Abstract
Aging is significantly associated with the development of comorbid chronic conditions. These conditions indicate the use of multiple medications, and are often warranted by clinical guidelines. The aim of the present study was to evaluate medication appropriateness and frailty among Malaysian aged care home residents with chronic disease. The participants were 202 elderly (≥65 years) individuals, a cross-sectional sample from 17 aged care homes. After ethics approval, each participant was interviewed to collect data on sociodemographics, frailty status (Groningen Frailty Indicator [GFI]), medication appropriateness (Medication Appropriateness Index (MAI), the 2015 Beers’ criteria (Potentially Inappropriate Medication [PIM]), and 2014 STOPP criteria (Potentially Inappropriate Prescribing [PIP]). The findings show that 81% (n = 164) and 42% (n = 85) were taking medications for cardiovascular and central nervous system-related conditions, respectively, and 34% were using medications for diabetes (n = 69). Each participant had a mean of 2.9 ± 1.5 chronic diseases, with an average GFI score of 6.4 ± 3.6. More than three-quarters of the participants (76%) were frail and polypharmacy was a factor in nearly half (48%); 41% and 36% were prescribed at least one PIP and PIM, respectively, whereas the average MAI score was 0.6 (range: 0–6). The number of medications used per participant correlated significantly and positively (0.21, P = .002) with GFI score. These findings reinforce the need for participants of aged care homes to receive periodic medication review aimed at minimizing morbidity associated with inappropriate pharmacotherapy.
Keywords: aged care, chronic diseases, elderly, frailty, medication appropriateness
1. Introduction
Globally, the proportion of individuals over the age of 65 continues to grow more rapidly than other age groups.[1] In 2015, Malaysia had approximately 2.4 million individuals (8.0%) aged 60 and above.[2] With increasing life expectancy and declining birth rates, the proportion of older people aged 65 and above is expected to make up over 15% of the total population by 2030.[2,3] As populations age, the importance of effective aged care grows significantly.[4] Although family remains the principal care and support for elderly, there are older people who are living alone or in aged care facilities.[5] The residents of aged care homes often have multiple comorbidities including frailty.[6]
Aging is significantly associated with the development of comorbid chronic conditions. These conditions indicate the use of multiple medications, and are often warranted by clinical guidelines. Many of the attributes of aging such as vulnerability, physical, and cognitive decline also apply to frailty.[7] Clinically, frailty is a condition characterized by reduced physiological reserve and increased vulnerability to adverse outcomes after stressor events.[8] Such outcomes include hospitalization, institutionalization, falls, disability, and mortality.[8–11] The decline in physiological reserve occurs with aging but, in frailty, this decline is accelerated and the ability to maintain homoeostasis starts to fail.[10,11] Frailty has been shown to be an important limitation or constraint upon drug choice in the elderly.[12] The frail elderly often have chronic conditions and progressive loss of functional capability. Both reduce quality of life, especially in loss of independence and dignity.[13,14] One study found 62% of participants as frail and suffering from cardiovascular disease (CVD).[15] Another common condition in the elderly is diabetes mellitus (DM). This was supported by a statistics showing approximately half of the patients with type 2 DM are over 65 years of age.[16,17] Diabetes is also associated with cognitive decline and physical disability that are linked to the development and worsening of frailty syndrome.[12]
Polypharmacy has been an ongoing concern among older patients in terms of the number of prescribed medications and the complexity of drug regimens increasing over time.[18] Thus, assessment of medication appropriateness has become increasingly important because of the increasing risk of adverse events associated with polypharmacy and inappropriate prescribing. For example, as many as 40% of prescriptions for oral anticoagulants in nursing home participants were reported as suboptimal or inappropriate [19–20] This raises questions about medication appropriateness among this population. These common problems are seldom addressed even though clinical practice guidelines emphasize appropriate prescription for standardizing management and managing medical problems.[21] Since older people with chronic diseases are vulnerable, treatment goals should not only focus on lowering risks, but also try to improve physical and mental health.[12] Hence, the present study aimed to evaluate medication appropriateness and frailty status among elderly aged care home residents in Malaysia with chronic disease.
2. Methods
2.1. Study design and participants
This cross-sectional study assessed medication appropriateness and frailty status among older residents (≥65 years) of aged care homes associated with at least one chronic medical condition. The sample size of participants aged 65 and above was estimated based on the prevalence of population aged 65 years and above in Malaysia (5.9%, 2015),[22] with 95% confidence level and 5% of margin of error. A total of 17 private aged care homes around Klang Valley in Malaysia were approached to recruit 202 participants, who met inclusion criteria. The criteria included being at least 65 years old, staying in the facilities for >3 months, with one chronic medical condition and receiving at least one long-term medication, provided consent to participate in this study and able to articulate. Individuals who were critically ill, bedridden, or had clinically diagnosed cognitive impairment were excluded. An interviewer–administered data collection form was used.
The validated English version of the data form was translated into Malay and Chinese using a forward–backward translation process, with the aim of obtaining versions conceptually equivalent to the English version. The forward translators were health professionals who were native speakers of the relevant language, proficient in English and with appropriate knowledge of the health concepts used in the study. Without prior exposure to the form, the Malay and Chinese versions of the form were translated back to English by 2 independent translators.
