Abstract
Rationale:
Understanding the status of internal mammary lymph nodes of breast cancer is critical in the accurate staging of breast cancer and the development of accurate therapeutic regimen for selected patients. Current techniques for dissection of internal mammary lymph node biopsy involve endoscopic or Traditional thoracic surgery, An important drawback of the current techniques is the great trauma caused by them.
Patient concerns:
Da Vinci robotic surgery system (Intuitive Surgical Inc. Sunnyvale, CA) was used to perform the internal mammary lymph chain excision for a breast cancer patient with left internal mammary lymph node metastasis.
Diagnoses:
Positron emission tomography-computed tomography examination and Ultrasonography examination.
Interventions:
In this paper, we introduce a Robot-assisted technique for dissection of internal mammary lymph node biopsy with only 3 small trocar ports. This technique reduces the incision size and considerably reduce the trauma.
Outcomes:
The operation lasted a duration of 1.5 hours. The operation was carried out smoothly with removal of 9 internal mammary lymph nodes in total. The amount of intra operative bleeding was less than 10 ml. The patient's postoperative recovery was fast. 11-month postoperative follow-up showed that the patient recovered well after surgery, no local recurrence or distant metastasis was found, and no obvious discomfort was reported.
Lessons:
Robot-assisted excision of internal mammary lymph chain in breast cancer is a safe, effective and simple operation with minimal invasion.
Keywords: breast cancer, Da Vinci robotic system, internal mammary lymph node, minimally invasive, robotic-assisted surgery
1. Introduction
Internal mammary lymph nodes (IMLNs) are an important part of breast cancer metastasis. Understanding the status of IMLN in breast cancer is critical in the accurate staging of breast cancer and the selection of the most appropriate therapeutic regimen.[1–3] Because extensive radical mastectomy causes great trauma and has no benefits for patients as compared with modified radical mastectomy, it is being used less frequently. Traditional thoracic surgery can be used for IMLN chain excision. However, for left breast cancer patients with IMLN metastasis, the operation is very difficult because of obstruction by the heart.[4] Furthermore, these procedures necessitate an incision in the costa, which breaks the thoracic structural integrity. Therefore, there is definitely a pressing need for minimally invasive techniques for IMLN dissection. In this paper, we describe and discuss the outcome of a robot-assisted technique. We present a case of IMLN dissection using the Da Vinci robotic surgery system (Intuitive Surgical inc. Sunnyvale, CA).
2. Methods
The patient was placed in a horizontal position under general anesthesia with double-lumen endotracheal intubation. The diseased side was blocked up to approximately 15 degrees, and the left upper limb flexion was 90 degrees and fixed to the lathe-head. After conventional disinfection, trocar locations were marked. The location of trocar was selected between midclavicular line and posterior axillary line in the third, fifth, and seventh intercostal spaces, making the 3 entrances to form a triangular shape, and also ensuring that the distance between each trocar was longer than 8 cm (Fig. 1).
Figure 1.
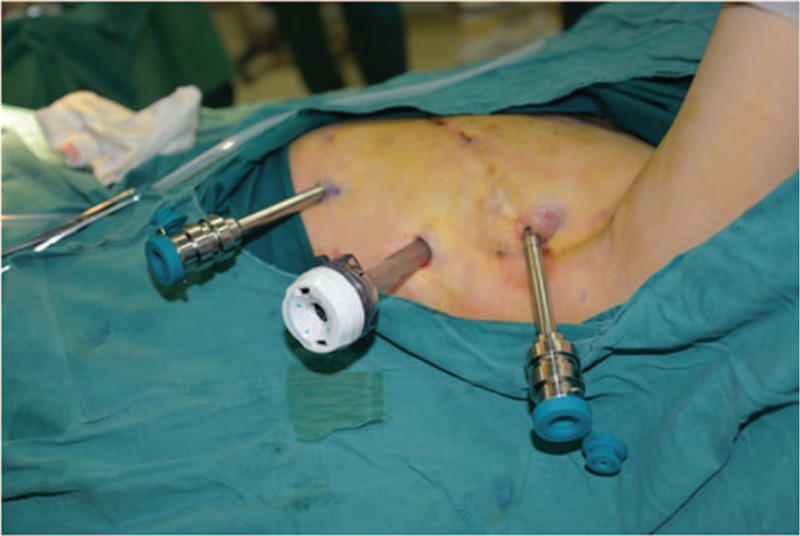
The location of trocar was selected between midclavicular line and posterior axillary line in the third, fifth, and seventh intercostal space, making the 3 entrances to form a triangular shape, and also ensuring that the distance between each trocar is longer than 8 cm.
