Abstract
The prevalence of Klebsiella pneumonia bloodstream infections (KP-BSIs) is increasing worldwide. Few study reports focus on the KP-BSIs published in Mainland China over the previous years. This study aimed to describe the risk factors of mortality from KP-BSIs.
A retrospective study was conducted in a teaching hospital in Shanghai, China, for a period of 4 years. Risk factors related to the patient mortality were analyzed using the binary logistic regression model.
Of 104 patients with KP-BSIs, the overall 30-day mortality rate was 25%. The logistic regression analysis revealed that thrombocytopenia (TB) (odds ratio [OR]: 1.007, 95% confidence interval [CI]: 1.002–1.013), pancreaticobiliary tract (PBT) (OR: 4.059, 95% CI: 1.398–11.78), and intra-abdominal infection (OR: 6.816, 95% CI: 1.806–25.716) were powerful risk factors leading to the mortality associated with KP-BSIs. Although prior antibiotic exposure, inappropriate empirical antibiotics, and inappropriate definitive antibiotics were not associated with mortality, multidrug-resistant (MDR) of KP-BSIs in the present study was high in both survivors and nonsurvivors (67.9% and 88.5%, respectively).
TB, PBT, and intra-abdominal infection caused significant mortality rates increase in KP-BSIs during the study period.
Keywords: bloodstream infection, Klebsiella pneumonia, mortality, multidrug-resistant, risk factors
1. Introduction
Klebsiella pneumonia (KP) has been reported as the second overall cause of gram-negative bloodstream infections (BSIs) after Escherichia coli.[1]KP is a common pathogen that causes infections of the bloodstream, urinary tract, lungs, intra-abdominal, and other sites.[2–4] Meatherall et al[5] conducted a population-based surveillance study in the Calgary Heath Region (population 1.2 million) for a period of 8 years. A total of 640 episodes of KP-BSIs were identified for an overall annual population incidence of 7.1 per 100,000.[5] Death was significantly more common in patients with BSIs than in patients with other infections.[6,7]
BSIs were chosen for the study to confirm that patients were truly infected, as it is rare that patients with KP-positive blood cultures have the negative laboratory result.[2] Multidrug-resistant (MDR) and the increase in the incidence of infections due to gram-negative bacilli producing extended-spectrum β-lactamase (ESBL) have led to the intensive use of Carbapenem.[8] Most of the reports analyzed the molecular epidemiological aspects [9,10] or focused on the special group such as ESBL-producing K pneumonia (ESBL-KP) of the isolates. Less attention has been focused on the mortality of KP, ESPL-KP as the risk factor.
ESBL pathogens pose an increasing challenge to physicians worldwide. Studies have analyzed the relation between the rate of mortality and ESBL-producing infections due to KP-BSIs, which still remains controversial.[11–14] However, the clinical relevance of the mortality resistance of KP isolates is of great concern due to the limited therapeutic options and increased risk of treatment failure in patients infected with such strains.[15] The prevalence of multidrug-resistant K pneumonia (MDR-KP) has increased dramatically.[16,17] Patients with MDR-KP infection have limited treatment options. Therefore, it would be useful to determine differential risks, if any that can predict the mortality in an infected patient. Furthermore, intensive care intervention, laboratory findings, nosocomial infections, and disease severity can be used to evaluate increased mortality rates of KP-BSIs patients.[2–7] Few studies have investigated the mortality caused by KP-BSIs in mainland China.[18,19] The objective of this study was to determine the risk factors and predictors of mortality caused by KP-BSIs.
2. Methods
2.1. Study design
A retrospective study of Chinese patients with KP bacteremia was conducted in Ren Ji Hospital affiliated to Shanghai Jiao Tong University of Medicine, an 1800-bed tertiary care university teaching hospital in Shanghai, China. The study period was between June 1, 2011, and June 30, 2015. Recurrent infections were excluded; only the first KP BSIs episode per patient was included in our analysis. Clinical manifestations were determined from medical charts. The study was observational in that administration of antimicrobial agents and the therapeutic managements were controlled by patient's physicians, and not by the investigators.