2.2. Measurement of frailty
Frailty was measured using Groningen Frailty Indicator (GFI), which was developed by Steverink et al, and has been validated in institutionalized elderly.[23] GFI that contains 15 dichotomous items can range from a total score of 0 (normal activity without restriction) to 15 (completely disabled). Participants with a GFI score of 4 and above were considered frail.
2.3. Assessment of medication appropriateness
The medication appropriateness of participants was assessed using Medication Appropriateness Index (MAI), Beers’ criteria (2015), and START/STOPP criteria (2014).[24–26] Beers criteria and START/STOPP criteria were applied to identify potentially inappropriate medication (PIM) and potentially inappropriate prescribing (PIP), respectively. On the contrary, MAI assesses the appropriateness of a medication on 10 criteria: indication, effectiveness, dosage, correct directions, practical directions, drug–drug interaction, drug–disease interaction, duplication, duration, and cost. Appropriate responses were scored 1, whereas inappropriate, do not know, and not applicable responses were scored 0.[24] The 10 criteria were then combined with the weights of 3 for indication and effectiveness; 2 for dosage, correct directions, drug–drug interaction, and drug–disease interaction; and 1 for practical directions, duplication, duration, and cost. For each medication, the weighted sum across the 10 criteria was calculated, with an MAI range of 0 (fully appropriate) to 18 (fully inappropriate) for each medication. The average MAI per medication for each participant was then calculated. A higher score indicates less appropriate prescribing.
2.4. Number of medications and chronic diseases
The clinical parameters including list of current medical conditions and medications were gathered. The data were obtained from participants’ medical records. For this study, polypharmacy was defined as individuals who are dispensed with 5 or more concurrent medicines at one time during the study period. The frequency of chronic diseases experienced by participants was also recorded.
2.5. Data collection and analysis
Before baseline interview, consent was obtained after explaining research goals to the participants who corresponded to the inclusion and exclusion criteria. Each interview was conducted in the participant's preferred language (English, Malay, or Chinese). Sociodemographic information included age, ethnicity, education, occupation, alcohol consumption, and cigarette smoking (nonsmokers, past smokers, and current smokers). These were collected from participants. Statistical analysis was performed using SPSS version 23 and STATA IC version 12 with statistical significance set at a P value of .05. The results are presented as percentages or means and standard deviations. The differences between the variables were examined using independent t test (e.g., occurrence of polypharmacy and medication appropriateness parameters). Pearson's correlation test was used to assess the correlation between the variables (e.g., between GFI score and medication appropriateness parameters).
The International Medical University Joint-Committee on Research and Ethics (Project ID: BPI-1-13-(52)) gave ethical approval for the study. Participants’ personal data were stored in a password-protected file accessible only to the researchers. No personal data was disclosed, and the study results are reported as deidentified data.
3. Results
3.1. Sociodemographic characteristics of participants
A total of 202 participants from 17 aged care homes participated in this study. Of these participants, 126 (62%) were female. Most of the study participants (n = 164, 81%) were taking medications for CVD; 42% (n = 85) were taking medications for central nervous system (CNS)-related diseases and 34% (n = 69) for DM. Table 1 shows sociodemographic characteristics of the participants, stratified by types of medications. The mean age of all participants was 76.8 ± 7.8 years old. Although most of the participants taking medications for CVD and CNS diseases were between the age of 75 and 84 years old, participants taking hypoglycaemic medications were mostly between the ages of 65 to 74 years old. Almost all the participants were of Chinese ethnicity (n = 189, 93%). More than half of the participants were married (n = 106, 52%). Nearly one-quarter of participants did not receive any formal education (n = 54, 27%).
Table 1.
Sociodemographic characteristics of study residents.
