Abstract
The aim of this study was to evaluate the efficacy and acute toxicity of definitive chemoradiotherapy (CRT) and radiotherapy (RT) alone as initial treatment in patients aged 75 years and older with locally advanced esophageal squamous cell carcinoma (ESCC) who are not eligible for surgery.
Between February 2009 and February 2015, 122 patients older than 75 years with locally advanced ESCC were retrospectively reviewed, in whom 52 patients allocated to the CRT group were treated with at least 2 cycles of platinum and 5-fluorouracil, 70 patients allocated to the RT group were treated with RT alone, all patients were received a total radiation dose of 54–66 Gy, with 1.8 or 2-Gy/fraction. Response rate (RR), progression-free survival (PFS), overall survival (OS), and acute toxicities were compared between the 2 different treatment groups.
In the CRT group, the median PFS and OS were 15.3 and 24.6 months, while 10.6 and 19.4 months in the RT group (P = .008 and P = .018). The 1-year survival rates of the 2 groups were 78.8% versus 64.3% (P = .081), and the 2-year survival rates were 48.1% and 30.0% (P = .042), respectively. The objective RR was 55.8% in the CRT group with 18 complete response (CR) and 18.6% in the RT group with 13 CR. Acute toxicity in the CRT group was higher than in the RT group, especially the grade 3 to 4 acute toxicities.
Compared with RT alone, definitive CRT in the treatment of locally advanced ESCC can prolong the survival time in elderly patients. Definitive CRT should be considered the first-treatment choice for elderly patients like the younger patients who are not eligible for surgery.
Keywords: chemoradiotherapy, elderly patients, esophageal carcinoma, radiotherapy, survival, toxicity
1. Introduction
Esophageal carcinoma (EC) is the eighth most common cancer worldwide and the sixth leading cause of death from cancer.[1] The elderly is recognized as the high risk of cancer with a mean age of cancer at diagnosis of 67 years older.[2] In the United States, approximately 44% of EC occurs in patients over 60 years older, and 30% older than 75 years of age,[3] and in France, more than half of EC occurs in patients older than 60 years and more than a quarter over 75 years older.[4] In recent years, with the prolongation of life expectancy and the aging of the population increase, elderly patients with esophageal carcinoma are increasing gradually. Surgical resection is considered to be the standard treatment in EC patients; however, for most patients aged 75 years and older are rarely referred for surgery which was partially explained by their comorbidities or a high postoperative mortality rate.[5–8] Despite the lack of clear rule, most of the researches treated age as a limit of esophagectomy, and older patients are often excluded from it. For these patients, definitive chemoradiotherapy (CRT) or radiotherapy (RT) alone for curative intent are regarded as viable options. Randomized trials[9–13] of definitive CRT and RT alone for locally advanced EC have shown that both treatments can cure a portion of patients. However, most of these studies typically included few patients aged ≥ 75, data on the feasibility or efficacy of both therapeutic regimens in the elderly patients are limited. The Radiation Therapy Oncology Group trial 85–01[14] established the superiority of chemoradiation with cisplatin and 5-fluorouracil over RT alone in patients treated without surgery and showed that CRT improved both 5-year survival (26% vs 0% at 5 years) and median survival (12.5 vs 9 months) compared with RT alone. However, only 23% of patients enrolled were over age 70, and no information concerning this population was reported. Furthermore, a series of studies revealed that the definitive CRT treatment generally results in significant acute and chronic treatment-related toxic side effects.[10,14,15] Therefore, the standard treatment for the elder EC patients has not reached a consensus yet. The purpose of our study was to compare the survival and major toxicity of elderly locally advanced esophageal squamous cell carcinoma (ESCC) patients (≥75 years) who received definitive CRT or RT alone, thus to offer scientific basis for clinical treatment for elder patients with locally advanced ESCC.
2. Patients and methods
2.1. Patients
We retrospectively analyzed the medical records of patients with locally advanced EC ≥75 years of age who were treated in Shandong Cancer Hospital from February 2009 to February 2015, and 122 patients were included according to the following selection criteria: age 75 years and older; histologically confirmed as ESCC; Eastern Cooperative Oncology Group (ECOG) performance status of 0 to 2; clinical stages from II to III based on the 7th UICC-TNM classification; at least 2 cycles of first-line chemotherapy with cisplatin and 5-FU concurrent with 54 to 66 Gy radiation in the CRT group, and 54 to 66 Gy radiation alone in the RT group; no prior cancer therapy or concomitant malignancy; no surgical treatment related to esophageal carcinoma during the follow-up period. This study was approved by the Medical Ethics Committee of the Shandong Cancer Hospital.
