines, such as in the volume of hematoma and GCS, which might have affected the clinical outcomes. Furthermore, 1 study suggested the craniotomy affect the hemodynamic and metabolism,[35] especially decreasing the cerebral blood flow (CBF), which implied the size of bone flap or bur hole had effect on the CBF. This meta-analysis included the included articles with different size of craniotomy or bur hole and the results may be affected. Lastly, some heterogeneity existed in articles that reported the data for the evacuation rates and operation time, and a random-effects model was used to estimate the effect more conservatively.
4.3. Future study
In conclusion, our results suggest that NE significantly improves clinical outcomes and reduces the rate of complications in patients with HICH, when compared with craniotomy. High quality trials are needed to identify patients who may qualify for a NE procedure, keeping the volume of the hematoma, GCS, age, and time of onset in mind. In addition, more data from RCTs are required to compare the effects of stereotactic aspiration and NE on patients with HICH.
Acknowledgments
The authors thank the reviewers for their constructive comments.
Footnotes
Abbreviations: ADL = activities of daily living, BI = Barthel Index, CI = confidence interval, GCS = Glass coma scale, GOS = Glasgow Outcome Scale, HICH = hypertensive intracerebral hemorrhage, mRS = modified Rankin Scale, NE = neuroendoscopy, RCTs = randomized controlled studies, RR = relative risk, SMD = standard mean differences.
ZY, XA, and XH have contributed equally to this work.
Funding: Supported by Science and technology supportive project of Sichuan Province. Project: Intracerebral haemorrhage prevention and diagnostic treatment skills [grant number 2015SZ0051]; Outstanding subject development 135 project: An international, multicentre, large sample randomized controlled trial of supratentorial deep intracerebral haematoma surgery and conservative treatment in adults [grant number ZY2016102].
The authors have no conflicts of interest to disclose.
References
- [1].Hemphill JC, 3rd, Greenberg SM, Anderson CS, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2015;46:2032–60. [DOI] [PubMed] [Google Scholar]
- [2].van Asch CJ, Luitse MJ, Rinkel GJ, et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 2010;9:167–76. [DOI] [PubMed] [Google Scholar]
- [3].Mayer SA, Rincon F. Treatment of intracerebral haemorrhage. Lancet Neurol 2005;4:662–72. [DOI] [PubMed] [Google Scholar]
- [4].Morgenstern LB, Hemphill JC, 3rd, Anderson C, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2010;41:2108–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Mendelow AD, Gregson BA, Rowan EN, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet 2013;382:397–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Feng Y, He J, Liu B, et al. Endoscope-assisted keyhole technique for hypertensive cerebral hemorrhage in elderly patients: a randomized controlled study in 184 patients. Turk Neurosurg 2016;26:84–9. [DOI] [PubMed] [Google Scholar]
- [7].Yamashiro S, Hitoshi Y, Yoshida A, et al. Effectiveness of endoscopic surgery for comatose patients with large supratentorial intracerebral hemorrhages. Neurol Med Chir (Tokyo) 2015;55:819–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wang WH, Hung YC, Hsu SP, et al. Endoscopic hematoma evacuation in patients with spontaneous supratentorial intracerebral hemorrhage. J Chin Med Assoc 2015;78:101–7. [DOI] [PubMed] [Google Scholar]
- [9].Zhang HZ, Li YP, Yan ZC, et al. Endoscopic evacuation of basal ganglia hemorrhage via keyhole approach using an adjustable cannula in comparison with craniotomy. Biomed Res Int 2014;2014:898762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Huang Y, Cheng XL, Huang JR, et al. A comparison study of endoscopic surgery versus craniotomy in hypertensive basal ganglia intracerebral hemorrhage. Chin J Pract Nervous Dis 2014;17:1–4. [Google Scholar]
- [11].Nishihara T, Morita A, Teraoka A, et al. Endoscopy-guided removal of spontaneous intracerebral hemorrhage: comparison with computer tomography-guided stereotactic evacuation. Child Nerv Syst 2007;23:677–83. [DOI] [PubMed] [Google Scholar]
- [12].Auer LM, Deinsberger W, Niederkorn K, et al. Endoscopic surgery versus medical treatment for spontaneous intracerebral hematoma: a randomized study. J Neurosurg 1989;70:530–5. [DOI] [PubMed] [Google Scholar]