2.2. Definitions
2.2.1. KP-BSIs was defined as the isolation of KP in a blood culture specimen
The onset of bacteremia was defined as the date when the first positive blood culture was obtained.
The primary site of infection was determined using clinical criteria and isolation of the infecting organism from sources other than blood.[20]
MDR was defined as resistance to at least 1 member of the following 3 classes of antibiotics: aminoglycosides (amikacin, gentamicin, or netilmicin), fluoroquinolones (ofloxacin or ciprofloxacin), and cephalosporins (cefazolin, cefotaxime, cefoxitin, ceftriaxone, ceftazidime, or cefepime).
Clinical variables collected from patients with bacteremia included age, gender, underlying medical conditions (including malignancy, leukemia, chronic renal disease, and diabetes mellitus), smoking, alcohol consumption, laboratory findings (including C-reactive protein [CRP], Procalcitonin [PCT], erythrocyte sedimentation rate [ESR], leukopenia, thrombocytopenia [TB], hemoglobin, blood glucose, serum creatinine [Scr], bilirubin, albumin, cereal third transaminase [ALT], and glutamic-oxaloacetic transaminase [AST]), and insertion of invasive devices (i.e., drainage catheter, central venous catheter, mechanical ventilation, and urinary catheter). TB was defined as platelet count less than 150 × 109/L. Neutropenia was defined as a peripheral absolute neutrophil count of less than 500 cells/mL.
Bacteremia was considered as hospital acquired if the blood culture was collected less than 48 hours after admission of the patient or within hours of discharge from the hospital or an infection that existed in patients who had been admitted to another hospital in 2 weeks before the current admission.
Community-acquired infections were those in which the first positive culture was obtained in less than 48 hours after hospital admission or in more than 48 hours after discharge from the hospital.
Prior antibiotic exposure was defined as administration of an antibiotic within 30 days prior to the culture date and for a 1-day period to the culture date.
The antimicrobial therapies were classified into empirical and definitive, the former being defined as the initial therapy before the results of blood culture were available, and the latter as therapy after the result of antibiotic susceptibility tests had been received. The antimicrobial therapy was considered “appropriate” if the treatment regimen included at least 1 antimicrobial agent active in vitro against KP, and if the dosage and route of administration conformed to current medical standards. We considered antimicrobial therapy to be “inappropriate” if the drugs used did not have in vitro activity against the isolated strain or if the patient did not receive antimicrobial therapy.
Mortality was defined as death of any cause within 30 days from the onset of symptoms.
2.2.2. Microbiology
KP isolates were identified using the Vitek 2 Advanced Expert System (bioMèrieux, Marcy l’Etoile, France), and antibiotic susceptibility was tested by the Kirby-Bauer agar disk diffusion method. Antibiotic susceptibility was interpreted according to the European Committee on Antimicrobial Susceptibility Testing guidelines.[21]
Ren Ji Hospital Ethics Committee approved the study (Shanghai Jiao Tong University School of Medicine).
2.2.3. Statistical analysis
Student's t test was used to compare continuous variables, and the chi-square test or Fisher's exact test was used to compare categorical variables. A stepwise logistic regression model was used to identify independent risk factors for 30-day mortality. Risk factors with a P value less than .10 in the univariate analysis for 30-day mortality were included in the initial model, and forward stepwise selection was performed to develop the final model. A P value less than .05 were considered statistically significant. All data were analyzed using the IBM SPSS Statistics for Windows (version 19.0). Odds ratio (OR) and 95% confidence interval (CI) were calculated to evaluate the strength of any association.
3. Results
A total of 104 KP blood isolates were identified during the study period. Patient demographics, clinical characteristics, type of infections, and prior antibiotic exposures for both survivors and nonsurvivors of KP-BSIs are shown in Table 1. The median age of patients was 56.5 years (range 15–96 years) and 39 (37.5%) were women. Of the 104 patients, 78 (75%) survived and 26 (25%) died within 30 days of onset.