3. Treatment
3.1. Chemotherapy
In the CRT group, chemotherapy consisted of cisplatin at a dose of 75 mg/m2 delivered on days 1 to 2 and 5-FU 1000 mg/m2 delivered on days 1to 4, with concurrent RT. Three weeks for a cycle of chemotherapy and each patient received at least 2 cycles. Antiemetic drugs were used to prevent vomiting in the course of chemotherapy. Before and after every cycle of chemotherapy, a complete blood count was obtained. Once severe hematologic toxicity happened, dose would be adjusted in the next chemotherapy cycle. If the white blood cell (WBC) under 3000/mm3 after the chemotherapy, treated with the Recombinant Human Granulocyte Colony-stimulating Factor Injection. If the WBC count down to 2000/mm3 to 2500/mm3, dose reduction for cisplatin from 75 to 40 mg/m2, and 5-FU from 1000 to 500 mg/m2 at the beginning of the next course, the RT was continued as before. If there was a Grade 4 hematological, or Grade 3/4 gastrointestinal reaction occurred, the dose would reduced by 25% in the subsequent course. Once severe radiation pneumonitis/esophagitis appeared, both chemotherapy and RT would discontinue until recovery. Treatment was stopped when disease progression was observed, or patients’ own condition was not suitable for continue.
3.2. Radiation
The prescribed dose was specified according to the International Commission on Radiation Units and Measurement, Report 83.[16] A total radiation dose of 54 to 66 Gy was given in 30 to 33 fractions of 1.8 to 2 Gy/fraction with 5 fractions administered per week in both CRT group and RT group. And all patients were treated with three-dimension conformal radiotherapy (3D-CRT). Gross tumor volume (GTV) was defined as the macroscopic primary tumor and regional lymph node metastases, based on all available information, like endoscopy, endoscopic ultrasonography, computerized tomography, and from 18F-fluorodeoxyglucose positron emission tomography, and so on. A margin of 5 cm from GTV to field margin in the caudal/cranial direction and 2 cm in the transversal plane was used during the direct simulation. The clinical target volume (CTV) was defined as the GTV plus the volume of a 3-cm margin along the length of the esophagus and 1-cm radical margin. Around the pathological lymph nodes, a 1-cm margin was used. The planning target volume was defined as the CTV plus 1-cm margin. To the spinal cord, the maximal dosage had to be ≦44 Gy and 30% of the lung volume was to receive not more than 20 Gy [volume of lung receiving at least 20 Gy (V20) ≦30%]. And for the heart, the corresponding value V40 had to be ≦30%. To ensure that the spinal cord, lung, and heart exposure were within organ tolerance, verification images were performed weekly during treatment.
3.3. Evaluation methods
About 3 months after the completion of the treatment, tumor response was evaluated with physical examination, barium X-ray, and computerized tomography scan based on the new Response Evaluation Criteria in Solid Tumors (revised RECIST guideline, version 1.1),[17] which was classified into complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). CR: All target lesions disappeared completely. PR: The total diameters of the target lesions decreased at least 30%, taking as reference the baseline sum diameters. PD: The total diameters of the target lesions increased at least 20%, taking as reference the smallest sum on study. Not only that, the total diameters must also demonstrate an absolute increase of at least 5 mm. And SD: Neither the extent of lesions shrinkage low to PR nor sufficient increase up to PD, taking as reference the smallest sum diameters while on study. CR plus PR was defined as RR, and RR plus SD was defined as disease-control rate (DCR). Toxicity was evaluated according to the Common Terminology Criteria for Adverse Events (CTCAE 4.0).
3.4. Follow-Up
Follow-up was performed in all patients. In most cases, for the first 2 years, patients had a follow-up visit every 3 months, and then every 6 months until death or the cut-off date, February, 2017. The median duration of follow-up was 39.7 months.
3.5. Statistical analysis
With respect to the base-line characteristics, the 2 treatment groups were compared with use of the χ2 test or the t test. The Fisher exact test was also used when necessary. Overall survival (OS) and progression-free survival (PFS) rates were calculated according to the Kaplan–Meier method and tested by the log-rank test. OS was determined from the starting day of (chemo) radiotherapy to the date of death or the last follow-up. And PFS was defined as the time interval between the starting date of first-line (chemo) radiotherapy treatment and the date of disease progression, last follow-up or death of any cause. The Cox regression analysis and log-rank test were used to univariate and multivariate analyses, respectively. Two-sided P value < .05 was considered significant. Statistical Package for Social Sciences (version 17.0, SPSS, Chicago, IL) software package was used for all statistical analyses.
4. Results
4.1. Patient and tumor characteristics
One hundred twenty-two patients over 75 years of age were analyzed. All of them had received the complete dose of RT in about 5 weeks, and all the patients in the CRT group also received the prescribed dose of chemotherapy. Clinical baseline characteristics are detailed in Table 1. The median age of the patients was 78.0 years (range 75–91 years), with 87 being males (71.3%). Majority of patients (92.6%) had a good ECOG performance status (0 or 1). Twenty-two patients (18.0%) had an initial weight lost ≥ 10% and all the patients had cytopathologically diagnosed esophageal squamous cell carcinoma, with stage II/III, 24 of 98 patients, respectively. The majority of tumors (80.3%) were stage III, and more than half of them (55.7%) were over 5 cm in tumor diameter. The most common comorbidities are cardiovascular and pulmonary diseases. Among them 34 patients had cardiovascular comorbidity with 15 patients (28.8%) in the CRT group and 19 (27.1%) in the RT group (P = .836), respectively. Eight patients had pulmonary comorbidities with 5 patients (9.6%) in the CRT group and 3 (4.3%) in the RT group (P = .284), respectively. There is no statistical difference between the 2 groups associated with comorbidities.
