Abstract
The objective of this study was to summarize the incidence of postoperative cognitive dysfunction (POCD) after 7days following liver transplantation (LT), and to evaluate the effectiveness of bispectral index (BIS) guided anesthetic intervention in reducing POCD. Additional serum concentrations of S100β and neuron-specific enolase (NSE) were detected during surgery to determine whether they were reliable predictors of POCD.
Patients who underwent LT at Beijing YouAn Hospital Affiliated to Capital University of Medical Science from January 2014 to December 2015 were enrolled. BIS monitor was needed during surgery. Patients who underwent LT without BIS monitoring during August 2012 to December 2014 served as historical controls. A battery of 5 neuropsychological tests were performed and scored preoperatively and 7days after surgery. POCD was diagnosed by the method of one standard deviation (SD). The blood samples of BIS group were collected at 5 time points: just before induction of general anesthesia (T0), 60 minutes after skin incision (T1), 30 minutes after the start of the anhepatic phase (T2), 15 minutes after reperfusion of the new liver (T3), and at 24 hours after surgery (T4).
A total of 33 patients were included in BIS group, and 27 in the control group. Mean arterial pressure was different between 2 groups at 30 minutes after the start of the anhepatic phase (P = .032). The dose of propofol using at anhepatic phase 30 min and new liver 15 min was lower in the BIS group than control group (0.042 ± 0.021 vs. 0.069 ± 0.030, P < .001; 0.053 ± 0.022 vs. 0.072 ± 0.020, P = .001). Five patients were diagnosed as having POCD after 7 days in the BIS group and the incidence of POCD was 15.15%. In the control group, 9 patients had POCD and the incidence of POCD was 33.33%. The incidence of POCD between 2 groups had no statistical difference (P = .089). S100β increased at stage of anhepatic 30 minutes (T2) and new liver 15 minutes (T3) compared with the stage of before anesthesia (T0) (1.49 ± 0.66 vs. 0.72 ± 0.53, P < .001; 1.92 ± 0.78 vs. 0.72 ± 0.53, P < .001). NSE increased at stage of anhepatic 30 minutes (T2) and new liver 15 minutes (T3) compared with the stage of before anesthesia (T0) (5.80 ± 3.03 vs. 3.58 ± 3.24, P = .001; 10.04 ± 5.65 vs. 3.58 ± 3.24, P < .001). At 24 hours after surgery, S100β had no difference compared to one before anesthesia (1.0 ± 0.62 vs. 0.72 ± 0.53, P = .075), but NSE still remained high (5.19 ± 3.64 vs. 3.58 ± 3.24, P = .043). There were no significant differences in the serum concentrations of S100β between patients with and without POCD at 5 time points of operation (P > .05). But at 24 hours after surgery, NSE concentrations were still high of patients with POCD (8.14 ± 3.25 vs. 4.81 ± 3.50, P = .035).
BIS-guided anesthesia can reduce consumption of propofol during anhepatic and new liver phase. Patients in BIS group seem to have a mild lower incidence of POCD compared to controls, but no statistical significant. The influence of BIS-guided anesthesia on POCD needs to be further confirmed by large-scale clinical study. S100β protein and NSE are well correlative with neural injury, but NSE is more suitable for assessment of incidence of postoperative cognitive deficits after surgery.
Keywords: bispectral index-guided anesthesia, liver transplantation, neuron-specific enolase, optimizing intraoperative management, postoperative cognitive dysfunction, S100β protein
1. Introduction
Liver transplantation (LT) is the only curative treatment in patients with end-stage liver disease. One-year survival rate after surgery has been 80% to 85% since the first LT was performed in 1963,[1–3] but few researches pay close attention to neurological complications. Postoperative cognitive dysfunction (POCD) is a kind of neurological complications after anesthesia and surgery. It often shows up as new cognitive impairment of memory, disability to combine tasks, psychomotor dexterity, among others. The diagnosis of POCD depends on a neuropsychiatric test or group of tests. The risk factors of developing POCD mainly include larger and more invasive operations, duration and depth of anesthesia, advanced age, lasted low hypotension and cerebral anoxia, and other factors.[4,5]
Previous studies reported a higher rate of POCD after LT.[6] The incidence of POCD after LT is not so high in our LT center, possibly thanks to improvement of surgical procedure, closer monitoring during operation, specially the depth of anesthesia, and cautious dose initiation and adjustment. Some researchers have proved that intraoperative monitoring of anesthetic depth is a pragmatic intervention to reduce postoperative cognitive impairment.[7] But this benefit in LT has been reported rarely.