Table 1.
Risk factors associated with 30-day mortality in patients with KP-BSI (univariate analysis)∗.
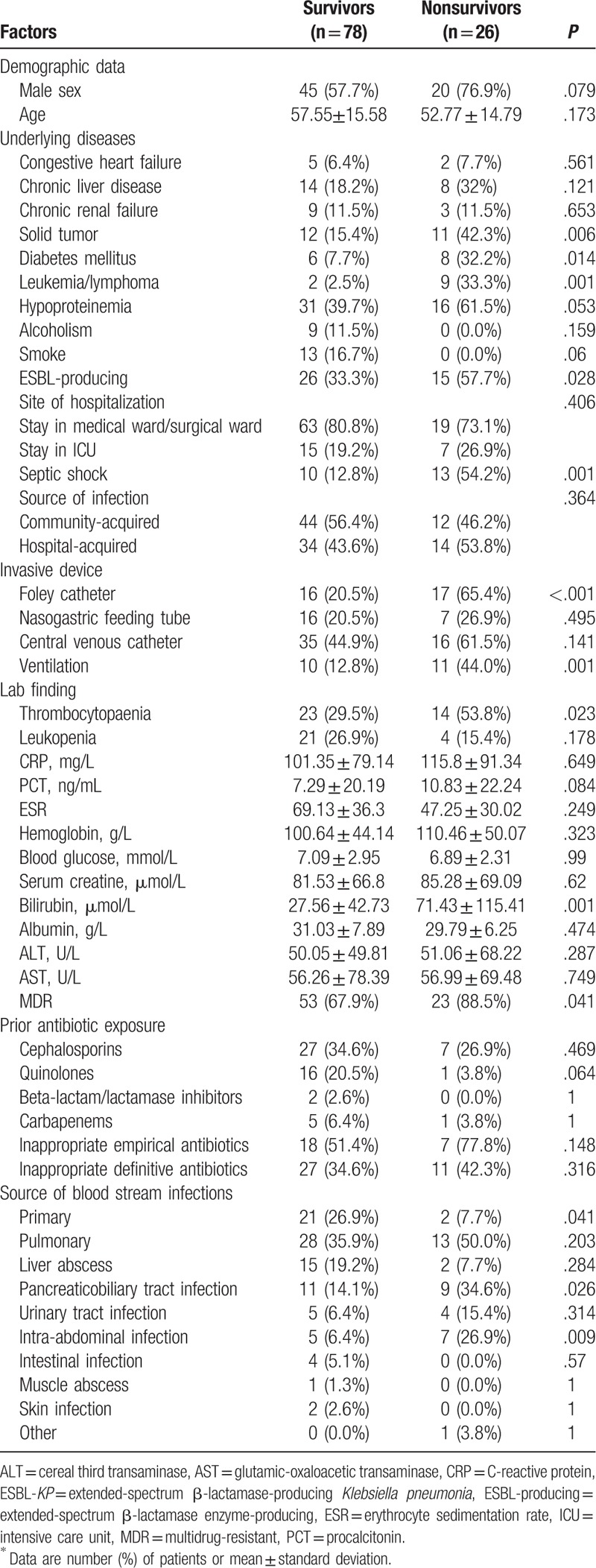
When co-morbid conditions of the 2 groups were compared by univariate analysis, the non-survivor group was significantly more likely to have diabetes mellitus (P = .014) and leukemia/lymphoma (P = .001) than controls. The rate of mortality was higher in the ESBL-producing KP cases (P = .028). The nonsurvivor group was also more frequently intubated (a Foley catheter ventilation). The nonsurvivor group had higher incidence of leukopenia and higher blood platelet and bilirubin levels than the survival group (P = .007, .030, and .001, respectively). MDR in nonsurvivor and survivor groups was serious (88.5% and 67.9%, respectively). No difference was found in the prior antibiotic exposure between the 2 groups.