3.2. Utilization of medications for chronic conditions
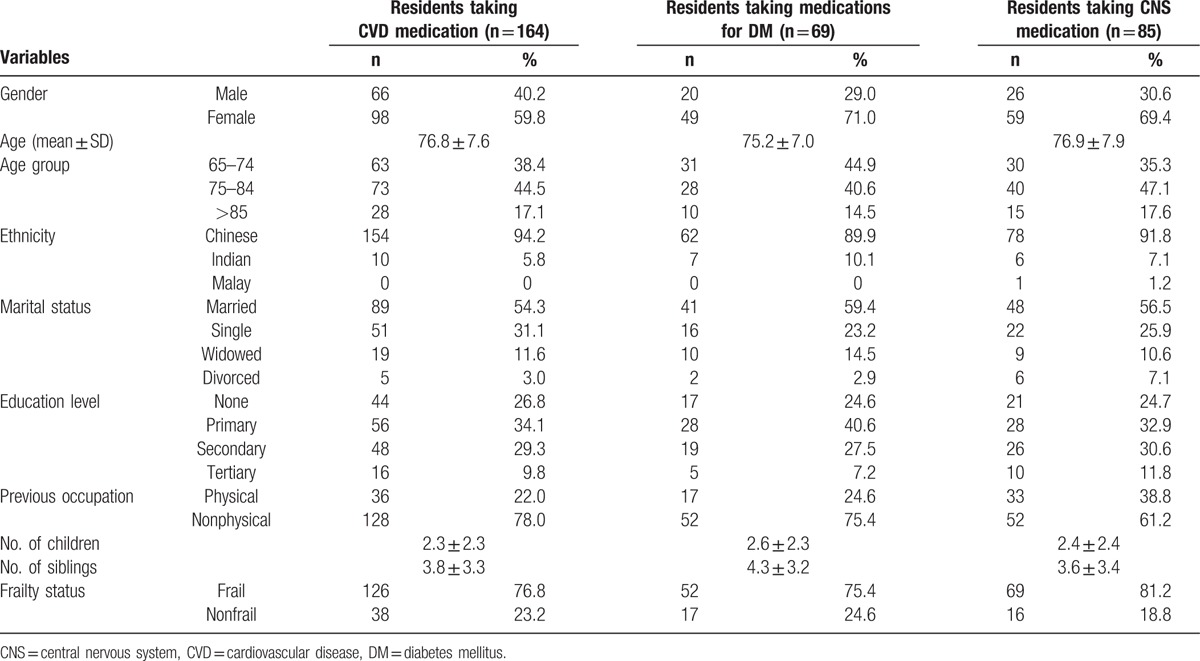
Pattern of CVD medication utilization is shown in Figure 1. Antihyperlipidemic agents (n = 93, 46%) were most frequently prescribed to the participants, with most being statins. This was followed by CCBs (n = 86, 42%). There were 80 (39%) participants taking angiotensin inhibitors, with participants taking ARBs (n = 27, 13%) about half of those taking ACE inhibitors (n = 53, 26%). Participants taking antiplatelet dose aspirin (n = 42, 21%) almost doubled those taking other antiplatelet agents (n = 23, 11%). Of the participants taking diuretics (n = 33, 16%), most were on thiazides (n = 21, 10%).
Figure 1.
Percentage use of medications for CVS-related disorders.
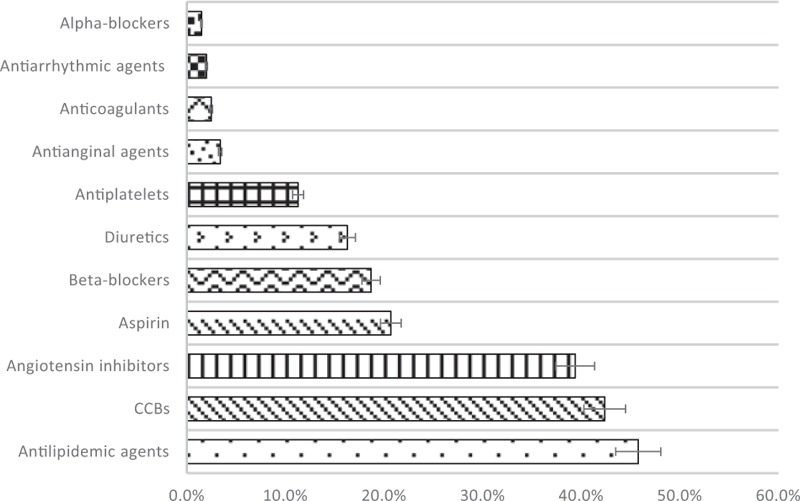
Hypoglycaemic agent use is shown in Figure 2. Participants prescribed with biguanides (n = 56, 28%) were double of those prescribed with sulfonylureas (n = 28, 14%). Similar number of participants (n = 7, 3.4%) were prescribed with DPP-4 inhibitors and insulin. Participants prescribed with short-acting insulin (n = 5, 3%) also concurrently prescribed with intermediate-acting insulin.
Figure 2.
Percentage use of medications for diabetes.
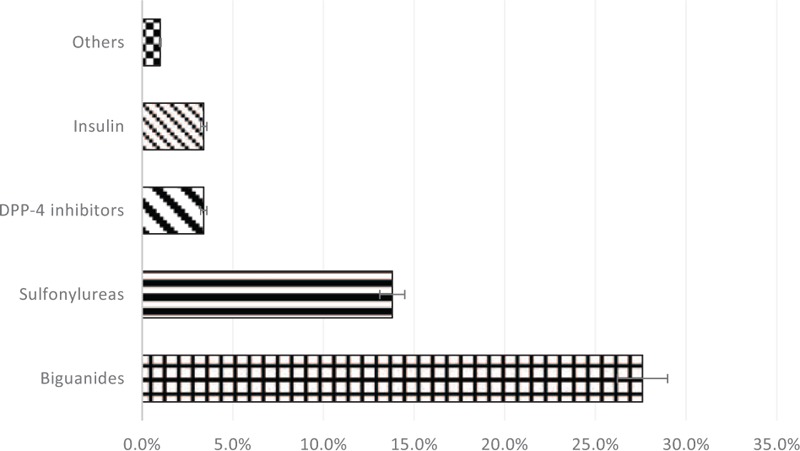
Pattern of CNS medications utilization is shown in Figure 3. CNS medications used most frequently were first-generation antihistamines (n = 29, 14%). This was followed by antipsychotics (n = 19, 9%) and anti-Parkinson agents (n = 18, 9%). Among participants who received antipsychotics, second-generation agents were being favored over the first-generation agents (n = 12, 5.9% vs n = 7, 3.4%). In addition, SSRI was the most commonly prescribed class of antidepressants (n = 13, 6%). In case of benzodiazepines, the intermediate-acting agents were most commonly prescribed to the participants (n = 12, 6%).
