Abstract
Rationale:
Clostridium difficile bacteremia (CDB) and liver abscess is a quite rare presentation of C. difficile infection.
Patients concerns:
A 74-year-old male with primary biliary cirrhosis and hepatocellular carcinoma who underwent transarterial chemoembolization (TACE) developed a high fever on post-TACE day 14. Intravenous ceftriaxone and following meropenem were administered, however, his clinical response was poor. On post-TACE day 24, 2 sets of blood culture were taken due to elevation of C-reactive protein levels.
Diagnosis:
CDB, caused by bacterial translocation.
Interventions:
Intravenous vancomycin and oral metronidazole were administered for two weeks.
Outcomes:
One month after recurrent CDB, the patient was re-admitted due to a liver abscess at the same site of TACE. C. difficile was isolated from the liver abscess and the patient received 6 weeks of oral metronidazole treatment. CDB and liver abscess have not recurred since completion of antibiotic treatment.
Lessons:
The spore-forming ability of C. difficile may contributed to the recurrent CDB episodes and liver abscess formation in necrotic liver tissue following TACE, and long-term metronidazole therapy was considered to be effective to C. difficile liver abscess.
Keywords: Clostridium difficile, Clostridium difficile bacteremia, extraintestinal Clostridium difficile infection, liver abscess, recurrent Clostridium difficile bacteremia
1. Introduction
Clostridium difficile is a well-known anaerobic, spore-forming pathogen that causes transmissible antibiotic-associated diarrhea and is one of the most common healthcare-associated infections worldwide. Thus, treatment and prevention of C difficile infection (CDI) remains a challenging issue worldwide.[1]
In contrast to typical CDI, extraintestinal manifestation of CDI (Ex-CDI) has been rarely reported.[2,3]Clostridium difficile bacteremia (CDB) is considered a severe form of Ex-CDI, and several case series have been reported.[4,5] To our knowledge, only 2 cases of liver abscess caused by C difficile have been reported.[6,7] Herein, we present a case of recurrent bacteremia and liver abscess caused by C difficile.
2. Case report
A 74-year-old male with primary biliary cirrhosis (PBC) complicated with hepatocellular carcinoma (HCC) from 6 years previously was admitted for transarterial chemoembolization (TACE) of segment 2 (S2) and S7 for HCC. PBC was diagnosed at the age of 60 years, and then HCC developed in S8 and S6 during follow-up periods in prior hospital. Subsegmentectomy of S8 and radiofrequency ablation of HCC in S6 had been performed at the age of 68 and 70 years, respectively. Since then, the patient was referred to our hospital to follow up PBC and HCC. Two months before admission, antibiotics had been prescribed to the patient at another hospital, although specific details were unavailable. However, rabeprazole was known to have been prescribed to the patient before admission.
TACE of S2 (12 mm in diameter) and S7 (13 mm in diameter) was performed with combination epirubicin and lipiodol following gelatin-sponge particle. The patient developed a fever >39°C on post-TACE day 14, and he had no specific symptoms without fever. Intravenous ceftriaxone (2000 mg q24 h) was administered after collecting blood cultures. Fever still persisted, and thus, meropenem (1000 mg q8 h) was administered to treat suspected bacterial infection caused by drug-resistant gram-negative rods and anaerobes on the TACE day 16. However, a computed tomography (CT) scan at this point did not reveal any focus of infection (Fig. 1A). Blood culture results were negative. Due to his poor clinical response and new onset of diarrhea (2–3 times/day), meropenem was discontinued on post-TACE day 21. Glutamate dehydrogenase (GDH) in a stool specimen was negative at this time, and a stool culture was not performed. On post-TACE day 