Abstract
Rationale:
Primary intestinal lymphangiectasia (PIL) is a rare disease characterized by dilated intestinal lacteals resulting in lymph leakage into the small bowel lumen. Main clinical features include intermittent diarrhea, hypoproteinemia. Scattered case reports suggested that PIL is compatible to pregnancy, but with increased complications.
Patient concerns:
A 34-year-old woman with endoscopically diagnosed PIL presented to antenatal our clinic at 10 weeks into gestation. She reported strict adherence to low-fat/high-protein diet with medium-chain triglycerides (MCTs) supplementation. She was general well except for moderate edema and hypoalbuminemia. At 33 weeks, she developed diarrhea, nausea, and vomiting, with decreased fetal movements. One week later, she had an asthma attack. Nonstress test showed frequent variable deceleration.
Diagnoses:
The diagnosis of PIL was established endoscopically 8 years earlier.
Interventions:
Hypoalbuminemia was corrected with intravenous albumin administration. She also received corticosteroid therapy to promote fetal lung maturation in anticipation to early termination of the pregnancy.
Outcomes:
A cesarean section was carried out at 34 weeks due to fetal distress. The baby girl was apparently healthy: weighing 2160 g, with an Apgar score of 9 at both 1 and 5 minutes. Symptoms dissipated rapidly after the delivery. The last follow-up visit at 15 months was unremarkable for both the mother and infant.
Lessons:
PIL could be compatible with pregnancy, but requires strict adherence to dietary treatment, proper management of the symptoms (e.g., hypoalbuminemia), particularly during late gestation.
Keywords: hypoalbuminemia, pregnancy, primary intestinal lymphangiectasia
1. Introduction
Primary intestinal lymphangiectasia (PIL), first described by Waldmann in 1961,[1] is a rare disease characterized by dilated intestinal lacteals resulting in lymph leakage into the small bowel lumen. Major clinical features include lymphopenia, hypoalbuminemia, and hypogammaglobulinemia.[2–4] Other symptoms include nausea/vomiting, weight loss, chronic fatigue, edema, intermittent diarrhea, steatorrhea, and abdominal pain. Fat-soluble vitamin deficiencies may occur. Symptoms typically emerge in childhood; cases with adult onset have only been occasionally reported. Definitive diagnosis is established on the basis of endoscopic examination of the small bowel. Mainstay treatment consists of long-term low-fat diet and supplementation of medium-chain triglycerides (MCTs).[2,3] Here, we report a case of a PIL with childhood onset. Symptoms exacerbated during late pregnancy and dissipated after delivery.
2. Case report
A 34-year-old woman started attending antenatal clinic 10 weeks into pregnancy. Stating from infancy, she had intermittent diarrhea, edema, and occasional tetany. She also had asthma and is allergic to erythromycin and aminophylline. A diagnosis of PIL was established by endoscopy (Fig. 1) in the First Affiliated Hospital, Zhejiang University 8 years ago (at an age of 26). She was then placed on a low-fat/high-protein diet with MCTs supplementation. Compliance was reasonably good. She became pregnant 3 years earlier; antenatal clinical visit noticed subclinical hypothyroidism, but she did not receive replacement therapy, and the pregnancy ended with miscarriage. For the current episode of pregnancy, subclinical hypothyroidism was confirmed again (Table 1), and she received levothyroxine treatment (125 mg daily).
Figure 1.