After 1 lung ventilation, the left lung gradually collapsed. The trocars were inserted through the marked locations into the intercostal space via a puncture along the midaxillary line and connected to the mechanical arm along with placement of the camera and special surgical instruments.
The surgeon sat at the control table to operate the robot to complete the surgery. One assistant was responsible for wiping the camera lens, replacing instruments, taking out the specimen, rinsing the operation field, and so forth. In combination with body surface location, the enlarged lymph nodes were located 2 cm of the left border of the sternum within the intercostal space under a 3-dimensional (3D) visual field (Fig. 2). The origin of the internal mammary vessels was found, and the partial pleural layer was cut at approximately 2 cm from the vena subclavia along the internal mammary vessels. The internal mammary artery and vein were dissociated and cut off by using an ultrasonic scalpel. The partial pleural layer was cut longitudinally 2 cm on both sides along the internal mammary vessels to open the intercostal space. The internal mammary vessels and the fatty lymph tissues on both sides were completely excised by using the electric coagulation hook combined with the ultrasonic knife. Bagged specimens were taken out through a trocar port. A thoracic drainage tube connected with a water-sealed bottle for closed drainage was placed into the intercostal space. The drainage tube was fixed, double-lung ventilation was then applied, and each incision was closed.
Figure 2.
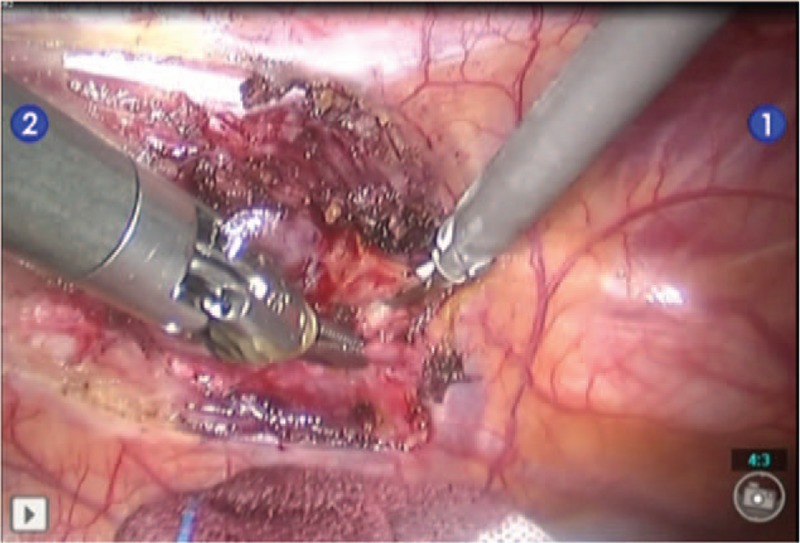
The enlarged lymph nodes were located at 2 cm of the left border of sternum within the second intercostal space.
3. Results
The patient is a 34-year-old woman, with a single, progressive, increscent mass in the upper inner quadrant of the left breast, who had undergone breast lumpectomy and was diagnosed with invasive ductal carcinoma of the left breast; modified radical mastectomy was performed for left breast cancer in another hospital. Table 1 shows the clinical pathology feature after the first operation. Three months later, positron emission tomography-computed tomography examination showed highly metabolic lymph nodes in the left internal mammary region, with suspected invasion (Fig. 3). So, she visited our hospital on June 24, 2016, for further treatment.
Table 1.
Clinical pathology feature of the patient after the first operation.

Figure 3.