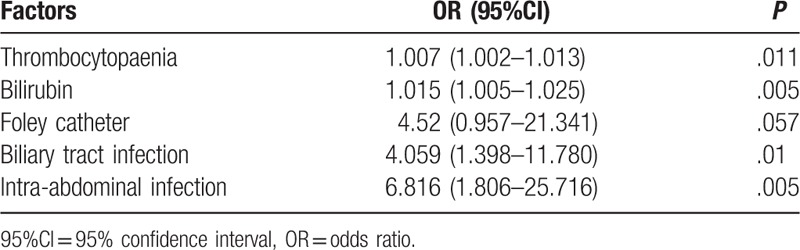
The results of multivariate logistic regression analysis of risk factors for mortality are shown in Table 1. TB (OR, 1.007; 95%CI, 1.002–1.013; P = .011), and bilirubin remained as independent risk factors for mortality caused by KP-BSI. An association between mortality and implanted Foley catheter was observed (OR, 4.520; 95% CI, 0.957–21.341; P = .057).
The most common source of BSI was pulmonary (39%). Liver abscess, primary bacteremia, and biliary tract infection were also frequent sources of infection (Table 2). Nonsurvivors had a higher rate of biliary tract (34.6%) and intra-abdominal infections (26.9%) caused by KP. Pulmonary infection was more common in survivors than in nonsurvivors. Only biliary tract and intra-abdominal infections were found to be associated with mortality on multivariate logistic regression analysis (Table 2).
Table 2.
Multivariate logistic regression analysis of risk factors for mortality.
4. Discussion
This study aimed to determine differential risk factors, if any, which could predict the mortality of KP-BSIs. The 30-day mortality rate was 25% in the present study. Few population-based reports have recorded the mortality rate of KP. A report from Canada identified an overall annual population incidence of 1.3 per 100,000. The crude mortality rate reported in previous studies was within the range 23% to 46%.[8] Hospital-acquired infection carried a higher mortality rate compared with community-acquired infection in these studies, which was consistent with the present study findings.[4,18,22–24] A study of Korean population found the mortality rate of hospital-acquired and community-acquired infections to be 22% and 11%, respectively. A study of patients in Hong Kong found the mortality rate of hospital-acquired and community-acquired infections to be 43% and 20.2%, respectively.[4,22] Similar studies reported the crude mortality rate to be 20% to 45% in European and North American populations [8,23] and 26% in China.[18] The high range might be related to the population studied and the source of infection.
ESBL-producing KP isolates have been increasing worldwide. The prevalence of these isolates varied from 2% to 50% in prior investigations.[24,25] It was observed that nonsurvivors had a higher rate of ESBL-producing isolates (57.7%) than survivors (33%), and this difference was not found to be statistically significant by multivariate logistic regression analysis. Previous studies showed that KP-BSIs did not increase mortality due to ESBL-producing.[9–11,26] A case–control study lasted10 years in Mexico and attributed the irrelevance to small number of isolates; the appropriate and prompt definitive therapy almost always included a β-lactam and an aminoglycoside.[24] Gürntke et al[27] believed that studies on the impact of ESBL production on the mortality of KP-BSIs could not prove that ESBL-related increased mortality was directly attributable to ESBL-producing infections. The authors found the effect of length of stay (LOS) before BSI onset on mortality. The present study did not evaluate the effect of LOS before and after BSI onset. However, it supported the conclusion by Gürntke et al that ESBL-KP bacteremia was not associated with a worse clinical outcome. No correlation was found between MDR and the mortality caused by KP-BSIs. China had a high rate of overall antibiotic use. The rate of MDR was 88.5% and 67% in the nonsurvivors and survivors, respectively. No significant difference was observed in the prior antibiotic exposure and mortality. This might be due to a low number of patients included in this study.
The presence of indwelling catheters had been previously reported as a significant risk factor for KP-BSIs.[28–30] The role of invasive devices had been implicated in colonization and infection by destroying the continuum of the skin or mucosa.[31] An association between mortality and implanted Foley catheter was observed in the present study.