S100β protein and neuron-specific enolase (NSE) are well known as potential markers of neural injury. Of those, S100β protein is an acidic calcium-binding protein, which is found in astrocytes and Schwann cells, and physiological serum levels of S100β protein are low. In the early stages of brain injury, glial cells are activated, and S100β is released into the blood after neural damage.[8] Therefore, S100β protein seems to be a potential biochemical marker of POCD.[9] NSE is a glycolytic protein with a serum half-life longer than 20 h. It is primarily located in the cytoplasm of neurons and involved in increasing neuronal chloride levels during onset of neuronal activity.[10–12] Some studies have indentified POCD using serum levels of S100β protein and NSE. We have not found any data on the relationship between plasma S100β or NSE and POCD after LT.
The objective of this study was to summarize the incidence of POCD after 7days following LT in our center, and to evaluate the effectiveness of bispectral index (BIS, one of the several technologies used to monitor depth of anesthesia) intra-operative anesthetic intervention in reducing post-operative cognitive impairment. And additional analysis examined concentrations of S100β and NSE in serum during surgery to determine whether they were reliable predictors of POCD.
2. Materials and methods
2.1. Patients
From January 2014 to December 2015, patients who underwent LT at Beijing You, an Hospital Affiliated to Capital University of Medical Science were enrolled. This hospital is a high-volume hepatobiliary unit. Since 2003, more than 1000 LTs have been performed. The exclusion criteria were the presence of overc hepatic encephalopathy (MMES score <23),[13] clinical history of a previous neurologic or psychiatric disorder, episodes of gastrointestinal bleeding, alcohol or neuroactive drug intake in the previous 1 months, presence of malnutrition, cardiac, respiratory, or renal insufficiency, severe focal or diffuse brain atrophy upon computed tomography/magnetic resonance imaging cerebral scan, and disability of finish neuropsychological tests. BIS monitor was needed during surgery. Patients who underwent LT without BIS monitoring during August 2012 to December 2014 served as historical controls. The data of these patients were collected retrospectively from the electronic medical records. The study was approved by the institutional research ethics committee.
2.2. General anesthesia
No patients had premedication. Anesthesia was induced with midazolam 0.05 mg/kg, etomidate 0.3 mg/kg, sufentanil 0.5 mcg/kg, and rocuronium 0.9 mg/kg. All patients needed intubation and mechanical ventilation. PaCO2 was maintained between 35 and 45 mmHg. Invasive hemodynamic monitoring included pressure measurement by arteria radialis puncture and the Swan–Ganz catheter of right internal jugular vein. Anesthesia was maintained with sufentanil 0.3–0.5 μg/kg/h, rocuronium 0.3 to 0.5 mg/kg/h and propofol. In BIS group, the dose of propofol was guided by a BIS (Aspect A-2000) value between 45 and 55 from the commencement of anesthesia to the end of surgery. In the control group, the dose of propofol was guided by clinical signs of depth of anesthesia. This was generally to maintain arterial pressure within 15% to 20% of the baseline and the heart rate within 40 to 90 beats/min range. Body temperature was kept between 35.5°C and 37°C using warming blankets. Ulinastatin of 1 million unit was given intravenously after induction of anesthesia. Methylprednisolone (0.5 g) was administered in an anhepatic phase. We maintained a tight control of blood pressure and kept the mean arterial pressure (MAP) within 10% t o 20% of the preoperative value by the administration of continuous infusion of norepinephrine (0.01–0.5 μg/kg/min) or bolus doses of epinephrine (10–100 μg). The electrolyte and acid-base balance were kept within normal range during surgery. After the surgery, all patients were admitted to a transplant intensive care unit (ICU).