24, the patient was afebrile; however, he complained of mild increased abdominal distension. His C-reactive protein levels were elevated up to 16.9 mg/dL (normal range: ≤0.3 mg/dL); thus, 2 sets of blood cultures were taken to rule out bacteremia. Both of anaerobic blood cultures became positive after 13-hour incubation. Gram staining revealed long and thin gram-positive rods. A CT scan revealed edematous colon and increased ascites without free air, and slightly decreased lipiodol accumulation. Ampicillin/sulbactam was subsequently started after collecting an ascites specimen for culture and analysis. The neutrophil count in the ascites was 5504/μL (total cell count: 5520/μL). Gram-positive rods were detected in blood cultures and identified as C difficile by conventional identification and matrix-assisted laser desorption/ionization time-of flight mass spectrometry (MALDI-TOF MS) using the VITEK MS system (Sysmex bioMérieux Co., Ltd, Tokyo, Japan). Ampicillin sulbactam was subsequently switched to intravenous vancomycin (1000 mg q12 h) and oral metronidazole (250 mg QID). At this time, C. DIFF QUIK CHEK COMPLETE (Tech Lab, Blacksburg, VA) showed positivity for GDH and toxin in the stool; however, no pathogen was detected from the ascites culture. Though vancomycin was discontinued on day 9 due to nephrotoxicity, oral metronidazole was continued for 14 days. The patient had no apparent diarrhea since then. Twelve days after discontinuation of oral metronidazole (post-TACE day 48), he suddenly developed a high fever. Clostridium difficile was again isolated from 2 sets of anaerobic blood cultures at this time. A CT scan and transthoracic echocardiogram did not reveal any specific findings, including colon and liver findings. Oral metronidazole (250 mg QID) was administered for 7 days and then switched to oral vancomycin (125 mg QID, total 41 day of administration).
Figure 1.
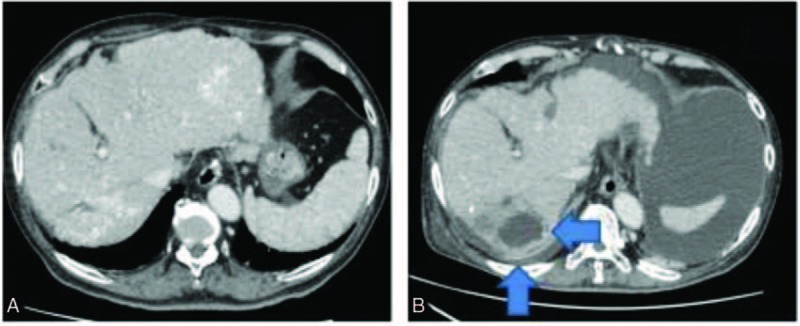
CT scan of the patient's liver showed lipiodol accumulation ((A) post-TACE day 14), and liver abscess formation (arrow) of S7 ((B) post-TACE day 87). CT = computed tomography, TACE = transarterial chemoembolization.
One month after recurrent CDB, the patient was re-admitted due to a liver abscess at the same site of TACE in S7 (post-TACE day 87, Fig. 1B). Intravenous ceftriaxone (2000 mg q24 h) was administered empirically. Three days later, needle aspiration of the liver abscess was performed, and C difficile was solely isolated from the aspirates. Blood cultures on admission were negative. One week later, the patient's fever persisted, and thus drainage of the liver abscess was performed. Gram staining of the pus showed a small number of gram-positive rods, and C difficile was isolated from a second drainage specimen. After re-isolation of C difficile, ceftriaxone and oral vancomycin were switched to oral metronidazole (250 mg QID, post-TACE day 103), and the patient's condition gradually improved. Metronidazole was continued for 6 weeks. CDB and liver abscess have not reoccurred since completion of metronidazole treatment.
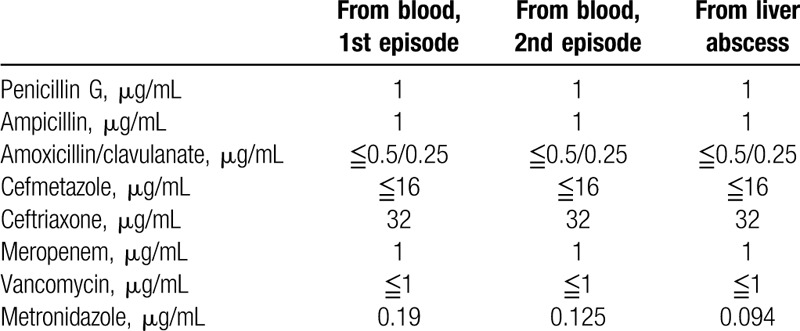
The sequential CT findings of S7 are shown in Fig. 1. Table 1 shows the results of susceptibility testing of C difficile strains. Antimicrobial susceptibility testing of C difficile was performed twice using a “dry plate EIKEN” (Eiken Chemical Co., Ltd, Tokyo, Japan) for 7 antibiotics by the microbroth dilution method. Susceptibility testing for metronidazole was performed using Etest strips (Sysmex bioMérieux Co., Ltd, Tokyo, Japan). Clostridium difficile toxin A and B and binary toxin production of strains isolated from the blood and liver abscess were identified by PCR using nonrepeating sequences of toxins A and B and the repeating sequence of toxin A and cdt (binary toxin).[8,9] All strains tested were positive for toxin A, toxin B, and binary toxin.