Table 1.
Patient and tumor characteristics.
4.2. Response to treatment, survival, and outcome
For the whole patients (n = 122), the median PFS and median OS were, respectively, 12.9 months (95% confidence interval [CI], 10.6–15.3 months), and 21.7 months (95% CI, 19.3–24.1 months). The median PFS for patients in CRT group (15.3 months, 95% CI, 12.7–17.9 months) was significantly longer compared with patients in RT group (10.6 months, 95% CI, 8.8–12.5 months, P = .008). Similarly, the median OS was also significantly longer in CRT group (24.6 months, 95% CI, 19.4–29.4 months) than that in the RT group (19.4 months, 95% CI, 13.7–25.0 months, P = .018). PFS and OS curves of the 2 groups are shown in Figs. 1 and 2, respectively. One-year and 2-year survival rates were 78.8% and 48.1% in the CRT group, as well as 64.3% and 30.0% in the RT group, respectively.
Figure 1.
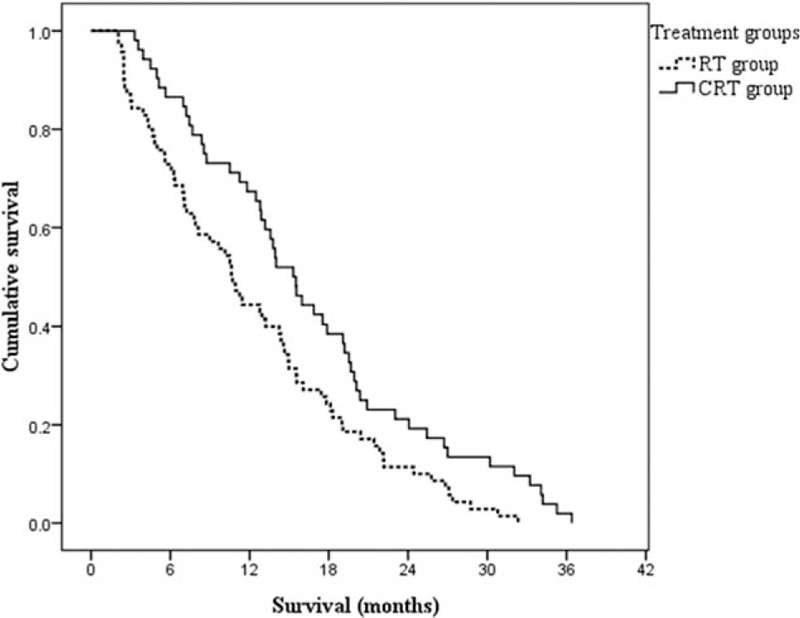
Kaplan–Meier estimates of progression-free survival according to different treatment groups. The elderly patients in the CRT group had a significantly longer median progression-free survival than that in the RT group, 15.3 versus 10.6 months, P = .008. CRT = chemoradiotherapy, RT = radiotherapy.
Figure 2.
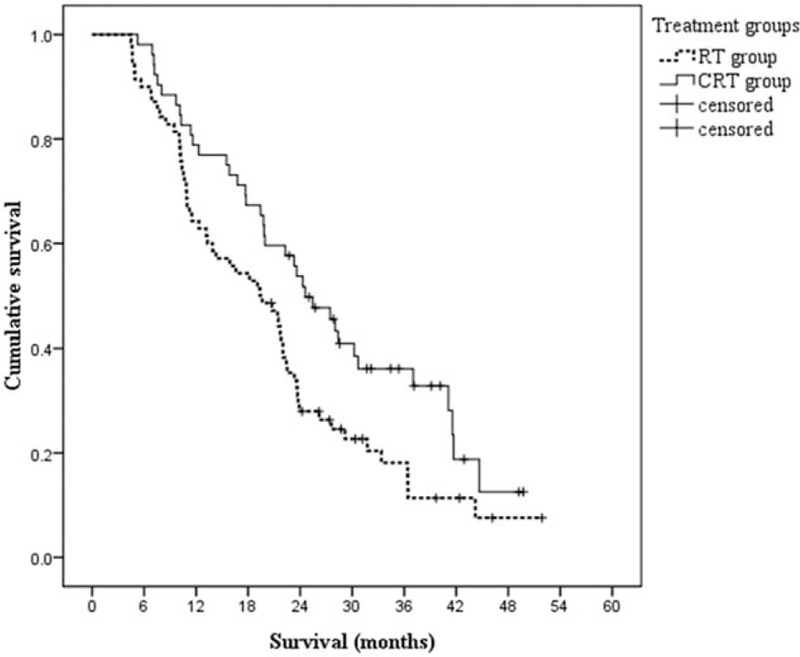
Kaplan–Meier estimates of overall survival according to different treatment groups. The elderly patients in the CRT group had a significantly longer median overall survival than that in the RT group, 24.6 versus 19.4 months, P = .018. CRT = chemoradiotherapy, RT = radiotherapy.