2.3. Neuropsychological assessment
Transplanted patients underwent repeated neuropsychological assessments before and 7 days after LT. The validated cognitive tests include 5 tests. First is Mini Mental State Examination, which is intended as a screening test for dementia. It contains questions relating to temporal and spatial orientation, tasks relating to retentiveness, recollection, attention and correctness, and an assessment of language and the ability to write and draw. Second is Digit Span Test. It assesses patients’ memory and immediate attention/recall, including the Digit Span Forward and Digit Span Backward tests. Third is Digit Symbol Test. The participants need to write the number referred to each symbol in 90 seconds. Number of hits are registered. The test measures short-term memory, visual-spatial skills and attention. Fourth is Trail Making Test. The study participant must draw lines connecting consecutively numbered circles. Time spent is assessed. It involves complex visual and motor speed. Fifth is Short Story Memory Test. The test assesses immediate memory span, learning, susceptibility to interference, and recognition memory, including 2 short stories. Each of them consists of a certain number of nouns. The subjects needed to repeat the stories and the nouns. We evaluated the number of words recalled made for each presentation. A standard deviation (SD) was calculated for each test score. The SD was used to measure changes in cognitive function during the time interval between the day of surgery and postoperative day 7. The diagnosis of POCD was accorded to the method of 1 SD. When there were significant findings in ≥2 postoperative neurocognitive tests, then the patients were diagnosed as having POCD.[14]
2.4. Blood assays
Venous blood (3 mL) was collected from a right internal jugular catheter and placed into vacuum tubes containing sodium heparin. The blood samples were collected at 5 time points: just before induction of general anesthesia (T0), 60 minutes after skin incision (T1), 30 minutes after the start of the anhepatic phase (T2), 15 minutes after reperfusion of the new liver (T3), and at 24 hours after surgery (T4). Samples were placed in dry tubes and centrifuged; serum was removed and stored at −80°C until the analysis. Molecular analysis was conducted using a commercial Human Soluble Protein-100 (S-100) ELISA Kit (CUSABIO, Wuhan, China) and Human Neural specificity enolization enzyme ELISA kit (CUSABIO). We used Wuhan Huamei Biological Technology Company (Wuhan, China) to construct reaction standard curves. The protein level was calculated while comparing the optical density value of samples with the standard curve.
2.5. Data collection
Data on characteristics of patients, including age, sex, body mass index (BMI), education status, the model for end-stage liver disease (MELD) score, and American Society of Anesthesiologists classifications (ASA); general data of surgery, including duration of operation time, anhepatic time, blood loss, blood transfusion volume, input fluid volume; MAP, hemoglobin (HB), SVO2, and dosage of propofol at T0, T1, T2, T3; the score of every neuropsychological tests calculated before and 7 days after LT; serum concentration of S100β protein and NSE at 5 time points were collected.
2.6. Statistical analysis
Data were shown as mean ± SD. The Kolmogorov-Smirnov test was used to establish deviation from a normal distribution. Comparison between the BIS group and control groups was made using the Fisher exact test, independent sample t test and Mann–Whitney U test as appropriate. Univariate analysis of variance was used for the dose of propofol, S100β, and NSE of BIS group during LT. Paired sample t test was used for S100β and NSE in patients with and without POCD during different phases of operation. A P value of .05 was considered significant. Statistical analysis was performed using the Statistical Package for the Social Sciences version 16.0 (SPSS Inc, Chicago, IL).
3. Results
3.1. Demographic factors and clinical characteristics
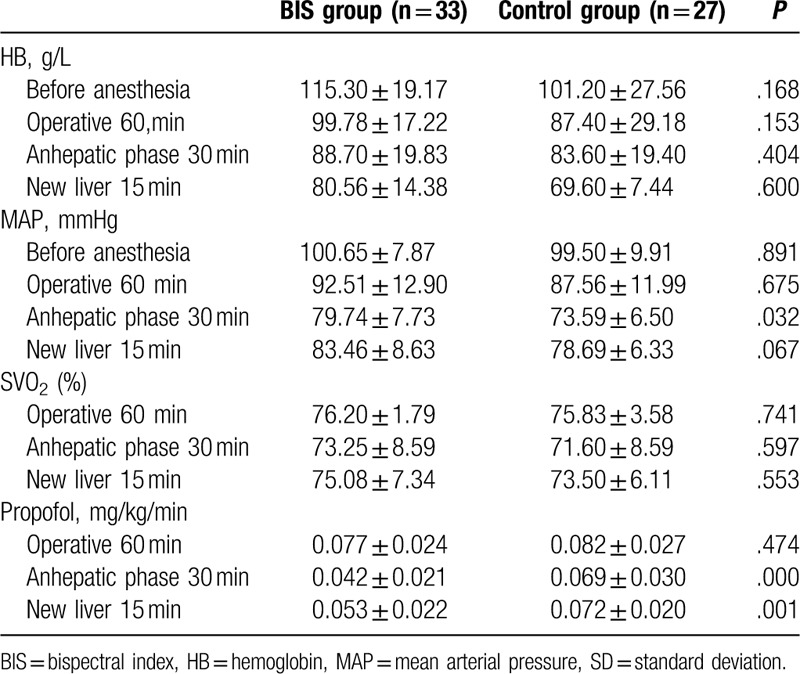
Thirty-three patients were included in BIS group, and 27 in the control group. All operations and neuropsychological tests had gone well. All of them received whole liver, which was donated by donation after cardiac death patients. Characteristics of patients, including age, sex, BMI, education status, MELD score, ASA, were not different between 2 groups (P > .05, Table 1). General data of surgery, including duration of operation time, anhepatic time, blood loss, blood transfusion volume, and input fluid volume were not different between 2 groups (P > .05, Table 1). There were no significant differences in HB and SVO2 between 2 groups during surgery (P > .05, Table 2), but MAP was different at 30 minutes after the start of the anhepatic phase (T2) (P = .032, Table 2). The dose of propofol using at anhepatic phase 30 minutes and new liver 15 minutes was lower in the BIS group than control group (0.042 ± 0.021 vs. 0.069 ± 0.030, P < .001; 0.053 ± 0.022 vs. 0.072 ± 0.020, P = .001, Table 2).