- [13].Xu X, Chen X, Li F, et al. Effectiveness of endoscopic surgery for supratentorial hypertensive intracerebral hemorrhage: a comparison with craniotomy. J Neurosurg 2017;126:1–7. [DOI] [PubMed] [Google Scholar]
- [14].Zhu H, Wang Z, Shi W. Keyhole endoscopic hematoma evacuation in patients. Turk Neurosurg 2012;22:294–9. [DOI] [PubMed] [Google Scholar]
- [15].Chi FL, Lang TC, Sun SJ, et al. Relationship between different surgical methods, hemorrhage position, hemorrhage volume, surgical timing, and treatment outcome of hypertensive intracerebral hemorrhage. World J Emerg Med 2014;5:203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Balu S. Differences in psychometric properties, cut-off scores, and outcomes between the Barthel Index and Modified Rankin Scale in pharmacotherapy-based stroke trials: systematic literature review. Curr Med Res Opin 2009;25:1329–41. [DOI] [PubMed] [Google Scholar]
- [17].Shih MM, Rogers JC, Skidmore ER, et al. Measuring stroke survivors’ functional status independence: five perspectives. Am J Occup Ther 2009;63:600–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol 2010;25:603–5. [DOI] [PubMed] [Google Scholar]
- [19].Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–2. [DOI] [PubMed] [Google Scholar]
- [20].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [21].Wu R, Li B. A multiplicative-epistatic model for analyzing interspecific differences in outcrossing species. Biometrics 1999;55:355–65. [DOI] [PubMed] [Google Scholar]
- [22].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [23].Stovold E, Beecher D, Foxlee R, et al. Study flow diagrams in Cochrane systematic review updates: an adapted PRISMA flow diagram. Syst Rev 2014;3:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nagasaka T, Tsugeno M, Ikeda H, et al. Early recovery and better evacuation rate in neuroendoscopic surgery for spontaneous intracerebral hemorrhage using a multifunctional cannula: preliminary study in comparison with craniotomy. J Stroke Cerebrovasc Dis 2011;20:208–13. [DOI] [PubMed] [Google Scholar]
- [25].Cho DY, Chen CC, Chang CS, et al. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol 2006;65:547–55. discussion 555–546. [DOI] [PubMed] [Google Scholar]
- [26].Juttler E, Steiner T. Treatment and prevention of spontaneous intracerebral hemorrhage: comparison of EUSI and AHA/ASA recommendations. Expert Rev Neurotherap 2007;7:1401–16. [DOI] [PubMed] [Google Scholar]
- [27].Zhou X, Chen J, Li Q, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke 2012;43:2923–30. [DOI] [PubMed] [Google Scholar]
- [28].Wang JW, Li JP, Song YL, et al. Stereotactic aspiration versus craniotomy for primary intracerebral hemorrhage: a meta-analysis of randomized controlled trials. PLoS ONE 2014;9:e107614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zhou H, Zhang Y, Liu L, et al. Minimally invasive stereotactic puncture and thrombolysis therapy improves long-term outcome after acute intracerebral hemorrhage. J Neurol 2011;258:661–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nakano T, Ohkuma H, Ebina K, et al. Neuroendoscopic surgery for intracerebral haemorrhage—comparison with traditional therapies. Minim Invasive Neurosurg 2003;46:278–83. [DOI] [PubMed] [Google Scholar]
- [31].Hsieh PC, Cho DY, Lee WY, et al. Endoscopic evacuation of putaminal hemorrhage: how to improve the efficiency of hematoma evacuation. Surg Neurol 2005;64:147–53. discussion 153. [DOI] [PubMed] [Google Scholar]
- [32].Nagasaka T, Tsugeno M, Ikeda H, et al. A novel monoshaft bipolar cautery for use in endoscopic intracranial surgery. A short technical note. Clin Neurol Neurosurg 2011;113:607–11. [DOI] [PubMed] [Google Scholar]
- [33].Nagasaka T, Tsugeno M, Ikeda H, et al. Balanced irrigation-suction technique with a multifunctional suction cannula and its application for intraoperative hemorrhage in endoscopic evacuation of intracerebral hematomas: technical note. Neurosurgery 2009;65:E826–7. discussion E827. [DOI] [PubMed] [Google Scholar]
- [34].Nishihara T, Teraoka A, Morita A, et al. A transparent sheath for endoscopic surgery and its application in surgical evacuation of spontaneous intracerebral hematomas. Technical note. J Neurosurg 2000;92:1053–5. [DOI] [PubMed] [Google Scholar]
- [35].Schaller B, Graf R, Sanada Y, et al. Hemodynamic and metabolic effects of decompressive hemicraniectomy in normal brain. An experimental PET-study in cats. Brain Res 2003;982:31–7. [DOI] [PubMed] [Google Scholar]