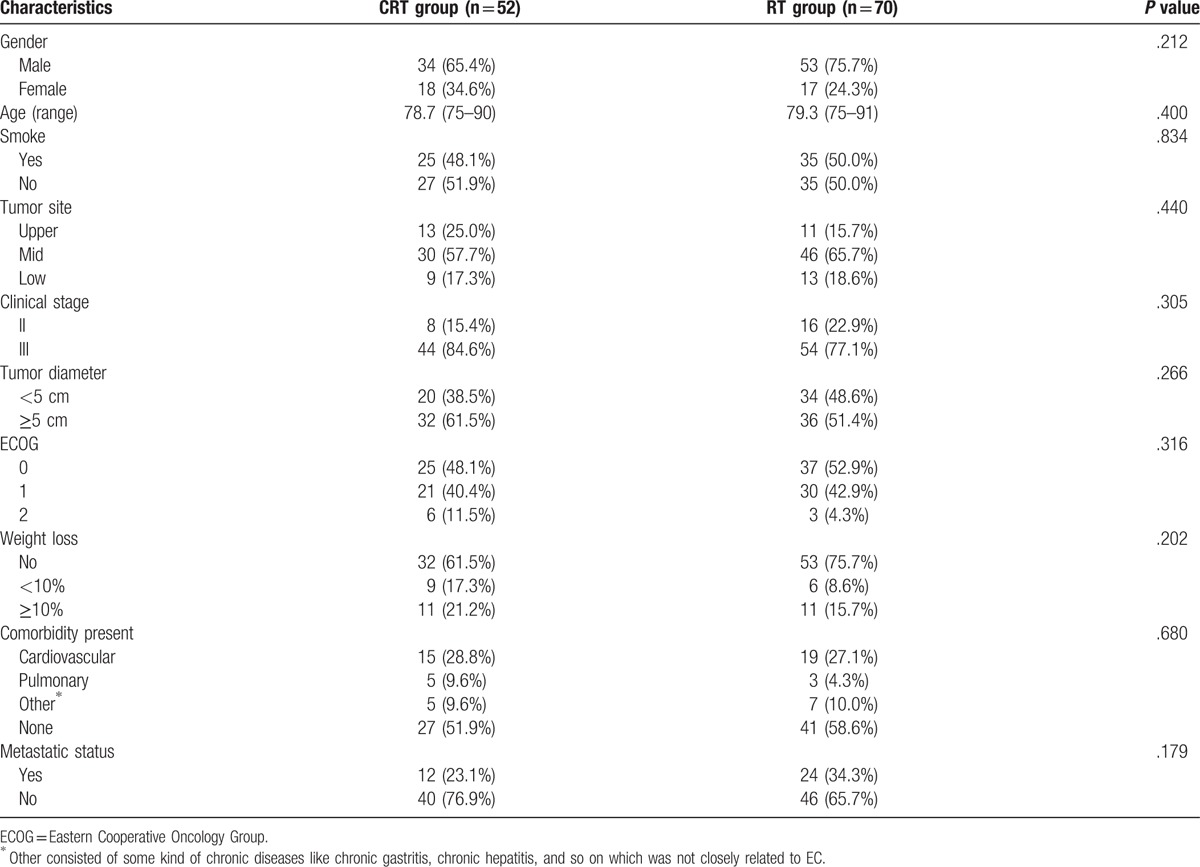
The treatment response of the 2 groups was shown and compared in Table 2. A CR was observed in 31 patients (25.4%), with 18 patients (34.6%) in the CRT group and 13 patients (18.6%) in the RT group (P = .044), respectively. Median OS in patients with CR was 36.4 months as compared with 16.6 months in non-CR (P < .001, Fig. 3). However, patients with metastases were usually accompanied by shortened survival. Although there was not a statistically significant difference in DCR, there was a trend toward a higher ratio in favor of the CRT group (94.2% vs 82.9%, P = .059).
Table 2.
Response rates.
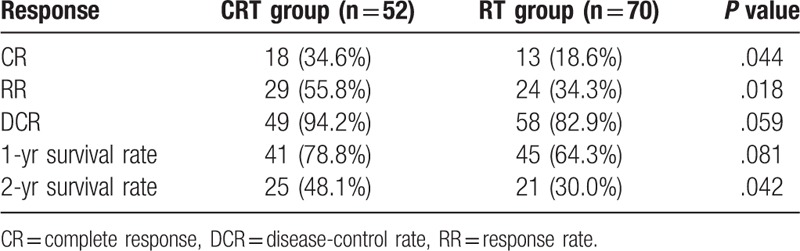
Figure 3.