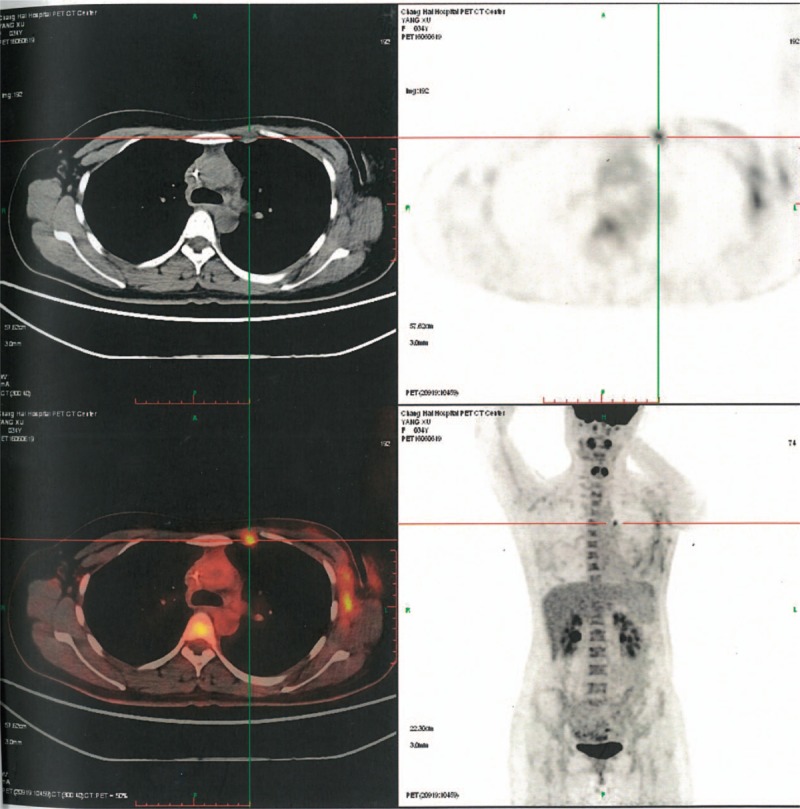
Positron emission tomography-computed tomography (PET-CT) examination showed high metabolic lymph nodes in the left internal mammary region.
Ultrasonography examination showed a low-echo signal in the second intercostal space of the left border of the sternum (Fig. 4), in the lining along the internal arteriovenous of the thorax, with no obvious lymph node structures or suspicious characteristics. After adequate preoperative preparation, Da Vinci-assisted left IMLN chain resection was performed on June 27, 2016. The operation lasted for a duration of 1.5 hours, including 30 minutes for docking. The operation was carried out smoothly with removal of 9 IMLNs in total, of which the diameter of the largest one was approximately 2 cm (Fig. 5). The amount of intraoperative bleeding was less than 10 mL. The thoracic closed drainage tube drained pale red liquid of 150 mL in total, and the liquid amount kept reducing each day. The thoracic closed drainage tube was removed on day 4 after the operation. The patient was hospitalized for 6 days.
Figure 4.
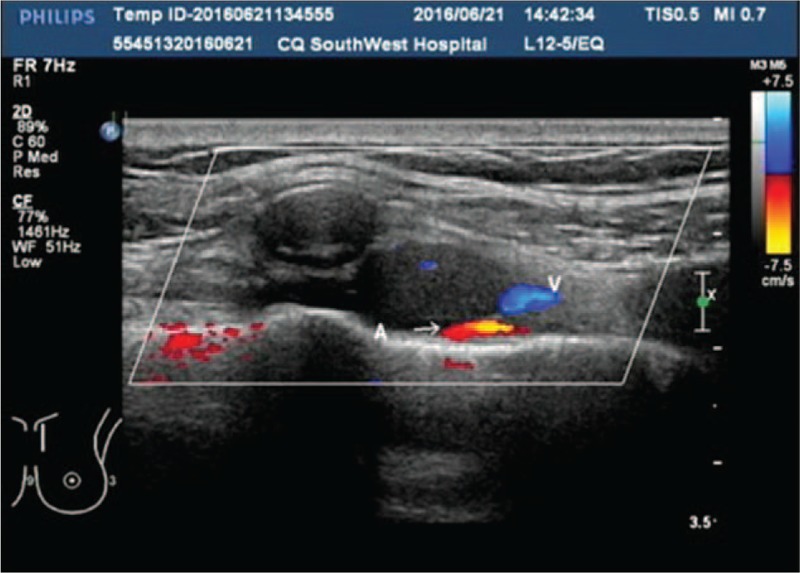
Ultrasonography examination showed a low-echo signal in the second intercostal space of the left border of the sternum, in the lining along the internal arteriovenous of the thorax, with no obvious lymph node structures or suspicious characteristics.
Figure 5.
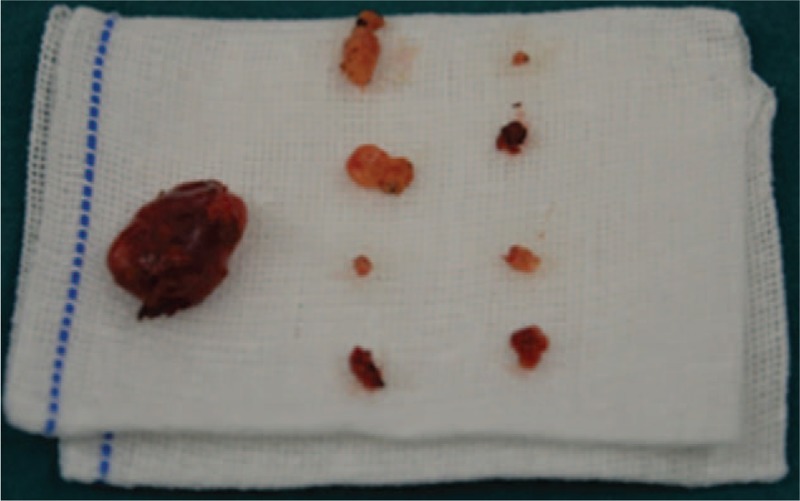
The operation was carried out smoothly with removal of 9 internal mammary lymph nodes in total, of which the diameter of the largest one was about 2 cm.