Table 1.
Characteristics of the sample (mean ± SD).
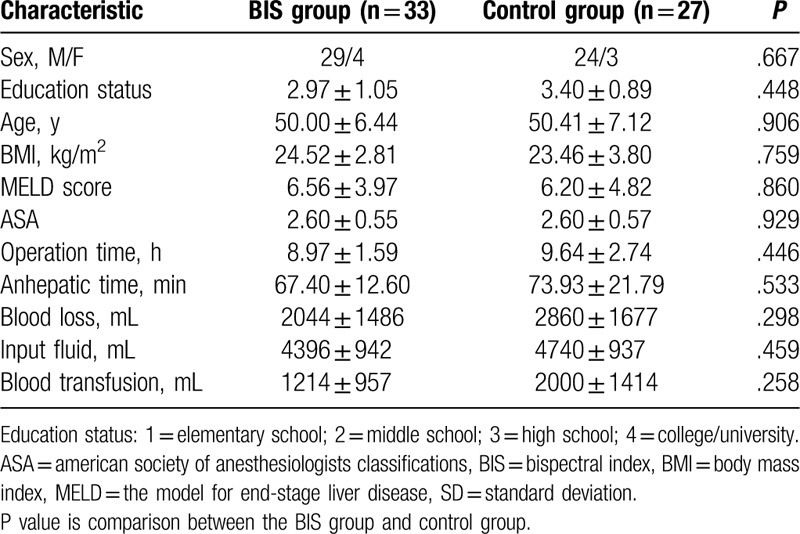
Table 2.
HB/MAP/SVO2/propofol of the sample (mean ± SD).
3.2. Neuropsychological tests
Five patients were diagnosed as having POCD after 7 days in the BIS group and the incidence of POCD was 15.15%. In the control group, 9 patients had POCD and the incidence of POCD was 33.33%. The incidence of POCD between 2 groups had no statistical difference (P = .089).
3.3. Blood assay
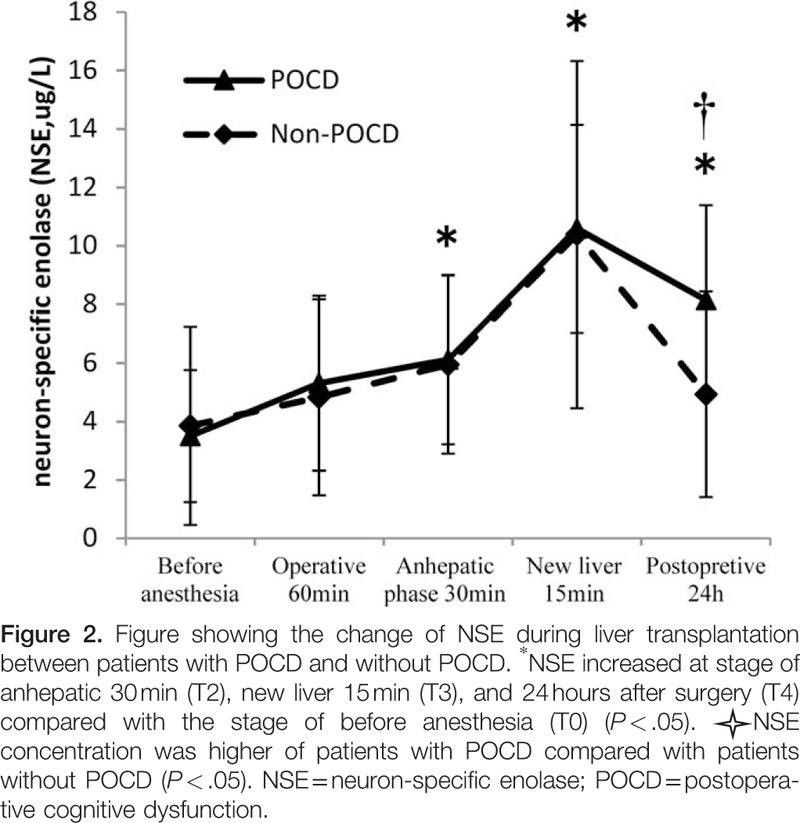
S100β increased at stage of anhepatic 30 minutes (T2) and new liver 15 minutes (T3) compared with the stage of before anesthesia (T0) (1.49 ± 0.66 vs. 0.72 ± 0.53, P < .001; 1.92 ± 0.78 vs. 0.72 ± 0.53, P < .001). NSE increased at stage of anhepatic 30 minutes (T2) and new liver 15 minutes (T3) compared with the stage of before anesthesia (T0) (5.80 ± 3.03 vs. 3.58 ± 3.24, P = .001; 10.04 ± 5.65 vs. 3.58 ± 3.24, P < .001). At 24 hours after surgery, S100β had no difference compared before anesthesia (1.0 ± 0.62 vs. 0.72 ± 0.53, P = .075), but NSE was still high (5.19 ± 3.64 vs. 3.58 ± 3.24, P = .043).There were no significant differences in the serum concentrations of S100β between patients with and without POCD at 5 time points of operation (P > .05, Fig. 1.). Although there were also no significant differences in the serum concentrations of NSE in patients with POCD compared with patients without POCD before anesthesia (T0), operative 60 minutes (T1), anhepatic stage 30 minutes (T2), and new liver 15 minutes (T3) (P > .05, Fig. 2), but at 24 hours after surgery (T4), NSE concentrations were still high in patients with POCD (8.14 ± 3.25 vs. 4.81 ± 3.50, P = .035, Fig. 2).