Figure 3.
Percentage use of medications for CNS-related disorders.
3.3. Medication appropriateness
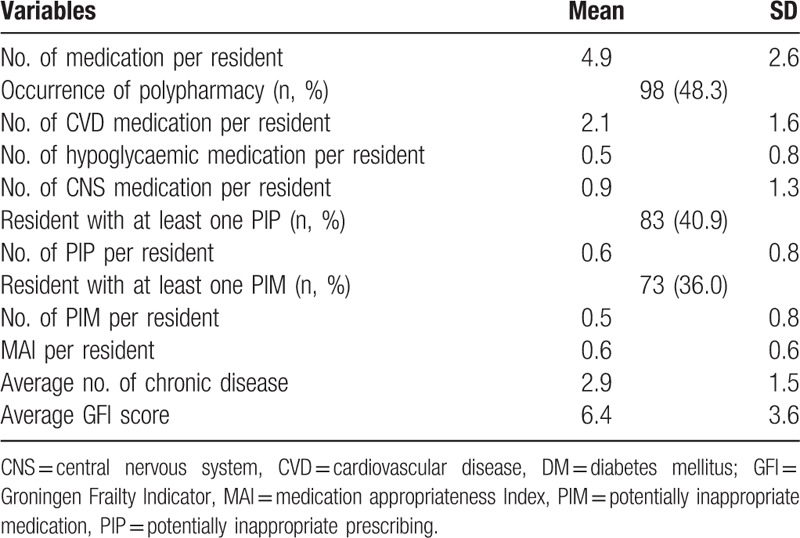
Table 2 presents information about medication appropriateness. On average, each participant received 2.1 ± 1.6 of CVD, 0.5 ± 0.8 of hypoglycaemic, and 0.9 ± 1.3 of CNS medications, respectively. Nearly half (n = 98, 48%) of the study participants were associated with polypharmacy. There were 41% of participants found to have at least one PIP, with a mean number of 0.6 ± 0.8 PIPs in each participant. Percentage of participants (36%) prescribed with at least one PIM was lower than those prescribed with at least one PIP, with a mean number of 0.5 ± 0.8 PIPs in each participant. On the contrary, the average MAI score of participants was 0.6 (range: 0–6).
Table 2.
Descriptive data of medication appropriateness parameters, GFI, and chronic diseases (n = 202).
3.4. Frailty status
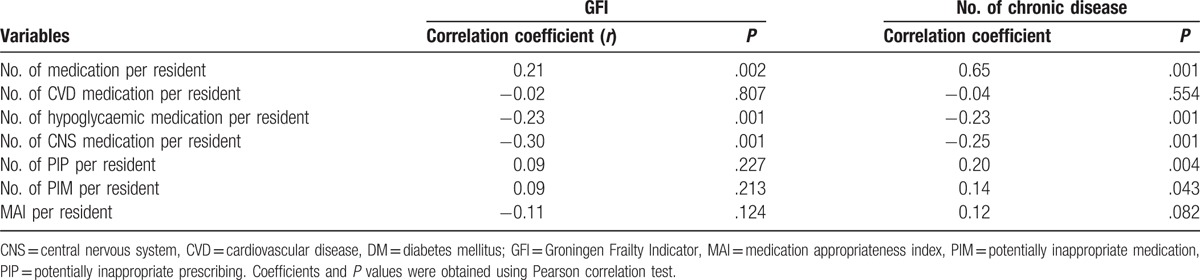
More than three-quarters of participants (n = 154, 76%) were frail, with an average GFI score of 6.4 ± 3.6. Table 3 shows the relationship between GFI and parameters of medication appropriateness. The number of medications used per participant correlated significantly and positively (r = 0.21, P = .002) with GFI score. The numbers of CVD, hypoglycaemic, and CNS medications per participant, correlated negatively with GFI score (r = −0.02, r = −0.23, r = −0.30, respectively). However, these correlations were statistically significant only for hypoglycaemic and CNS medications (both P = 0.001). PIP and PIM per participant correlated positively with GFI score, but were not statistically significant (r = 0.09, P > .05).
Table 3.
Relationships of medication appropriateness parameters with GFI and number of chronic diseases.
3.5. Comorbidity status
The mean number of chronic diseases of participants was 2.9 (SD = 1.5). Table 3 shows the relationship between number of chronic diseases and parameters of medication appropriateness. The number of medications per participant correlated significantly and positively (r = 0.65, P = .001) with GFI score. When analyzed according to the 3 groups of medications, we found that the number of medications (CVD, hypoglycaemic, and CNS) per participant correlated negatively with GFI score (r = −0.04, r = −0.23, r = −0.25, respectively). Among the 3 groups, only the numbers of hypoglycaemic and CNS medications per participant achieved statistical significance (both P = .001). Both the number of PIP and the number of PIM per participant correlated positively and significantly with GFI score (r = 0.20, P = .004; r = 0.14, P = .043). Although the MAI score per participant correlated positively with number of chronic diseases, the result was not statistically significant (r = 0.12, P = .082).