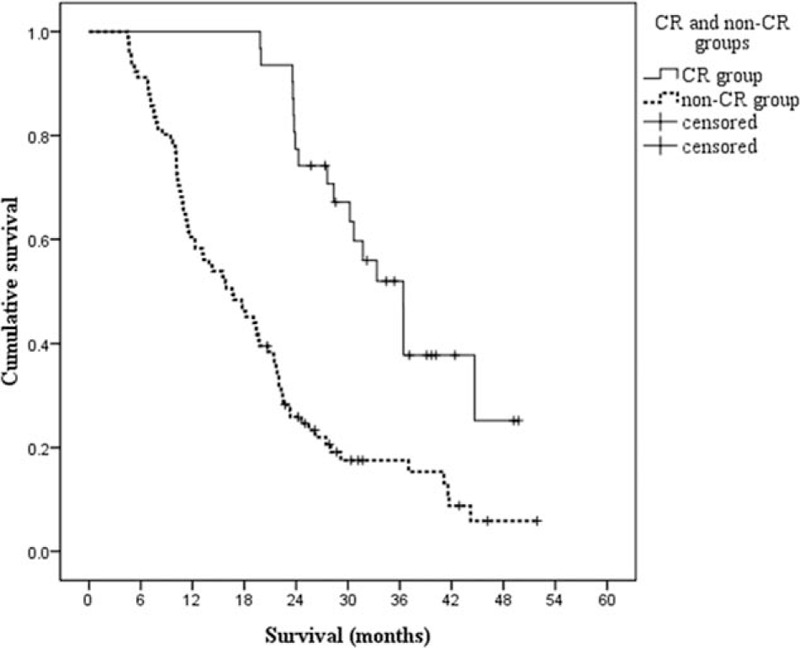
Kaplan–Meier estimates of overall survival according to CR or non-CR after treatment. The median overall survival for elderly patients achieved CR after treatment was 36.4 months, and 16.6 months for elderly patients achieved non-CR after treatment; P < .001. CR = complete response.
4.3. Univariable/multivariable analysis
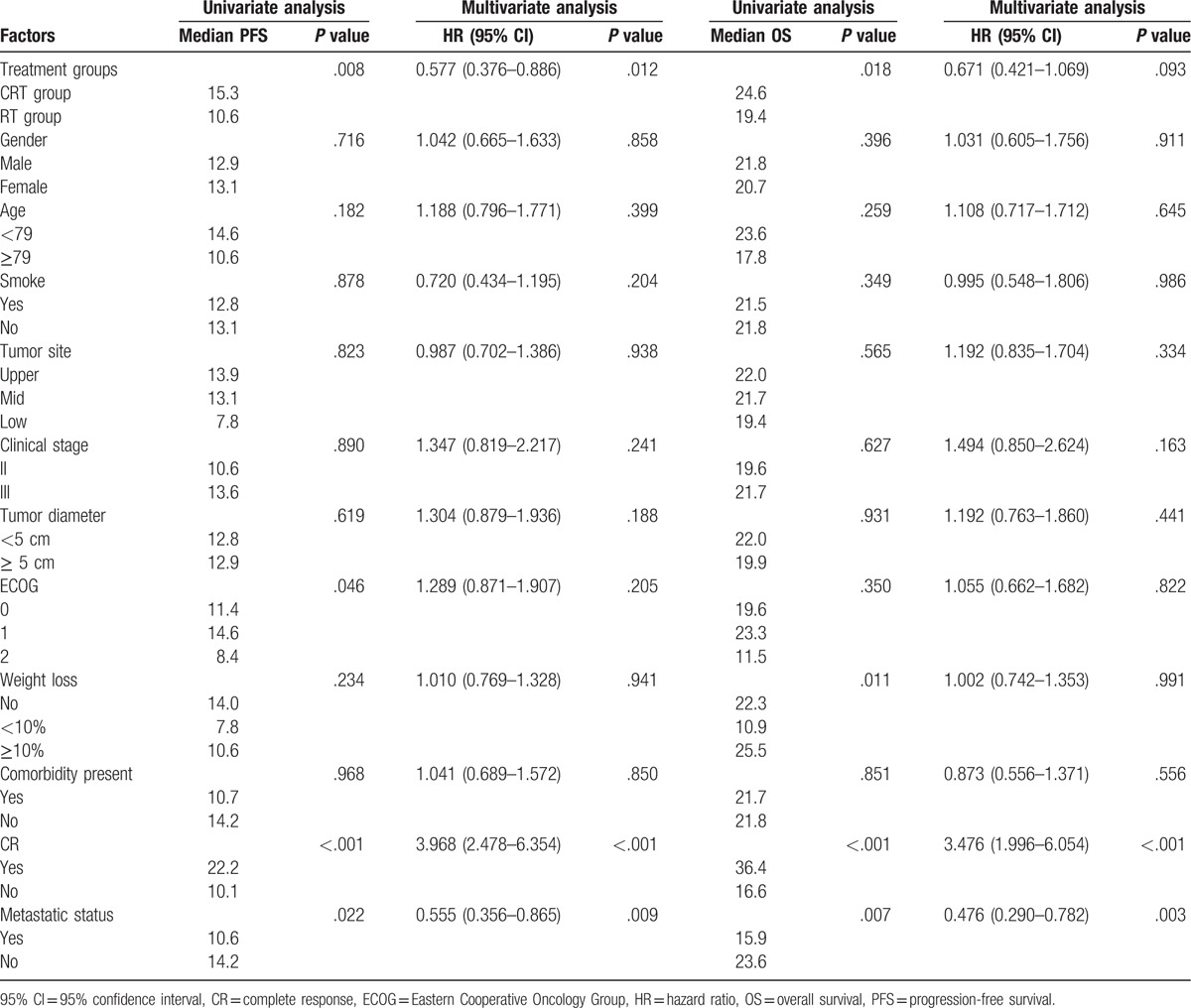
The various prognostic factors were evaluated by the univariate analysis first, and the data indicated that the important ones for PFS were different treatments (P = .008), ECOG performance status (P = .046), curative effect evaluation for CR after treatment (P < .001), and metastatic status (P = .022). In contrast, gender, age, smoking status, tumor site, clinical stage, tumor diameter, and weight loss were not independent prognostic factors of PFS. In the multivariate analysis, the different treatment groups, the curative effect evaluation for CR after treatment, and metastatic status were independent prognostic factors for PFS. As for the OS, univariate analysis identified different treatments, weight loss, curative effect evaluation for CR after treatment, and metastatic status as independent prognostic factors; however, gender, age, smoking status, tumor site, clinical stage, tumor diameter, ECOG performance, and comorbidity present were not independent prognostic factors of it. And further multivariate analysis showed that only curative effect evaluation for CR after treatment and metastatic status were independent prognostic factors for OS with hazard ratio of 3.476 (95% CI: 1.996–6.054) and 0.476 (95% CI: 0.290–0.782). Univariable and multivariable analyses for OS and PFS are shown in Table 3.