Figure 1.
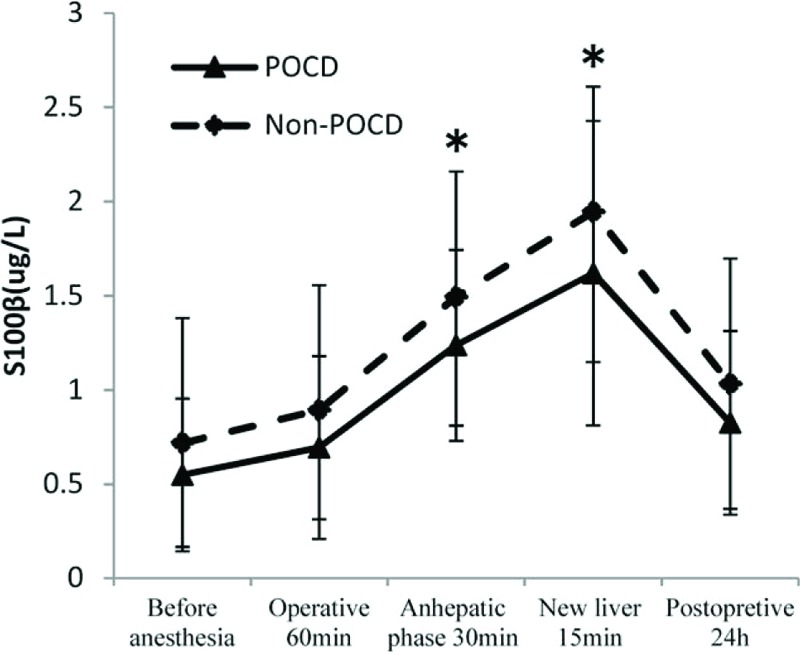
Figure showing the change of S100β during liver transplantation between patients with POCD and without POCD. ∗S100β increased at stage of anhepatic 30 min (T2) and new liver 15 min (T3) compared with the stage of before anesthesia (T0) (P < .05). POCD = postoperative cognitive dysfunction.
4. Discussion
POCD was recognized as a neurological complication secondary to anesthesia and surgery as early as 1955.[15] Its pathogenesis has not been properly clarified, and there are fewer validated risk models for POCD. Advanced age has been considered as an independent risk factor in several large cohort studies.[16,17] The other risk factors of developing POCD mainly include larger and more invasive operations, duration and depth of anesthesia, lasted low hypotension and cerebral anoxia, and other factors.[4,5]
The research of prevention and treatment for POCD are fewer. Several researches indicated that intraoperative anesthetic intervention could reduce the incidence of POCD. A systematic review about clinical evidence of anesthesia and cognitive disorders summarized clinical trials which were published between April 2010 and February 2016.[7] The review shows that intraoperative anesthetic monitoring could reduce the dose of anesthetic, mainly about propofol and inhaled anesthetics.[18,20,21] Similar conclusions have been drawn from our findings (Table 2). But the effects on cognition had different results. Jildenstål et al's[18] report showed that the group of using auditory evoked potential-guided anesthesia had lower occurrence of POCD at 1 day postoperatively. Another research about BIS-guided anesthetic also showed that mild and moderate POCD were reduced at 1 and 52 weeks in BIS group.[19] Chan et al's[20] study indicated that patients with delirium and POCD within 3 months was lower in the BIS group, even if cognitive performance was similar at 1 week postoperatively, but there was no statistical difference. A large sample study, which involved 1155 patients (≥60 years’ old), showed that BIS-guided anesthesia had an active effectiveness in reducing the rate of delirium 1 week and 3 months after operation, but not of POCD.[21] All of these studies suggest that elderly patients can get benefit of a reduction of cognitive dysfunction from intraoperative anesthetic monitoring. Although the incidence of POCD is lower in BIS group than the control group in our study (15.15% vs. 33.33%), there is no statistical significance. The study of the rate of early POCD after LT is rare. Li et al[22] reported that 44% patients had POCD 7 days after LT. His diagnostic criteria of POCD are as the same as ours. The rate of early POCD is lower in our study compared with his report, and the neuropsychological tests were mildly abnormal in 5 POCD patients. Maybe such result thanks to optimizing intraoperative management.