Table 1.
Results of susceptibility testing of C difficile isolates.
3. Discussion
The frequency of Ex-CDI comprises approximately 0.17% to 0.6% of all CDI.[2,3] Among Ex-CDI, CDB is the most frequently reported presentation. Lee et al reported 12 cases of CDB during a 20-year period, the current largest case series in the world. They reported all patients had chronic medical illnesses, especially diabetes mellitus and liver cirrhosis.[4] Antibiotic exposure and proton pump inhibitor use are also thought to be risk factors of CDB.[2] All reported CDB cases were classified as healthcare-associated infections. Primary bacteremia comprised half of CDB cases, followed by secondary bacteremia of intra-abdominal origin. Approximately half of CDB cases were polymicrobial bacteremia.[4] Toxin-nonproducing strains also caused CDB; thus, factors other than C difficile toxin may contribute to the development of bacteremia.[4,10] Susceptibility testing of C difficile is not standardized for clinical use; however, all C difficile strains isolated from blood were susceptible to vancomycin and metronidazole, whereas 90% of isolates were resistant to penicillin.[4] Therefore, intravenous vancomycin or systemic metronidazole may be superior to other antimicrobials.
Liver abscess formation is a well-known severe complication of TACE. Lv et al[11] reported 21 liver abscesses per 11,054 TACE procedures. TACE-related liver abscesses were diagnosed within 11 to 23 days after TACE, and 57.1% of the patients had a history of bilioenteric anastomosis or biliary stent placement. Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, and Enterococcus faecalis are commonly isolated from blood and aspirate cultures of such patients, similar to patients with liver abscess.[11,12] A few cases of liver abscess caused by Clostridium perfringens were found in the literature, and these cases developed within 2 to 5 days after TACE.[13,14] No other Clostridium species have been reported to cause liver abscess as a complication of TACE.
This case showed a unique clinical presentation. To our knowledge, this is the first case of recurrent CDB and C difficile liver abscess as a complication of TACE. The present patient had characteristic risk factors for CDB: liver cirrhosis, HCC, antibiotic exposure, and proton pump inhibitor use.[2,4] However, liver abscess formation as a complication of TACE was atypical as the patient did not undergo bilioenteric anastomosis and the onset of disease and causative organism were unusual.[11,12] We speculate that the first CDB episode was caused by bacterial translocation due to C difficile overgrowth in the intestine, which also caused spontaneous bacterial peritonitis based on ascites findings and CT findings of the colon. There are 2 potential etiologies of recurrent CDB: (1) early stage liver abscess, whose dissolution and liquefaction were not complete, led to recurrent CDB due to short treatment duration; (2) treatment failure of intestinal CDI led to overgrowth of C difficile in the intestine and bacterial translocation or ascending cholangitis. The latter etiology was thought to be less likely because of the lack of diarrhea and non-specific CT findings of the colon. The first CDB episode might have subsequently resulted in C difficile colonization of necrotic liver tissue following TACE, and the spore-forming ability of C difficile may have allowed it to survive during antimicrobial therapy. Long-term metronidazole therapy was considered to be effective because of excellent penetration to the liver and susceptibility of isolated C difficile strains.
CDIs are becoming more frequent worldwide. Ex-CDI may also be increasing; however, the precise trend of Ex-CDI is unclear. Clinicians may be confused when they encounter Ex-CDI because it is rare. A greater accumulation of Ex-CDI cases is necessary to determine its epidemiology for the proper treatment of Ex-CDI, including recurrent CDB and liver abscess.