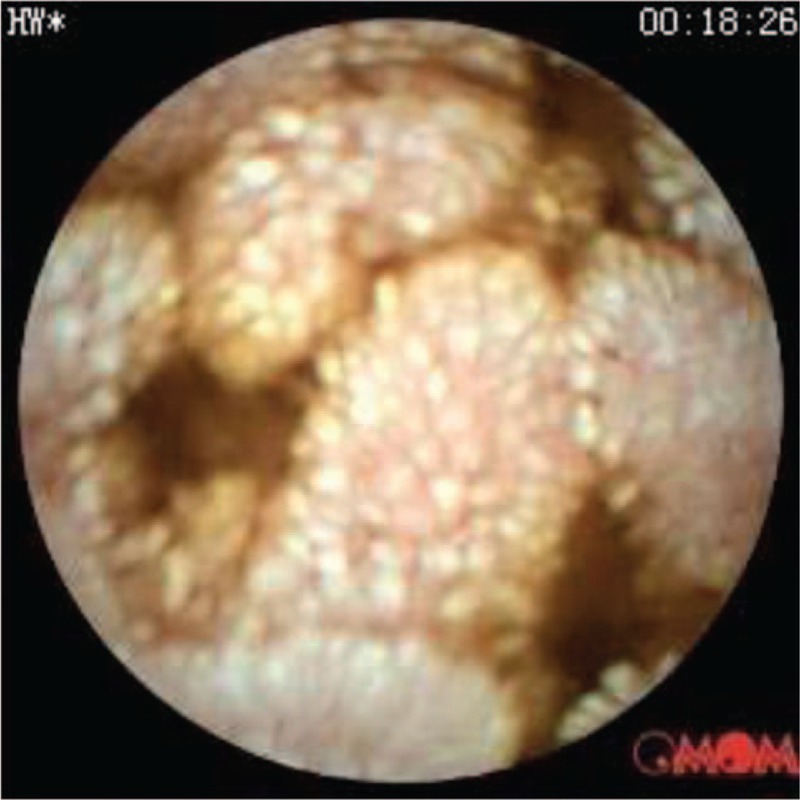
Capsule endoscopy showing lymphangiectasia in the small intestine.
Table 1.
Blood routine examination and biochemistry panel.

The antenatal clinical visit was conducted at a frequency of once every 2 weeks. She had persistent edema in both legs and eyelids. Serum albumin was between 20 and 24 g/L (normal reference range, 40–55 g/L). Urine examination did not show proteinuria.
33 weeks into the pregnancy, she developed diarrhea, nausea, and vomiting, and was admitted for decreased fetal movements. Blood pressure was normal (129/69 mm Hg). A nonstress test showed fetal distress. A physical examination revealed pitting edema in both legs and eyelids. Weight gain during the pregnancy was 12.5 kg. Body mass index was 25 kg/m2 (height: 160 cm; weight: 64 kg). Fundal height was 30 cm. Abdominal circumference was 95 cm. Fetal ultrasound showed biparietal diameter at 8.0 cm, and femur length at 6.1 cm.
Blood routine showed normal white blood cell count (8900/mm3), with a differential count of 96.5% neutrophils (normal range, 40–75%), and 1.6% lymphocytes (normal range, 20–50%). Hemoglobin was 13.8 g/dL with normal hematocrit (40.7%). Platelet count was 251,000/mm3. Serum total protein was 42.2 g/L, with hypoalbuminemia (albumin, 19.6 g/L) (Table 1). Urine routine did not reveal proteinuria. Stool occult blood was negative. Liver and renal function tests were normal. Ultrasound examination of the chest and abdominal cavities and organs was unremarkable.
The patient received dexamethasone (6 mg, i.m., every 12 hours for a total dose of 24 mg) to promote fetal lung maturation in anticipation to early termination of the pregnancy. She also received 5 10 mg doses of intravenous (IV) albumin every 24 hours to correct hypoalbuminemia (albumin, 19.6 g/L elevated to 25.3 g/L). One week after hospitalization (34 weeks of pregnancy), she had an asthma attack. Based on nonstress test (Fig. 2), cesarean section was performed. She delivered an apparently healthy girl, weighing 2160 g, with an Apgar score of 9 at both 1 and 5 minutes. Edema in the legs dissipated over a period of 3 days after the C-section. Hypoalbuminemia, however, persisted (albumin at 20.3 g/L and total proteins at 42.3 g/L upon discharge on 6 days after the C-section). Follow-up of the patient indicated that edema aggravated upon exclusive breast-feeding and alleviated significantly upon mixed feeding. The last follow-up at 15 months was not remarkable.
Figure 2.

Frequent variable deceleration at 34 weeks of gestation.