Table 3.
Univariate and multivariate analyses of prognostic factors to PFS and OS.
4.4. Toxicity and feasibility of different therapies
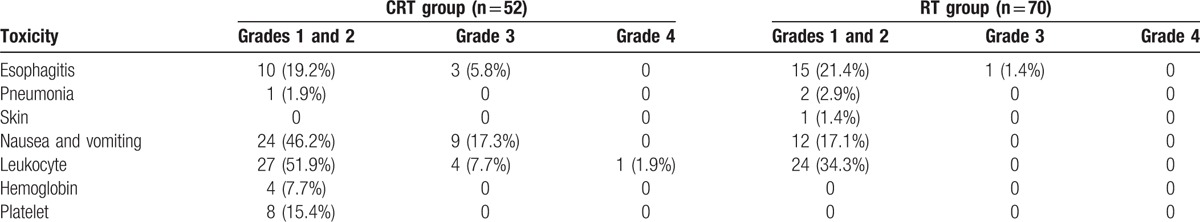
The incidence of toxicities in the CRT group was obviously higher than that in the RT group, especially for the gastrointestinal reaction (nausea and vomiting, 63.5% vs 17.1%) and the hematology toxicity (leukocyte, 61.5% vs 34.3%; hemoglobin, 7.7% vs 0; and platelet, 15.4% vs 0), the difference between them has statistical significance. No treatment-related mortality occurred in both the groups. The results are summarized in Table 4.
Table 4.
Acute toxicities of chemoradiotherapy and radiotherapy (%).
5. Discussion
With the prolongation of average life-span, the number of elderly EC patients is increasing year by year. The life expectancy of 75-year-older people was more than 10 years,[18] but in addition to the great pain that cancer brings to them, it may take them many years of meaningful life at the same time. So, to prolong survival and improve quality of life, it has a great significance to seek the suitable treatment for this age group. However, although the definitive CRT and RT alone treatments are both conventional ones, there are limited data on the efficacy and feasibility about them; no prospective clinical trials, as far as we know, have investigated definitive CRT or RT alone strategies specifically for the elderly. There are many possible reasons for the poor representation of older EC patients in clinical trials, like the following aspects: older patients are more likely to have other health problems (comorbidities); physicians, patients, and families may think that older patients are unlikely to benefit from intensive treatment, or patients’ own physical condition cannot tolerate the intensive treatment; around half of the primary tumors have been extended and/or distant metastases at the time of presentation, life expectancy cannot evaluate; the lack of financial is also one of the reasons; what is more, fewer trials are specifically designed for elderly patients.
Our study suggested that definitive CRT was an effective treatment in elderly patients with locally advanced ESCC, however, accompanied by increased toxic effects. Moreover, our results found that in elderly patients the CRT treatment produced a higher RR and a similar OS as usually reported in younger patients treated with the same regimen. In the literature, as a recommended nonsurgical treatment in young EC patients, CRT has a good clinical efficacy with 2-year survival rate of 47.6% to 66.7%.[19–22] What is interesting is that our results of the 2-year survival rate in elderly were within the range of those reported in these series, with a rate of 48.1%. These findings suggest that CRT treatment was associated with a survival advantage in the patients of all ages. The percentage of patients achieved CR after treatment was 34.6% in the CRT group compared with 18.6% in the RT group (P = .044). A retrospective cohort study[23] found that patients with complete pathological response had obviously longer survival (62.73 months ± 17.02 vs 41.42 months) compared with those with residual disease, and it was also the predictor of long-term survival. Another research[24] also found that complete pathological response significantly improved the median survival. Our study found that patients with CR after treatment had a longer survival than those with non-CR (36.4 vs 16.6 months, P < .001). According to the survival curves, the 1-year survival rate had no significant difference between the 2 groups, whereas the 2-year survival rate of the CRT group was higher than that of the RT group (P = .042). As follow-up becomes longer, the survival rate advantage of CRT gradually becomes apparent. There was a tendency for the 2 survival curves that the gap of them was gradually widened.