Optimizing patients during surgery was stated firstly by Kehlet in 1997, defined as enhanced recovery after surgery (ERAS).[23] It has been demonstrated that ERAS program could enhance postoperative recovery, shorter hospital length of stay, and reduce morbidity by adopting a series of optimization measures recommended by evidence-based medicine during the perioperative period.[24,25]
ERAS programs have also been used during hepatic surgery.[26,27] A meta-analysis result suggested that implementation of ERAS programs is safe and effective in liver surgery. Compared with traditional care, ERAS programs resulted in a significant reduction in complications and the length of hospital stay. But the literature supporting operative optimization to improve cognitive outcomes is sparse. Given the clear benefit of ERAS in colorectal surgery, it is difficult to justify performing large randomized and well-controlled studies of such protocols for LT. BIS-guided anesthesia as one of ERAS programs is not only the monitoring of the depth of anesthesia, but influencing of cardiovascular, internal environment, hepatic and renal insufficiency, and so on. That needs to be proved by more research and evidence-based medicine. Another meta-analysis included 10,761 patients evaluated the effect on BIS-guided anesthesia. It had benefits on reducing time to extubation and discharge from both the operating room and postanesthetic care unit. The rate of nausea and vomiting and cognitive impairment also reduced.[28] In our study, MAP was lower in the control group at 30 minutes after the start of the anhepatic phase (T2) compared with BIS group (Table 2). The result indicated that BIS-guided anesthesia could avoid low blood pressure during surgery.
S100β protein and NSE are well known as potential markers of neural injury.[8–12] Some studies have identified POCD using serum levels of S100β protein and NSE. But the relationship of S100β protein and NSE serum concentration with POCD is inconsistent and inconclusive. A correlation between neuropsychological function and the serum level of S100β protein and NSE has been found in patients after cardiac surgery[29–34]; such similar relationship was also found in noncardiac surgery. Linstedt et al found the higher serum concentrations of S100β in POCD patients compared with those without POCD 30 minutes postoperatively in abdominal and vascular surgery, but not in urological surgery. However, NSE was unchanged during the course of the study.[35] Another study including 149 patients who underwent shoulder surgery showed that there was no relationship between concentrations of S100β and NSE and POCD.[36] In our study, we detected S100β protein and NSE concentrations in the BIS group during surgery. We found that S100β and NSE increased significantly at 30 minutes after the start of the anhepatic phase and 15 minutes after reperfusion of the new liver. Pathophysiology is the most changeful during anhepatic phase and new liver phase of surgery. Cerebral metabolism is affected by blocking of portal vein or vena cava during anhepatic phase and ischemia-reperfusion injury during new liver phase. There is an undiscovered cerebral injury which could be detected by serum markers such as S100β and NSE during surgery. But in our study, the increased concentrations of S100β protein recovered soon at 24 hours after surgery; the concentration of NSE was still higher compared with induction of general anesthesia. These results may have connection with their metabolic character. The highest S100β protein serum level was observed just after an injury[37] and was then normalized within 24 hours, even in patients with poor outcomes.[38] But the half-life of NSE is 24 hours.[39]
In our study, POCD group and non-POCD group had no difference in S100β at 5 time points. But NSE in POCD group was still higher at 24 hours after surgery. Observations of patients undergoing LT revealed that the postreperfusion concentration of NSE correlated with decreased regional oxygen saturation.[39] The reason for this remains unclear, and the number of patients is not sufficient for statistical power.
The important limitations of this study include small sample size and nonparallel control. The study seems to suggest a lower incidence of POCD in BIS group compared to control group, but no statistical significance, which might be attributable to small sample size. However, it is really difficult for any medical center to enroll enough patients who underwent liver transplant. We have performed BIS-guided anesthesia instead of conventional one at our center since 2014, so we have to use historical data as control in the present study. Moreover, small sample size may weaken the importance of NSE in prediction of POCD. Although NSE in POCD group was higher at 24 hours after surgery than non-POCD group, its identity of reliable predictors of POCD needs more research because we are not so sure about the number of patients for statistical power and repeatability.