3.6. Polypharmacy
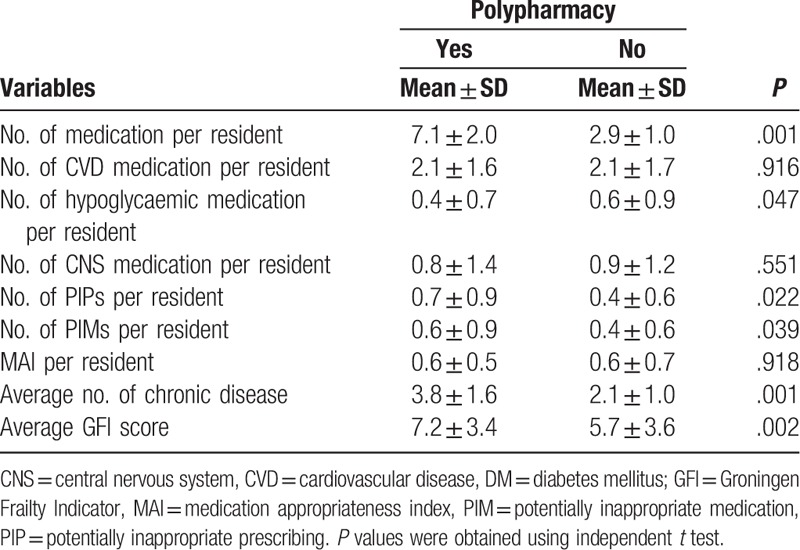
The number of hypoglycaemic medications was significantly lower among participants with polypharmacy than participants without polypharmacy (0.4 ± 0.7 vs 0.6 ± 0.9; P = .047) (Table 4). The other 2 variables (number of CVD medications and the number of CNS medications) did not achieve statistical significance (both P > .05). Although the number of PIP and the number of PIM were significantly higher in participants with polypharmacy (both P < .05), the MAI score was almost the same between participants with and without polypharmacy (P = .918). Both the number of chronic diseases and the GFI score were significantly higher in participants with polypharmacy (both P < .05).
Table 4.
Medication appropriateness parameters, by presence or absence of polypharmacy.
4. Discussion
This study evaluated medication appropriateness and frailty among residents of aged care homes in Malaysia. A significant proportion of study participants were on medications for CVD, CNS-related disease, and diabetes mellitus. Polypharmacy was a feature of the medication regimens of most of the participants and most had at least one PIP and one PIM. The average MAI score was 0.6 (range: 0–6). More than three-quarters of participants were frail as measured using GFI, with an average GFI score of 6.4 ± 3.6. The number of medications per participant correlated significantly and positively with GFI score.
There was a relatively high prevalence of potentially inappropriate medication use among study participants, with 36% and 41% of participants with at least one PIM and PIP, respectively. This prevalence was higher when compared with another Malaysian study conducted in similar settings, which reported a percentage of 24% to 33%.[27] This difference may be attributable to variations of prescribing or differences in the care provided. Nevertheless, the results were lower when compared with the global trend, in which an overall weighted point prevalence of 43.2% was reported in a recent systematic review (2016) which included studies from 18 different countries.[28] This might be because the review analyzed studies mainly from European countries which implement the multidose dispensing system that was associated with the use of potentially inappropriate medications.[28–30]
The mean number of medications was 4.9 ± 2.6, with almost half (48%) with polypharmacy. A similar study in Malaysia reported a slightly lower mean number of medications (4.7 ± 2.8) and percentage (44%) of participants experiencing polypharmacy.[31] The findings support the hypothesis that the problem of polypharmacy is quite prevalent among residents of aged care homes taking chronic medications in Malaysia.
Participants with polypharmacy in the present study had significantly higher numbers of PIP and PIM than their counterparts without polypharmacy. The finding is in accordance with other studies that report a relationship between the total number of medications and likelihood of receiving potentially inappropriate medications.[32–34] Similarly, the finding that study participants with higher number of chronic diseases (multiple comorbidities) had higher events of polypharmacy is also consistent with a study reporting multiple comorbidities as one factor associated with polypharmacy among aged care home residents.[35] In this study, polypharmacy was significantly lower among participants on hypoglycaemic medications than those on CVD or CNS medications. This may be ascribed to heightened awareness of prescribers of interaction between medications that can result in hypoglycaemia or hyperglycaemia, diminished autonomic warning symptoms of hypoglycaemia, and alteration of the blood glucose-lowering effect of hypoglycaemic medications.[36]
There can be significant adverse clinical consequences of polypharmacy in the elderly in care homes, as these individuals constitute the frailest segment of the geriatric population. In the present study occurrence of polypharmacy was significantly associated with higher frailty. There was significant correlation between number of medications per participant and GFI score. Polypharmacy has been associated with frailty among home-dwelling elderly in a French study.[37] This finding is consistent with the association between polypharmacy, cognitive impairment and impairment of physical fucntioning.[38,39]
About three-quarters (76%) of the study participants were frail. This prevalence was much higher than the prevalence of frailty (46.9%) among general aged care home participants as reported in a recent systematic review and meta-analysis (2015).[40] The difference in prevalence may be attributed to differences in the criteria used to define frailty, that is, according to the operational definition used.[41] Most of the studies included in the systematic review and meta-analysis defined frailty according to Field Frailty Indicator and the only included study which used GFI to define frailty reported a prevalence (62.1%) which was closer to this study.[40] Also, we included only elderly participants who were receiving long-term medications. This might contribute to the observed difference in prevalence since these participants may have multiple comorbidities and self-perceived impairments of physical fitness, which are 2 of the frailty indicators in GFI.