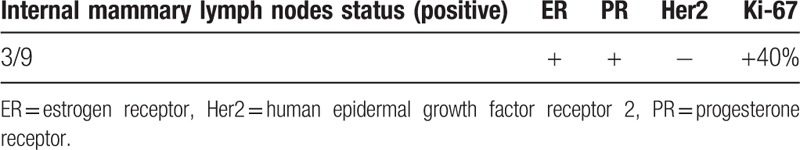
The postoperative pathology showed metastasis (Table 2), and we compared the number of metastatic lymph nodes and the number of resected lymph nodes (3/9). Postoperative follow-up at 10 months showed that the patient recovered well after surgery, no local recurrence or distant metastasis was found, and no obvious discomfort was reported.
Table 2.
Postoperative pathology of the patient.
4. Discussion
Understanding the status of IMLN of breast cancer is critical for the accurate staging of breast cancer and the selection of an appropriate therapeutic regimen. The National Comprehensive Cancer Network (NCCN) guidelines recommend that, for breast cancer patients with a suspicious positive IMLN or with axillary lymph node metastasis, radiotherapy should be carried out in the parasternal areas.[5] However, parasternal radiotherapy may lead to impaired function of the heart, lungs, and other vital organs, which will reduce patients’ quality of life.[6–9]
To explore a minimally invasive method for safe IMLN resection, researchers have conducted intercostal biopsy of internal mammary sentinel lymph nodes to estimate the status of IMLN.[10,11] However, because of the longitudinal distribution of the IMLN along the internal thoracic artery, every lymph node can be a sentinel node.
In theory, the IMLNs are equally distributed in the intercostal space and behind the costal cartilage. Intercostal biopsy without costal cartilage resection may miss lymph nodes. Reports of thoracoscopic surgery for IMLN resection have also been reported.[4,12–15] This method is easy for right IMLN chain resection. However, because of the location of the heart and the pivot effect of the rigid long straight instrument, it is difficult to remove the left IMLNs,[4] limiting the application and development of this technique.
The 3D imaging system and the larger magnification of the Da Vinci robotic surgical equipment can provide a clearer vision compared with open surgery or traditional laparoscopic surgery. The operating instrument with the special internal joint design (EndoWrist) has 7 degrees of freedom, which is beyond the human hand's limit and therefore is conducive to the completion of more detailed operations. Meanwhile, the vibration reduction system and the action calibration system of the robot system can effectively protect the blood vessels and nerves, further improving the safety of the surgery.
The main disadvantage of robotic surgery is increased cost and operation time. A recent cost analysis of robotic surgery based on medical costs in the United States showed that the robotic system extended the operative time and disposables costs, thus contributing to an increase in operative costs.[16,17] Cost can be minimized by the multidisciplinary use, especially in an institutional practice. In addition, lack of haptic feedback is another concern. Novice surgeons may find the absence of haptic feedback a difficulty. The visual clues must be relied to assess the surgical field.
The surgical results of this case show that except for the duration of operation preparation and docking, the operation lasted for only 1.5 hours. Blood loss was only 10 mL, and 9 IMLNs were removed. The patient's postoperative recovery was fast, and her post-traumatic stress reaction was mild. With 10 months of follow-up after the operation, the patient showed good recovery, without surgical complications.
These results indicate that it is safe, effective, simple, and fast to use the Da Vinci robotic system for internal mammary lymph chain resection in breast cancer cases. Although this case was a left operation, the surgical instrument with its special internal joint design effectively avoided being blocked by the heart. Therefore, the operation was completed safely and quickly. According to our literature searches, this is the first report of robot-assisted internal mammary lymph chain resection in a breast cancer patient.
5. Conclusions
Robot-assisted excision of IMLN in breast cancer is a safe, effective, and simple operation that is minimally invasive. It is helpful for accurate understanding of the status of the IMLN in breast cancer, and provides a new choice for breast cancer patients with IMLN metastasis. However, additional clinical experience is warranted for further validation of this technique.
Acknowledgments
We thank Ling Zhong for helping with the images collection and Junlan Liu for critical reading of the manuscript.