PBT infection has been considered as a factor leading to a good outcome. The mortality was significantly higher in patients having PBT BSI in the present study, which might be attributed to the underlying disease. Of 9 patients with PBT infection in this study, 5 (55.6%) were taking immunosuppressants after liver transplantation and 4 (44.4%) had septic shock onset. The mortality rate in PBT patients in the present study was 34.6% compared with other studies in which PBT was rare (4–21%).[4,8,32]
A low platelet count is a common laboratory abnormality in critically ill patients. Thrombocytopenia was found to be an independent risk factor for mortality in the present study, which was confirmed by several other studies.[33,34] The rate of thrombocytopenia (TB) in patients with bacteremia was 79.6%. The rate of mortality was higher in patients with bacteremia. The mechanism by which TB occurs in patients with infection is not clear. The most common cause of TB is severe infection and/or inflammation.[35] Other related causes of TB are thrombotic microangiopathy, disseminated intravascular coagulation, massive blood loss, and drug-induced thrombocytopenia.[35–38]
The present study had several limitations, including its retrospective design. BSIs were chosen for the study to confirm that patients were truly infected. However, patients who had bacteremia but did not have blood samples for culture were missed. Another limitation was that study pathogens were not collected. Therefore, data pertaining to strain genotype were not available. Finally, this study was performed in a single metropolitan area, including only 104 patients. Therefore, risk factors and bacterial population might have been different at other institutions.
The present study considered ESBL-KP, MDR, and laboratory findings as the risk factors for mortality caused by KP-BSIs. In conclusion, the study demonstrated that PBT, intra-abdominal infection, and TB represent strong risk factors for the mortality caused by KP-BSIs.
Footnotes
Abbreviations: ALT = cereal third transaminase, AST = glutamic-oxaloacetic transaminase, BSIs = bloodstream infections, CI = confidence interval, CRP = C-reactive protein, ESBL = extended-spectrum β-lactamase, ESBL-KP = extended-spectrum β-lactamase-producing Klebsiella pneumonia, ESR = erythrocyte sedimentation rate, KP = Klebsiella pneumonia, KP-BSIs = Klebsiella pneumonia bloodstream infections, LOS = length of stay, MDR = multidrug-resistant, MDR-KP = multidrug-resistant Klebsiella pneumonia, OR = odds ratio, PBT = pancreaticobiliary tract, PCT = procalcitonin, Scr = serum creatinine, TB = thrombocytopenia.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Uslan DZ, Crane SJ, Steckelberg JM, et al. Age-and sex-associated trends in bloodstream infection: a population-base study in Olmsted County, Minnesota. Arch Intern Med 2007;167:834–9. [DOI] [PubMed] [Google Scholar]
- [2].Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbial Rev 2007;20:440–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Marquez P, Terashita D, Dassey D, et al. Population-based incidence of carbapenem-resistant Klebsiella pneumonia along the continuum of care, Los Anfeles County. Infect Control Hosp Epidemiol 2013;34:144–50. [DOI] [PubMed] [Google Scholar]
- [4].Kang CI, Kim SH, Bang JW, et al. Community-acquired versus nosocomial Klebsiella pneumonia bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci 2006;21:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Meatherall BL, Gregson D, Ross T, et al. Incidence, risk factors, and outcomes of Klebsiella pneumonia bacteremia. Am J Med 2009;122:866–73. [DOI] [PubMed] [Google Scholar]