Acknowledgment
The authors thank Daisuke Sakanashi and Narimi Miyazaki (the Division of Microbiological Laboratory, Department of Infection Control and Prevention, Aichi Medical University) for their valuable assistance with analyzing C difficile toxin production.
Footnotes
Abbreviations: CDB = Clostridium difficile bacteremia, CDI = Clostridium difficile infection, CT = computed tomography, Ex-CDI = extraintestinal manifestation of Clostridium difficile infection, GDH = glutamate dehydrogenase, HCC = hepatocellular carcinoma, MALDI-TOF MS = matrix-assisted laser desorption/ionization time-of flight mass spectrometry, PBC = primary biliary cirrhosis, S = segment, TACE = transarterial chemoembolization.
Consent: This study adhered to the tenets of the Declaration of Helsinki, and ethic committee of Nagoya University Graduate School of Medicine (No. 8728). Informed consent was obtained from the patient for publication of this case and its related images.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Leffler DA, Lamont JT. Clostridium difficile infection. N Engl J Med 2015;372:1539–48. [DOI] [PubMed] [Google Scholar]
- [2].Gupta A, Patel R, Baddour LM, et al. Extraintestinal Clostridium difficile infections: a single-center experience. Mayo Clinic Proc 2014;89:1525–36. [DOI] [PubMed] [Google Scholar]
- [3].Mattila E, Arkkila P, Mattila PS, et al. Extraintestinal Clostridium difficile infections. Clin Infect Dis 2013;57:e148–53. [DOI] [PubMed] [Google Scholar]
- [4].Lee NY, Huang YT, Hsueh PR, et al. Clostridium difficile bacteremia, Taiwan. Emerg Infect Dis 2010;16:1204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Libby DB, Bearman G. Bacteremia due to Clostridium difficile—review of the literature. Int J Infect Dis 2009;13:e305–9. [DOI] [PubMed] [Google Scholar]
- [6].Sakurai T, Hajiro K, Takakuwa H, et al. Liver abscess caused by Clostridium difficile. Scand J Infect Dis 2001;33:69–70. [DOI] [PubMed] [Google Scholar]
- [7].Ulger Toprak N, Balkose G, Durak D, et al. Clostridium difficile: a rare cause of pyogenic liver abscess. Anaerobe 2016;42:108–10. [DOI] [PubMed] [Google Scholar]
- [8].Kato H, Kato N, Watanabe K, et al. Identification of toxin A-negative, toxin B-positive Clostridium difficile by PCR. J Clin Microbiol 1998;36:2178–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stubbs S, Rupnik M, Gibert M, et al. Production of actin-specific ADP-ribosyltransferase (binary toxin) by strains of Clostridium difficile. FEMS Microbiol Lett 2000;186:307–12. [DOI] [PubMed] [Google Scholar]
- [10].Elliott B, Reed R, Chang BJ, et al. Bacteremia with a large clostridial toxin-negative, binary toxin-positive strain of Clostridium difficile. Anaerobe 2009;15:249–51. [DOI] [PubMed] [Google Scholar]
- [11].Lv WF, Lu D, He YS, et al. Liver abscess formation following transarterial chemoembolization: clinical features, risk factors, bacteria spectrum, and percutaneous catheter drainage. Medicine 2016;95:e3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Woo S, Chung JW, Hur S, et al. Liver abscess after transarterial chemoembolization in patients with bilioenteric anastomosis: frequency and risk factors. AJR Am J Roentgenol 2013;200:1370–7. [DOI] [PubMed] [Google Scholar]
- [13].Li JH, Yao RR, Shen HJ, et al. Clostridium perfringens infection after transarterial chemoembolization for large hepatocellular carcinoma. World J Gastroenterol 2015;21:4397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Oshima S, Takaishi K, Tani N, et al. Two cases of liver abscess caused by Clostridium perfringens after transcatheter arterial chemoembolization. Cancer Chemother 2013;40:1795–7. [PubMed] [Google Scholar]