The advantages of the ERAS have been confirmed by many studies. The focus of the present study is on finding the specific measures of the ERAS. Anesthesia depth monitoring is an important measure of ERAS to prevent POCD. Although the etiology and pathogenesis of POCD are not yet clear, we can use effective means to prevent it, which is the meaning of this study.
5. Conclusion
In conclusion, our study finds that BIS-guided anesthesia can reduce consumption of propofol during anhepatic and new liver phase. Patients in BIS group seem to have a mild lower incidence of POCD compared to controls, but no statistical significant. The influence of BIS-guided anesthesia on POCD needs to be further confirmed by large-scale clinical study. Furthermore, S100β protein and NSE are well correlated with neural injury, but NSE is more suitable for assessment of incidence of postoperative cognitive deficits after surgery.
Footnotes
Abbreviations: BIS = bispectral index, LT = liver transplantation, NSE = neuron-specific enolase, POCD = postoperative cognitive dysfunction.
The authors report no conflicts of interest.
References
- [1].Starzl TE, Marchioro TL, Vonkaulla KN, et al. Homo transplantation of the liver in humans. Surg Gynecol Obstet 1963;117:659–76. [PMC free article] [PubMed] [Google Scholar]
- [2].Langham MR, Jr, Tzakis AG, Gonzalez-Peralta R, et al. Graft survival in pediatric liver transplantation. J Pediatr Surg 2001;36:1205–9. [DOI] [PubMed] [Google Scholar]
- [3].Azoulay D, Samuel D, Adam R, et al. Paul brousse liver transplantation: the first 1,500 cases. Clin Transpl 2000;273–80. [PubMed] [Google Scholar]
- [4].Liebert AD, Chow RT, Bicknell BT, et al. Neuroprotective effects against POCD by photobiomodulation: evidence from assembly/disassembly of the cytoskeleton. J Exp Neurosci 2016;10:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang W, Wang Y, Wu H, et al. Postoperative cognitive dysfunction: current developments in mechanism and prevention. Med Sci Monit 2014;20:1908–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Aceto P, Perilli V, Lai C, et al. Postoperative cognitive dysfunction after liver transplantation. Gen Hosp Psychiatry 2015;37:109–15. [DOI] [PubMed] [Google Scholar]
- [7].Federico B, Ega Q, Idit M. Anesthesia and cognitive disorders: a systematic review of the clinical evidence. Expert Rev Neurother 2016;16:1311–20. [DOI] [PubMed] [Google Scholar]
- [8].Scazfca M, Almeida OP, Vallada HP, et al. Limitations of the mini-Mental State Examination for screening dementia in community with low socioeconomic status: results from the Sao Paulo Ageing & Health Study. Eur Arch Psychiatry Clin Neurosci 2009;259:8–15. [DOI] [PubMed] [Google Scholar]
- [9].Ali MS, Harmer M, Vaughan R. Serum S100 protein as a marker of cerebral damage during cardiac surgery. Br J Anaesth 2000;85:287–98. [DOI] [PubMed] [Google Scholar]
- [10].Lima JE, Takayanagui OM, Garcia LV, et al. Use of neuron-specific enolase for assessing the severity and outcome of neurological disorders in patients. Braz J Med Biol Res 2004;37:19–26. [DOI] [PubMed] [Google Scholar]
- [11].Schmidt FM, Mergl R, Stach B, et al. Elevated levels of cerebrospinal fluid neuron-specific enolase (NSE) in Alzheimer's disease. Neurosci Lett 2014;570:81–5. [DOI] [PubMed] [Google Scholar]
- [12].Bekker A, Haile M, Kline R, et al. The effect of intraoperative infusion of dexmedet omidine on the quality of recovery after major spinal surgery. J Neurosurg Anesthesiol 2013;25:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Folstein MF, Folstein SE, McHugh PR. Mini-mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [14].Funder KS, Steinmetz J, Rasmussen LS. Detection of postoperative cognitive dysfunction. Ugeskr Laeger 2010;172:449–52. [PubMed] [Google Scholar]
- [15].Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet 1955;259–63. [DOI] [PubMed] [Google Scholar]
- [16].Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of PostOperative Cognitive Dysfunction. Lancet 1998;351:857–61. [DOI] [PubMed] [Google Scholar]
- [17].Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- [18].Jildenstål PK, Hallén JL, Rawal N, et al. Effect of auditory evoked potential-guided anaesthesia on consumption of anaesthetics and early postoperative cognitive dysfunction: a randomised controlled trial. Eur J Anaesthesiol 2011;28:213–9. [DOI] [PubMed] [Google Scholar]