The correlation between GFI score and the number of PIP and PIM demand attention. Although there is lack of evidence that associates the potentially inappropriate medication use as detected using Beers criteria and other implicit measures such as MAI with negative outcomes in clinically defined frail older adults, frail older adults are likely to be more susceptible to medication-related adverse effects owing to attenuated physiological changes observed in frailty.[42] Therefore, it is advisable to use caution in prescribing to aged care home residents who are more frail than their community counterparts. Nonetheless, it is encouraging to observe significant negative correlations between GFI score and number of CNS medications, which suggests safer prescribing of CNS medications to frail elderly patients. This finding is expected since psychotropic medications are notoriously associated with increased risk of fall and other adverse consequences.[43]
With regard to comorbidity status, it was not surprising to observe a significant correlation between number of chronic diseases and the number of PIP and the number of PIM because patients with multiple comorbidities are more likely to be at increased polypharmacy-associated risk for inappropriate prescribing. It was observed that the number of chronic diseases correlated significantly with the number of medications per participant. Although it would be instinctive to also expect a positive correlation between the number of each group of medications used and the number of chronic diseases, the opposite was true where the number of each type of medication used. CNS medications, correlated negatively and significantly with the number of chronic disease. A study reported that patients with new episodes of depression were less likely to be prescribed antidepressants if they had multiple comorbidities.[44]
The prescribing pattern of CNS medications merits discussion. Many of the Beers and START/STOPP criteria involve medications acting on the brain. We observed that more second-generation antipsychotic agents were prescribed than first-generation agents. Both “generations” have comparable clinical but second-generation agents have fewer extrapyramidal effects.[45] A plethora of studies have reported that more second-generation antipsychotics were prescribed to nursing home residents than first-generation antipsychotics.[46–48] Prescribing first-generation antipsychotics is considered potentially inappropriate in the STOPP criteria. Prescribing of both first- and second-generation antipsychotics is considered potentially inappropriate in the Beers criteria.[25,26] With regard to antidepressants, SSRIs were more commonly prescribed than tricyclic agents. Tricyclic agents are associated with fall risk due to anticholinergic side effects. SSRIs have fewer anticholinergic side effects and are generally well tolerated by elderly patients.[49,50] Tricyclic antidepressants are potentially inappropriate in both STOPP and Beers criteria.[25,26] Although short- to intermediate-acting benzodiazepine were more frequently used relative to long-acting agents in the present study, it is advised that benzodiazepine should be avoided if at all possible in elderly patients regardless of duration of benzodiazepine action. Increased fall risk is similar whether agents of short, intermediate, or long duration of action are used.[51] All benzodiazepines are thus regarded as potentially inappropriate in both the STOPP and Beers criteria.[25,26]
There are mainly 2 limitations in this study. First, the cross-sectional design does not allow the establishment of any cause–effect relationship. Second, the relatively small number of participants, from aged care homes in urban central Peninsular Malaysia, limit the generalizability. The reliability of medication-related data was ensured by all good medication storage and administration practice in the homes used in the study. The results of certain laboratory tests such as serum sodium and creatinine levels are required to assess several criteria of STOPP. These laboratory records were not available in the medical charts of some participants, which might lead to underreporting of PIP.
In summary, this study identifies a high prevalence of polypharmacy and frailty among Malaysian aged care homes participants with chronic diseases. Participants with polypharmacy had significantly higher numbers of both PIP and PIM than their counterparts without polypharmacy. Polypharmacy and long standing frailty could contribute to declining health, and vice versa. The findings reinforce the need for periodic medication review.
Acknowledgments
We thank the International Medical University for the support during this study and extend our appreciation to all participants who took part in the study.
Footnotes
Abbreviations: CNS = central nervous system, CVD = cardiovascular diseases, DM = diabetes mellitus, GFI = Groningen Frailty Index, MAI = medication appropriateness index, PIM = potentially inappropriate medication, PIP = potential inappropriate prescription, STOPP = screening tool of older people's potentially inappropriate prescriptions.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Faunce TA. Global health & global aging. JAMA 2008;299:2333–4. [Google Scholar]
- [2].Zawawi R. Active ageing in Malaysia. In: The second meeting of the committee on international cooperation on active ageing Malaysia. 2013. [Google Scholar]
- [3].Department of Statistics Malaysia Official Portal [Internet]. Available from: https://www.statistics.gov.my/ (Accessed June 22, 2016).