3. Discussion
PIL is a rare disease that typically manifests itself in childhood. The underlying pathophysiology and clinical features of this disease apparently overlaps with the physiological changes that happened during pregnancy (e.g., hormonal changes and the resulting fluid retention). However, only 7 cases of pregnancy of PIL patients have been reported. The characteristics of the 7 cases are summarized in Table 2.[5–11] Based on these reports, we believe that PIL is generally compatible with pregnancy, but requires heightened alert. In the current case, the patient was generally well except edema and hypoalbuminemia until 33 weeks into the gestation, when diarrhea, nausea, and vomiting emerged. The timing is apparently consistent with the rapid blood volume increase in 32 to 34 weeks of gestation. Another factor could be the rapidly increasing metabolic needs and food intake that in turn place additional burden onto deformed lymphatic vessels. Symptoms of nausea and vomiting, together with hypoproteinemia, could jeopardize nutrition supply to the developing fetus. Ghoshal et al[6] reported a low-birth-weight of an infant by a mother with PIL diagnosis established postpartum. In the current case, hypoproteinemia was managed with intravenous albumin. This may have contributed to the normal development of the fetus, as noted previously by Tourlakis et al.[9] Other symptoms of PIL, including weight-loss, iron deficiency anemia, fat-soluble vitamin deficiency,[12,13] could also complicate pregnancy if not managed properly. Dietary therapy is the cornerstone of PIL management.[14] Based on self-report, the patient in the current case strictly adhered to the low-fat/high-protein diet with supplementation of MCTs. We believe that good compliance may have contributed to the successful pregnancy and outcome to both the mother and infant in this case.
Table 2.
Summary of the 7 reported cases of PIL with pregnancy.

4. Conclusion
PIL could be compatible with pregnancy, but requires strict adherence to dietary treatment, proper management of the symptoms (e.g., hypoalbuminemia), particularly during late gestation.
Acknowledgments
The authors would like to thank the patient for her consent.
Footnotes
Abbreviations: MCTs = medium-chain triglycerides, NST = nonstress testing, PIL = primary intestinal lymphangiectasia.
The authors have no conflicts of interest to disclose.
References
- [1].Waldmann TA, Steinfeld JL, Dutcher TF, et al. The role of gastrointestinal system in “Idiopathic hypoproteinemia”. Gastroenterology 1961;41:197–207. [PubMed] [Google Scholar]
- [2].Alshikho MJ, Talas JM, Noureldine SI, et al. Intestinal lymphangiectasia: insights on management and literature review. Am J Case Rep 2016;17:512–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vignes S, Bellanger J. Primary intestinal lymphangiectasia (Waldmann's disease). Orphanet J Rare Dis 2008;3:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Xinias I, Mavroudi A, Sapountzi E, et al. Primary intestinal lymphangiectasia: is it always bad? Two cases with different outcome. Case Rep Gastroenterol 2013;7:153–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Liu DT, Sherman AM. Case report—pregnancy and intestinal lymphangiectasis (familial neonatal hypoproteinaemia). Aust N Z J Obstet Gynaecol 1980;20:58–9. [DOI] [PubMed] [Google Scholar]
- [6].Ghoshal UC, Gupta R, Aggarwal R, et al. Intestinal lymphangiectasia: presentation in pregnancy and association with herpes zoster and alopecia. Indian J Gastroenterol 1997;16:153–4. [PubMed] [Google Scholar]
- [7].Voigt HJ, Sailer D, Kolb S, et al. Conception and pregnancy without complications in parenteral home nutrition. Z Geburtshilfe Perinatol 1989;193:198–200. [PubMed] [Google Scholar]
- [8].Quemere MP, Descargues G, Verspyck E, et al. Waldmann disease and pregnancy. J Gynecol Obstet Biol Reprod (Paris) 2000;29:517–9. [PubMed] [Google Scholar]
- [9].Tourlakis D, Hatziveis K, Spiliopoulos E. Pregnancy and Waldmann disease. Clin Ter 2008;159:173–4. [PubMed] [Google Scholar]
- [10].Szũcs J, Köves P. Intestinal lymphangiectasis with hypoproteinemia decompensated by pregnancy and hepatitis. Orv Hetil 1972;113:2122–4. [PubMed] [Google Scholar]
- [11].Ibarguengoitia Ochoa F, Pizano Martínez E, Castelazo Morales E, et al. Intestinal lymphangiectasis and pregnancy. A case report. Ginecol Obstet Mex 1987;55:333–4. [PubMed] [Google Scholar]
- [12].Balaban VD, Popp A, Grasu M, et al. Severe refractory anemia in primary intestinal lymphangiectasia. A case report. J Gastrointestin Liver Dis 2015;24:369–73. [DOI] [PubMed] [Google Scholar]
- [13].Freeman HJ, Nimmo M. Intestinal lymphangiectasia in adults. World J Gastrointest Oncol 2011;3:19–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Martin CC, Garcia AF, Restrepo JM, et al. Sucessful dietetic-therapy in primary intestinal lymphangiectasia and recurrent chylous ascites: A case report. Nutr Hosp 2007;22:723–5. [PubMed] [Google Scholar]