- [19].Ballard C, Jones E, Gauge N, et al. Optimised anaesthesia to reduce post operative cognitive decline (POCD) in older patients undergoing elective surgery, a randomised controlled trial. PLoS One 2012;7:e37410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chan MT, Cheng BC, Lee TM, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013;25:33–42. [DOI] [PubMed] [Google Scholar]
- [21].Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013;110:98–105. [DOI] [PubMed] [Google Scholar]
- [22].Li X, Wen DX, Zhao YH, et al. Increase of beta-amyloid and C-reactive protein in liver transplant recipients with postoperative cognitive dysfunction. Hepatobiliary Pancreat Dis Int 2013;12:370–6. [DOI] [PubMed] [Google Scholar]
- [23].Kehlet H, Slim K. The future of fast-track surgery. Br J Surg 2012;99:1025–6. [DOI] [PubMed] [Google Scholar]
- [24].Kehlet H, Wilmore DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189–98. [DOI] [PubMed] [Google Scholar]
- [25].Ni TG, Yang HT, Zhang H, et al. Enhanced recovery after surgery programs in patients undergoing hepatectomy: a meta-analysis. World J Gastroenterol 2015;21:9209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hendry PO, van Dam RM, Bukkems SF, et al. Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 2010;97:1198–206. [DOI] [PubMed] [Google Scholar]
- [27].Dam RM, Hendry PO, Coolsen MM, et al. Initial experience with a multimodal enhanced recovery programme in patients undergoing liver resection. Br J Surg 2008;95:969–75. [DOI] [PubMed] [Google Scholar]
- [28].Oliveira CR, Bernardo WM, Nunes VM. Benefit of general anesthesia monitored by bispectral index compared with monitoring guided only by clinical parameters. Systematic review and meta-analysis. Braz J Anesthesiol 2017;67:72–84. [DOI] [PubMed] [Google Scholar]
- [29].Anderson RE, Hansson LO, Vaage J. Release of S100B during coronary artery bypass grafting is reduced by off-pump surgery. Ann Thorac Surg 1999;67:1721–5. [DOI] [PubMed] [Google Scholar]
- [30].Blomquist S, Johnssohn P, Lührs C, et al. The appearance of S-100 protein in serum during and immediately after cardiopulmonary bypass surgery: a possible marker for cerebral injury. J Cardiothorac Vasc Anesth 1997;11:699–703. [DOI] [PubMed] [Google Scholar]
- [31].Grocott HP, Croughwell ND, Amory DW, et al. Cerebral emboli and serum S100b during cardiac operations. Ann Thorac Surg 1998;65:1645–50. [DOI] [PubMed] [Google Scholar]
- [32].Johnsson P. Markers of cerebral ischemia after cardiac surgery. J Cardiothorac Vasc Anesth 1996;10:120–6. [DOI] [PubMed] [Google Scholar]
- [33].Johnsson P, Lundquist C, Lindgren A, et al. Cerebral complications after cardiac surgery assessed by S-100 and NSE levels in blood. J Cardiothorac Vasc Anaesth 1995;9:694–9. [DOI] [PubMed] [Google Scholar]
- [34].Rasmussen LS, Christiansen M, Hansen PB, et al. Do blood levels of neuron specific enolase and S-100 protein reflect cognitive dysfunction after coronary artery bypass? Acta Anaesthesiol Scand 1999;43:495–500. [DOI] [PubMed] [Google Scholar]
- [35].Linstedt U, Meyer O, Kropp P, et al. Serum concentration of S-100 protein in assessment of cognitive dysfunction after general anesthesia in different types of surgery. Acta Anaesthesiol Scand 2002;46:384–9. [DOI] [PubMed] [Google Scholar]
- [36].Rappold T, Laflam A, Hori D, et al. Evidence of an association between brain cellular injury and cognitive decline after non-cardiac surgery. Br J Anaesth 2016;116:83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ingebrigtsen T, Romner B. Biochemical serummarkers forbrain damage: a short review with emphasis on clinical utility in mild head injury. Restor Neurol Neurosci 2003;21:171–6. [PubMed] [Google Scholar]
- [38].Kleindienst A, Bullock MR. A critical analysis of the role of the neurotrophic protein S100B in acute brain injury. J Neurotrauma 2006;23:1185–200. [DOI] [PubMed] [Google Scholar]
- [39].Cata JP, Abdelmalak B, Farag E. Neurological biomarkers in the perioperative period. Br J Anaesth 2011;10:844–58. [DOI] [PubMed] [Google Scholar]