- [4].Dwyer D. Experiences of registered nurses as managers and leaders in participantial aged care facilities: a systematic review. Int J Evid Based Healthc 2011;9:388–402. [DOI] [PubMed] [Google Scholar]
- [5].Ng ST, Tey NP, Yew SY, et al. Effects of quality of service and activities on life satisfaction of participants in nursing homes. Wulfenia J 2012;19:153–63. [Google Scholar]
- [6].Kwong EW, Lai CK, Chan KS. Factors associated with quality of life in nursing home participants with frailty. Clin Nurs Stud 2014;2:1–5. [Google Scholar]
- [7].Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc 2005;53:1321–30. [DOI] [PubMed] [Google Scholar]
- [8].Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [9].Clegg A, Young J, Iliffe S, et al. Frailty in elderly people. Lancet 2013;381:752–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Basic D, Shanley C. Frailty in an older inpatient population: using the clinical frailty scale to predict patient outcomes. J Aging Health 2015;27:670–85. [DOI] [PubMed] [Google Scholar]
- [11].Alexa ID, Ilie AC, Moroşanu A, et al. Approaching frailty as the new geriatric syndrome. Rev Medico Chir Soc Med Natl Iaşi 2013;117:680–5. [PubMed] [Google Scholar]
- [12].Abbatecola AM, Paolisso G, Corsonello A, et al. Antidiabetic oral treatment in older people: is fraily matter? Drugs Aging 2009;26(Suppl. 1):53–62. [DOI] [PubMed] [Google Scholar]
- [13].Varela F.R. de A, Ciconelli RM, Campolina AG, et al. Quality of life evaluation of frail elderly in Campinas, São Paulo. Rev Assoc Médica Bras 2015;61:423–30. [DOI] [PubMed] [Google Scholar]
- [14].Kane RL, Rockwood T, Hyer K, et al. Rating the importance of nursing home participants’ quality of life. J Am Geriatr Soc 2005;53:2076–82. [DOI] [PubMed] [Google Scholar]
- [15].Chin A, Paw MJ, Dekker JM, et al. How to select a frail elderly population? A comparison of three working definitions. J Clin Epidemiol 1999;52:1015–21. [DOI] [PubMed] [Google Scholar]
- [16].Harris MI, Flegal KM, Cowie CC, et al. Prevalence of diabetes, impaired fasting glucose, and impaired glucose tolerance in U.S. adults: the third National Health and Nutrition Examination Survey, 1988–1994. Diabetes Care 1998;21:518–24. [DOI] [PubMed] [Google Scholar]
- [17].Gossain VV, Carella MJ, Rovner DR. Management of diabetes in the elderly: a clinical perspective. J Assoc Acad Minority Phys 1994;5:22–31. [PubMed] [Google Scholar]
- [18].Gurwitz JH. Polypharmacy: a new paradigm for quality drug therapy in the elderly? Arch Intern Med 2004;164:1957–9. [DOI] [PubMed] [Google Scholar]
- [19].Bahri O, Roca F, Lechani T, et al. Underuse of oral anticoagulation for individuals with atrial fibrillation in a nursing home setting in France: comparisons of participant characteristics and physician attitude. J Am Geriatr Soc 2015;63:71–6. [DOI] [PubMed] [Google Scholar]
- [20].St-Onge M, Glazer-Cavanagh M, Bell L, et al. The effect of bleeding risk and frailty status on anticoagulation patterns in octogenarians with atrial fibrillation: the FRAIL-AF study. Can J Cardiol 2016;32:169–76. [DOI] [PubMed] [Google Scholar]
- [21].Onder G, Landi F, Fusco D, et al. Recommendations to prescribe in complex older adults: results of the CRIteria to assess appropriate Medication use among Elderly complex patients (CRIME) project. Drugs Aging 2014;31:33–45. [DOI] [PubMed] [Google Scholar]
- [22].Department of Statistics. Population statistics [Internet]. Putrajaya: Population and Demographic Statistics Division; 2015. Available from: https://www.statistics.gov.my/dosm/uploads/files/3_Time%20Series/Malaysia_Time_Series_2015/22Perangkaan_Penduduk.pdf (Accessed June 9, 2016).