Footnotes
Abbreviation: IMLN = internal mammary lymph node.
Authors’ contributions: Study conception and design: L.F., J.J.; acquisition of data: J.D., H.M.; analysis and interpretation of data: J.D., H.M.; drafting of manuscript: J.D., H.M.; critical revision: L.F.; supervision: J.J. All authors read and approved the final manuscript.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Li Z, Gu X, Tong J, et al. A meta-analysis of internal mammary lymph node metastasis in breast cancer patients. Onkologie 2013;36:747–52. [DOI] [PubMed] [Google Scholar]
- [2].Madsen EVE, Gobardhan PD, Bonges V, et al. The impact on post-surgical treatment of sentinel lymph node biopsy of internal mammary lymph nodes in patients with breast cancer. Ann Surg Oncol 2007;14:1486–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Lee SK, Kim S, Choi MY, et al. The clinical meaning of intramammary lymph nodes. Oncology 2013;84:1–5. [DOI] [PubMed] [Google Scholar]
- [4].He Q, Jiang J, Yang X, et al. A pilot study on thoracoscopic internal mammary lymphatic chain dissection for breast cancer. Breast 2008;17:568–73. [DOI] [PubMed] [Google Scholar]
- [5].NCCN (2016). NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Breast Cancer, version I, 2016 [C/OL] (2015–12–18). Available at: http://www.NCCN.com. Accessed February, 1 2016. [Google Scholar]
- [6].Hooning MJ, Botma A, Aleman BM, et al. Long-term risk of cardiovascular disease in 10-year survivors of breast cancer. Natl Cancer Inst 2007;99:365–75. [DOI] [PubMed] [Google Scholar]
- [7].Nilsson G, Holmberg L, Garmo H, et al. Radiation to supraclavicular and internal mammary lymph nodes in breast cancer increases the risk of stroke. Br J Cancer 2009;100:811–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Matzinger O, Heimsoth I, Poortmans P, et al. Toxicity at three years with and without irradiation of the internal mammary and medial supraclavicular lymph node chain in stage I to III breast cancer (EORTC trial 22922/10925). Acta Oncol 2010;49:24–34. [DOI] [PubMed] [Google Scholar]
- [9].Groheux D, Moretti JL, Baillet G, et al. Effect of 18 F-FDG PET/CT imaging in patients with clinical stage II and III breast cancer. Int J Radiat Oncol Biol Phys 2008;71:695–704. [DOI] [PubMed] [Google Scholar]
- [10].Xiao S, Binbin C, Pengfei Q. Internal mammary sentinel lymph node biopsy in breast cancer patients with clinically positive axillary lymph nodes. Chin J Clin Oncol 2015;42:341–4. [Google Scholar]
- [11].Xiaoshan C, Inbin C, Xiao S. A retrospective stud y of axillary and internal mammary sentinel lymph node biopsy in breast cancer patients after neoadjuvant chemotherapy. China Oncol 2015;25:608–13. [Google Scholar]
- [12].Long H, Lin Z, Situ D, et al. Stage migration and therapy modification after thoracoscopic internal mammary lymph node dissection in breast cancer patients. Breast 2011;20:129–33. [DOI] [PubMed] [Google Scholar]
- [13].Conrado-Abrão F1, Das-Neves-Pereira JC, Fernandes A, et al. Thoracoscopic approach in the treatment of breast cancer relapse in the internal mammary lymph node. Interact Cardiovasc Thorac Surg 2010;11:328–30. [DOI] [PubMed] [Google Scholar]
- [14].Long H, Situ DR, Ma GW, et al. Thoracoscopic internal mammary lymph node dissection: a video demonstration. Ann Surg Oncol 2013;20:1311–2. [DOI] [PubMed] [Google Scholar]
- [15].Jaroszewski DE, Ewais MM, Pockaj BA, et al. Thoracoscopy for internal mammary node dissection of metastatic breast cancer. J Laparoendosc Adv Surg Tech A 2015;25:135–8. [DOI] [PubMed] [Google Scholar]
- [16].Terris DJ, Singer MC. Qualitative and quantitative differences between 2 robotic thyroidectomy techniques. Otolaryngol Head Neck Surg 2012;147:20–5. [DOI] [PubMed] [Google Scholar]
- [17].Cabot JC, Lee CR, Brunaud L, et al. Robotic and endoscopic transaxillary thyroidectomies may be cost prohibitive when compared to standard cervical thyroidectomy: a cost analysis. Surgery 2012;152:1016–24. [DOI] [PubMed] [Google Scholar]