- [6].Girometti N, Lewis RE, Giannella M, et al. Klebsiella pneumonia bloodsream infection: epidemiology and impact of inappropriate empirical therapy. Medicine (Baltimore) 2014;93:298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Tumbarello M, Trecarichi EM, De Rosa FG, et al. Infections caused by KPC-producing Klebsiella pneumonia: differences in therapy and mortality in a multicenter study. J Antimicro Chemother 2015;70:2133–43. [DOI] [PubMed] [Google Scholar]
- [8].Durdu B, Hakyemez IN, Bolukcu S, et al. Mortality markers in nosocomial Klebsiella pneumonia bloodstream infection. Springerplus 2016;5:1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lee CH, Liu JW, Su LH, et al. Hypermucoviscosity associated with Klebsiella pneumonia-mediated invasive syndrome: a prospective cross-sectional study in Taiwan. Int J Infec Dis 2010;14:e688–92. [DOI] [PubMed] [Google Scholar]
- [10].Luk S, Wong WK, Ho AY, et al. Clinical features and molecular epidemiology of plasmid-mediated DNH-type AmpCβ-lactamase-producing Klebsiella pneumonia blood culture isolates, Hong Kong. J Glo Antimicrob Resist 2016;7:37–42. [DOI] [PubMed] [Google Scholar]
- [11].Schwaber MJ, Carmeli Y. Mortality and delay in effective therapy associated with extended-spectrum beta-lactamase production in Enterobacteriaceae bacteraemia: a systematic review and meta-analysis. J Antimicrob Chemother 2007;60:913–20. [DOI] [PubMed] [Google Scholar]
- [12].Lin YT, Liu CJ, Fung CP, et al. Nosocomial Klebsiella pneumonia bacteraemia in adult cancer patients-characteristics of neutropenic and non-neutropenia patients. Scand J Infect Dis 2011;43:603–8. [DOI] [PubMed] [Google Scholar]
- [13].Nasa P, Juneja D, Singh O, et al. An observational study on bloodstream extended-spectrum beta-lactamase infection in critical care unit: incidence, risk factors and its impact on outcome. Eur J Intem Med 2012;23:192–5. [DOI] [PubMed] [Google Scholar]
- [14].Ben-David D, Kordevani R, Keller N, et al. Outcome of carbapenenm resistant Klebsiella pneumonia bloodstream infections. Clin Microbiol Infect 2012;18:54–60. [DOI] [PubMed] [Google Scholar]
- [15].Hyle EP, Lipworth AD, Zaoutis TE, et al. Risk factors for increasing multidrug resistance among Extended-Spectrum beta–Lactamase-Producing Escherichia coli and Klebsiella species. Clin Infect Dis 2005;40:1317–24. [DOI] [PubMed] [Google Scholar]
- [16].Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem-hydrolyzing beta-lactamase, KPC-1, from a carbapenem-resistant strain of Klebsiella pneumonia. Antimicrob Agents Chemother 2001;45:1151–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hirsch EB, Tam VH. Detection and treatment options for Klebsiella pneumonia carbapenemases (KPCs): an emerging cause of multidrug-resistant infection. J Antimicrob Chemother 2010;65:1119–25. [DOI] [PubMed] [Google Scholar]
- [18].Tian L, Tan R, Chen Y, et al. Epidemiology of Klebsiella pneumonia bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist and Infection control 2016;5:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Liu YM, Li BB, Zhang YY, et al. Clinical and molecular characteristics of emerging Hypervirulent Klebsiella pneumonia bloodstream infections in Mainland China. Antimicro Agents Chemothe 2014;58:5379–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].The European Committee on Antimicrobial Susceptibility Testing (EUCAST) (2016) Breakpoint tables for interpretation of MICs and zone diameters. Version 6.0. Available at: http://www.eucast.org. [Google Scholar]
- [21].Blot S, Vandewoude K, De Bacquer D, et al. Nosocomial bacteremia caused by antibiotic-resistant gram-negative bacteria in critically ill patients: clinical outcome and length of hospitalization. Clin Infect Dis 2002;34:1600–6. [DOI] [PubMed] [Google Scholar]
- [22].Pau CK, Ma FF, Ip M, et al. Characteristics and outcomes of Klebsiella pneumonia bacteraemia in Hong Kong. Infect Dis (Lond) 2015;47:283–8. [DOI] [PubMed] [Google Scholar]