Many clinical studies have shown that age was not a risk factor of survival,[25–27] which is exactly the same result at our study. Tougeron et al[26] investigated 109 patients older than 70 years with esophageal squamous cell carcinoma treated with CRT. Chemoradiotherapy was based on radiotherapy combined with a cisplatin-based chemotherapy. Among them, 63 patients received CR, and 2-year survival rate was 35.5%. Meanwhile, a CR to CRT, a dose of RT ≥ 80%, and a Charlson score ≤ 2 were independent prognostic factors of OS in the multivariate analysis, but age was not.
In the present study, the incidence of toxicity in the CRT group was higher than that in the RT group; the main reason was that chemotherapy drugs usually associated with more gastrointestinal reaction (nausea and vomiting) and hematology toxicity (leukocyte, hemoglobin, and platelet decline). Fortunately, due to the wide use of antiemetic drugs and granulocyte colony-stimulating factor, most of the patients could be controlled well. However, this study is limited by its retrospective design and small numbers. Although the characteristics of patient and tumor were well matched when select cases were enrolled in this study, its retrospective nature still has potential selection bias.
So far there is no evidence to suggest that advanced age is an independent contraindication for CRT in the retrospective studies. Nevertheless, some studies[28–30] may suggest that CRT can result in serious side effects in patients older than 75 years of age who even have a good health body and organ function. Further study is required to establish predictive factors that can identify the risk factors for elderly patients, and thus to develop different selection criteria for candidates who would benefit from different effective therapies.
6. Conclusions
CRT with platinum and 5-FU was well tolerated and more effective than RT alone for elderly patents older than 75 years with locally advanced ESCC.
Footnotes
Abbreviations: CR = complete response, CRT = chemoradiotherapy, CT = computerized tomography, CTV = clinical target volume, DCR = disease-control rate, EC = esophageal carcinoma, ECOG = Eastern Cooperative Oncology Group, ESCC = esophageal squamous cell carcinoma, EUS = endoscopic ultrasonography, FDG-PET = 18F-fluorodeoxyglucose positron emission tomography, GTV = gross tumor volume, OS = overall survival, PD = progressive disease, PFS = progression-free survival, PR = partial response, PTV = planning target volume, RR = response rate, RT = radiotherapy, SD = stable disease, TNM = tumor-node-metastasis, UICC = Union for International Cancer Control, WBC = white blood cell.
QZ and GH carried out the systematic research. WX independently reviewed the titles and abstracts of the studies. QZ, YC, and MS conducted the full-text reading to identify the included studied. QZ, GH, and QT conducted data extraction and participated in statistical analysis. XN examined the manuscript and proposed suggestions to improve quality of the article. All authors read and approved the final manuscript and agreed to be named.
The authors have no conflicts of interest to disclose.
References
- [1].Di Pardo BJ, Bronson NW, Diggs BS, et al. The global burden of esophageal cancer: a disability-adjusted life-year approach. World J Surg 2016;40:395–401. [DOI] [PubMed] [Google Scholar]
- [2].Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003;349:2241–52. [DOI] [PubMed] [Google Scholar]
- [3].Trivers KF, Sabatino SA, Stewart SL. Trends in esophageal cancer incidence by histology, United States, 1998-2003. Int J Cancer 2008;123:1422–8. [DOI] [PubMed] [Google Scholar]
- [4].Remontet L, Esteve J, Bouvier AM, et al. Cancer incidence and mortality in France over the period 1978-2000. Rev Epidemiol Sante Publique 2003;51:3–0. [PubMed] [Google Scholar]
- [5].Moskovitz AH, Rizk NP, Venkatraman E, et al. Mortality increase for octogenarians undergoing esophagogastrectomy for esophageal cancer. Ann Thorac Surg 2006;82:2031–6. [DOI] [PubMed] [Google Scholar]
- [6].Stahl M, Stuschke M, Lehmann N, et al. Chemoradiation with and without surgery in patients with locally advanced squamous cell carcinoma of the esophagus. J Clin Oncol 2005;23:2310–7. [DOI] [PubMed] [Google Scholar]
- [7].Kinugasa S, Tachibana M, Yoshimura H, et al. Esophageal resection in elderly esophageal carcinoma patients: improvement in postoperative complications. Ann Thorac Surg 2001;71:414–8. [DOI] [PubMed] [Google Scholar]
- [8].Steyerberg EW, Neville B, Weeks JC, et al. Referral patterns, treatment choices, and outcomes in locoregional esophageal cancer: a population-based analysis of elderly patients. J Clin Oncol 2007;25:2389–96. [DOI] [PubMed] [Google Scholar]