- [23].Steverink N, Slaets JPJ, Schuurmans H, et al. Measuring frailty: development and testing of the Groningen Frailty Indicator (GFI). Gerontologist 2001;41:236–7. [Google Scholar]
- [24].Hanlon JT, Schmader KE, Samsa GP, et al. A method for assessing drug therapy appropriateness. J Clin Epidemiol 1992;45:1045–51. [DOI] [PubMed] [Google Scholar]
- [25].American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–46. [DOI] [PubMed] [Google Scholar]
- [26].O’Mahony D, O'Sullivan D, Byrne S, et al. STOPP/START criteria for potentially inappropriate prescribing in older people: version 2. Age Ageing 2015;44:213–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Chen LL, Tangiisuran B, Shafie AA, et al. Evaluation of potentially inappropriate medications among older participants of Malaysian nursing homes. Int J Clin Pharm 2012;34:596–603. [DOI] [PubMed] [Google Scholar]
- [28].Morin L, Laroche ML, Texier G, et al. Prevalence of potentially inappropriate medication use in older adults living in nursing homes: a systematic review. J Am Med Dir Assoc 2016;17:862.e1-9. [DOI] [PubMed] [Google Scholar]
- [29].Johnell K, Fastbom J. Multi-dose drug dispensing and inappropriate drug use: a nationwide register-based study of over 700,000 elderly. Scand J Prim Health Care 2008;26:86–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wallerstedt SM, Fastbom J, Johnell K, et al. Drug treatment in older people before and after the transition to a multi-dose drug dispensing system: a longitudinal analysis. PLoS One 2013;8:e67088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Al Aqqad SM, Chen LL, Shafie AA, et al. The use of potentially inappropriate medications and changes in quality of life among older nursing home participants. Clin Interv Aging 2014;9:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ryan C, O’Mahony D, Kennedy J, et al. Potentially inappropriate prescribing in older participants in Irish nursing homes. Age Ageing 2013;42:116–20. [DOI] [PubMed] [Google Scholar]
- [33].O'Sullivan DP, O’Mahony D, Parsons C, et al. A prevalence study of potentially inappropriate prescribing in Irish long-term care participants. Drugs Aging 2013;30:39–49. [DOI] [PubMed] [Google Scholar]
- [34].Ubeda A, Ferrándiz L, Maicas N, et al. Potentially inappropriate prescribing in institutionalised older patients in Spain: the STOPP-START criteria compared with the Beers criteria. Pharm Pract (Granada) 2012;10:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Bronskill SE, Gill SS, Paterson JM, et al. Exploring variation in rates of polypharmacy across long term care homes. J Am Med Dir Assoc 2012;13:309.e15-21. [DOI] [PubMed] [Google Scholar]
- [36].Ligthelm RJ, Kaiser M, Vora J, et al. Insulin use in elderly adults: risk of hypoglycemia and strategies for care. J Am Geriatr Soc 2012;60:1564–70. [DOI] [PubMed] [Google Scholar]
- [37].Herr M, Robine JM, Pinot J, et al. Polypharmacy and frailty: prevalence, relationship, and impact on mortality in a French sample of 2350 old people. Pharmacoepidemiol Drug Saf 2015;24:637–46. [DOI] [PubMed] [Google Scholar]
- [38].Magaziner J, Cadigan DA, Fedder DO, et al. Medication use and functional decline among community-dwelling older women. J Aging Health 1989;1:470–84. [Google Scholar]
- [39].Jyrkkä J, Enlund H, Lavikainen P, et al. Association of polypharmacy with nutritional status, functional ability and cognitive capacity over a three-year period in an elderly population. Pharmacoepidemiol Drug Saf 2011;20:514–22. [DOI] [PubMed] [Google Scholar]
- [40].Kojima G. Prevalence of frailty in nursing homes: a systematic review and meta-analysis. J Am Med Dir Assoc 2015;16:940–5. [DOI] [PubMed] [Google Scholar]
- [41].Collard RM, Boter H, Schoevers RA, et al. Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 2012;60:1487–92. [DOI] [PubMed] [Google Scholar]
- [42].Bergman H, Ferrucci L, Guralnik J, et al. Frailty: an emerging research and clinical paradigm: issues and controversies. J Gerontol A Biol Sci Med Sci 2007;62:731–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hartikainen S, Lönnroos E, Louhivuori K. Medication as a risk factor for falls: critical systematic review. J Gerontol A Biol Sci Med Sci 2007;62:1172–81. [DOI] [PubMed] [Google Scholar]
- [44].Gill JM, Klinkman MS, Chen YX. Antidepressant medication use for primary care patients with and without medical comorbidities: a national electronic health record (EHR) network study. J Am Board Fam Med 2010;23:499–508. [DOI] [PubMed] [Google Scholar]
- [45].Miller CH, Mohr F, Umbricht D, et al. The prevalence of acute extrapyramidal signs and symptoms in patients treated with clozapine, risperidone, and conventional antipsychotics. J Clin Psychiatry 1998;59:69–75. [DOI] [PubMed] [Google Scholar]
- [46].Azermai M, Elseviers M, Petrovic M, et al. Geriatric drug utilisation of psychotropics in Belgian nursing homes. Hum Psychopharmacol 2011;26:12–20. [DOI] [PubMed] [Google Scholar]
- [47].Olazarán J, Valle D, Serra JA, et al. Psychotropic medications and falls in nursing homes: a cross-sectional study. J Am Med Dir Assoc 2013;14:213–7. [DOI] [PubMed] [Google Scholar]
- [48].Simoni-Wastila L, Wei YJ, Luong M, et al. Quality of psychopharmacological medication use in nursing home participants. Res Social Adm Pharm 2014;10:494–507. [DOI] [PubMed] [Google Scholar]
- [49].de Jong MR, Van der Elst M, Hartholt KA. Drug-related falls in older patients: implicated drugs, consequences, and possible prevention strategies. Ther Adv Drug Saf 2013;4:147–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Martin RM, Hilton SR, Kerry SM, et al. General practitioners’ perceptions of the tolerability of antidepressant drugs: a comparison of selective serotonin reuptake inhibitors and tricyclic antidepressants. BMJ 1997;314:646–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Landi F, Onder G, Cesari M, et al. Psychotropic medications and risk for falls among community-dwelling frail older people: an observational study. J Gerontol A Biol Sci Med Sci 2005;60:622–6. [DOI] [PubMed] [Google Scholar]