- [23].Neuner EA, Yeh JY, Hall GS, et al. Treatment and outcomes in Carbapenenm-resistant Klebsiella pneumonia bloodstream infections. Diagn Microbiol Infect Dis 2011;69:357–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Laupland KB, Gregson DB, Church DL, et al. Incidence, risk factors and outcomes of Escherichia coli bloodstream infections in a large Canadian region. Clin Microbiol Infect 2008;14:1041–7. [DOI] [PubMed] [Google Scholar]
- [25].Goossens H. MYSTIC Study Group (Europe). MYSTIC program: summary of European data from 1997 to 2000. Diagn Microbial Infect Dis 2001;41:183–9. [DOI] [PubMed] [Google Scholar]
- [26].Gürntke S, Kohler C, Steinmetz I, et al. Molecular epidemiology of extended-spectrum beta-lactamase (ESBL)-positive Klebsiella pneumonia from bloodstream infections and risk factors for mortality. J Infect Chemother 2014;20:817–9. [DOI] [PubMed] [Google Scholar]
- [27].Mosqueda-Gómez JL, Montaño-Loza A, Rolón AL, et al. Molecular epidemiology and risk factors of bloodstream infections caused by extended-spectrum (-lactamase-producing Klebsiella pneumonia: a case-control study. Int J Infect Dis 2008;12:653–9. [DOI] [PubMed] [Google Scholar]
- [28].Chopra T, Marchaim D, Johnson PC, et al. Risk factors for bloodstream infection caused by extended-spectrum β-lactamase-producing Escherichia coli and Klebsiella pneumonia: a focus on antimicrobials including cefepime. Am J Infect Control 2015;43:719–23. [DOI] [PubMed] [Google Scholar]
- [29].Borer A, Saidel-Odes L, Eskira S, et al. Risk factors for developing clinical infection with carbapenem-resistant klebsiella pneumonia in hospital patients initially only colonized with carabapenem-resistant K penumoniae. Am J Infect Control 2012;40:421–5. [DOI] [PubMed] [Google Scholar]
- [30].Correa L, Martino MD, Siqueira I, et al. A hospital-based matched case-control study to identify clinical outcome and risk factors associated with carbapenem-resistant Klebsiella pneumonia infection. BMC Infect Dis 2013;13:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Schechner V, Kotlovsky T, Kazma M, et al. Asymptomatic rectal carriage of blaKPC producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect 2013;19:451–6. [DOI] [PubMed] [Google Scholar]
- [32].Jung Y, Lee MJ, Sin HY, et al. Difference in characteristics between healthcare-associated and community-acquired infection in community-onset Klebsiella pneumonia bloodstream infection in Korea. BMC Infect Dis 2012;12:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Togawa A, Toh H, Onozawa K, et al. Influence of the bacterial phenotypes on the clinical manifestations in Klebsiella pneumonia bacteremia patients: A retrospective cohort study. J Infect Chemother 2015;21:531–7. [DOI] [PubMed] [Google Scholar]
- [34].Kang CI, Kim SH, Bang JW, et al. Community-acquired versus nosocomial Klebsiella pneumonia bacteremia: clinical features, treatment outcomes, and clinical implication of antimicrobial resistance. J Korean Med Sci 2006;21:816–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Levi M. Platelets in critical illness. Semin Thromb Hemost 2016;42:252–7. [DOI] [PubMed] [Google Scholar]
- [36].Kaur A, Sethi GK, Goyal RK, et al. Thrombocytopenia in peadiatric ICU: Incidence, transfusion requirement and role as prognostic indicator. J Clin Diagn Res 2015;9:SC05–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Hui P, Cook DJ, Lim W, et al. The frequency and clinical significance of thrombocytopenia complicating critical illess: a systematic review. Chest 2011;139:271–8. [DOI] [PubMed] [Google Scholar]
- [38].Thiolliere F, Serre-Sapin AF, Reignier J, et al. Epidemiology and outcome of thrombocytopenic patients in the intensive care unit: results of a prospective multicenter study. Intensive Care Med 2013;39:1460–8. [DOI] [PubMed] [Google Scholar]