- [9].Qiu G, Tao Y, Du X, et al. The impact of prior radiotherapy on fact complications after self-expandable metallic stents (SEMS) for malignant dysphagia due to esophageal carcinoma. Dis Esophagus 2013;26:175–81. [DOI] [PubMed] [Google Scholar]
- [10].Han J, Zhu W, Yu C, et al. Clinical study of concurrent chemoradiotherapy or radiotherapy alone for esophageal cancer patients with positive lymph node metastasis. Tumori 2012;98:60–5. [DOI] [PubMed] [Google Scholar]
- [11].Higuchi K, Komori S, Tanabe S, et al. Definitive chemoradiation therapy with docetaxel, cisplatin, and 5-fluorouracil (DCF-R) in advanced esophageal cancer: a phase 2 trial (KDOG 0501-P2). Int J Radiat Oncol Biol Phys 2014;89:872–9. [DOI] [PubMed] [Google Scholar]
- [12].Servagi-Vernat S, Créhange G, Roullet B, et al. Phase II study of a platinum-based adapted chemotherapy regimen combined with radiotherapy in patients 75 years and older with esophageal cancer. Drugs Aging 2015;31:487–93. [DOI] [PubMed] [Google Scholar]
- [13].Servagi-Vernat S, Bosset M, Crehange G, et al. Feasibility of chemoradiotherapy for oesophageal cancer in elderly patients aged > or = 75 years: a prospective, single-arm phase II study. Drugs Aging 2009;26:255–62. [DOI] [PubMed] [Google Scholar]
- [14].Cooper JS, Guo MD, Herskovic A, et al. Chemotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 8501). Radiation Therapy Oncology Group. JAMA 1999;281:1623–7. [DOI] [PubMed] [Google Scholar]
- [15].Kato K, Nakajima TE, Ito Y, et al. Phase II study of concurrent chemoradiotherapy at the dose of 50.4 Gy with elective nodal irradiation for Stage II-III esophageal carcinoma. Jpn J Clin Oncol 2013;43:608–15. [DOI] [PubMed] [Google Scholar]
- [16].Hodapp N. [The ICRU Report 83: prescribing, recording and reporting photon-beam intensity-modulated radiation therapy (IMRT)]. Strahlenther Onkol 2012;188:97–9. [DOI] [PubMed] [Google Scholar]
- [17].Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–47. [DOI] [PubMed] [Google Scholar]
- [18].Arias E. United States life tables, 2003. Natl Vital Stat Rep 2006;54:1–40. [PubMed] [Google Scholar]
- [19].Hu G, Wang Z, Wang Y, et al. Comparison of cisplatinum/paclitaxe with cisplatinum/5-fluorouracil as first-line therapy for nonsurgical locally advanced esophageal squamous cell carcinoma patients. Drug Des Devel Ther 2016;10:2129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yang H, Yao J, Wen H, et al. Clinical evaluations of neoadjuvant chemotherapy with DN and FP regimen for patients with middle or lower thoracic locally advanced esophageal squamous cell carcinoma. Zhong hua Yi Xue Za Zhi 2015;95:1530–3. [PubMed] [Google Scholar]
- [21].Nomura M, Oze I, Abe T, et al. Impact of docetaxel in addition to cisplatin and fluorouracil as neoadjuvant treatment for resectable stage III or T3 esophageal cancer: a propensity score-matched analysis. Cancer Chemother Pharmacol 2015;76:357–63. [DOI] [PubMed] [Google Scholar]
- [22].Courrech Staal EF, Aleman BM, van Velthuysen ML, et al. Chemoradiation for esophageal cancer: institutional experience with three different regimens. Am J Clin Oncol 2011;34:343–9. [DOI] [PubMed] [Google Scholar]
- [23].Rizvi FH, Syed AA, Khattak S, et al. Complete pathological response after neoadjuvant treatment in locally advanced esophageal cancer predicts long term survival: a retrospective cohort study. Int J Surg 2014;12:621–5. [DOI] [PubMed] [Google Scholar]
- [24].Scheer RV, Fakiris AJ, Johnstone PA. Quantifying the benefit of a pathologic complete response after neoadjuvant chemoradiotherapy in the treatment of esophageal cancer. Int J Radiat Oncol Biol Phys 2011;80:996–1001. [DOI] [PubMed] [Google Scholar]
- [25].Anderson SE, Minsky BD, Bains M, et al. Combined modality chemoradiation in elderly oesophageal cancer patients. Br J Cancer 2007;96:1823–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Tougeron D, Di Fiore F, Thureau S, et al. Safety and outcome of definitive chemoradiotherapy in elderly patients with oesophageal cancer. Br J Cancer 2008;99:1586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nallapareddy S, Wilding GE, Yang G, et al. Chemoradiation is a tolerable for older adults with esophageal cancer. Anticancer Res 2005;25:3055–60. [PubMed] [Google Scholar]
- [28].Mak RH, Mamon HJ, Ryan DP, et al. Toxicity and outcomes after chemoradiation for esophageal cancer in patients age 75 or older. Dis Esophagus 2010;23:316–23. [DOI] [PubMed] [Google Scholar]
- [29].Li QQ, Liu MZ, Hu YH, et al. Definitive concomitant chemoradiotherapy with docetaxel and cisplatin in squamous esophageal carcinoma. Dis Esophagus 2010;23:253–9. [DOI] [PubMed] [Google Scholar]
- [30].Takeuchi S, Ohtsu A, Doi T, et al. A retrospective study of definitive chemoradiotherapy for elderly patients wth esophageal cancer. Am J Clin Oncol 2007;30:607–11. [DOI] [PubMed] [Google Scholar]


