ABSTRACT
The development of a functional biomarker assay in the tuberculosis (TB) field would be widely recognized as a major advance in efforts to develop and to test novel TB vaccine candidates efficiently. We present preliminary studies using mycobacterial growth inhibition assays (MGIAs) to detect Mycobacterium bovis BCG vaccine responses across species, and we extend this work to determine whether a standardized MGIA can be applied in characterizing new TB vaccines. The comparative MGIA studies reviewed here aimed to evaluate robustness, reproducibility, and ability to reflect in vivo responses. In doing so, they have laid the foundation for the development of a MGIA that can be standardized and potentially qualified. A major challenge ahead lies in better understanding the relationships between in vivo protection, in vitro growth inhibition, and the immune mechanisms involved. The final outcome would be a MGIA that could be used with confidence in TB vaccine trials. We summarize data arising from this project, present a strategy to meet the goals of developing a functional assay for TB vaccine testing, and describe some of the challenges encountered in performing and transferring such assays.
KEYWORDS: mycobacterial growth inhibition assay, MGIA, tuberculosis, correlates of immunity, vaccines
INTRODUCTION
The tuberculosis (TB) vaccine field is severely hampered by the lack of defined immune parameters that correlate with vaccine efficacy in human studies. There has been recent progress, such as the finding that antigen-specific gamma interferon (IFN-γ) enzyme-linked immunosorbent spot assay (ELISpot) responses correlate with reduced risk of developing disease in Mycobacterium bovis BCG-vaccinated South African infants (1). However, heterogeneous BCG vaccine responses are observed and may be associated with the immune environment at the time of vaccination, including the proportions of monocytes and activated HLA-DR+ CD4+ T cells (1, 2). Development of a validated functional biological response assay capable of assessing the potential of a vaccine-induced immune response to protect against Mycobacterium tuberculosis infection or TB disease would represent a major advance in the effort to develop TB vaccines. Such an assay would circumvent the need to identify or fully understand the specific aspects of the immune response contributing to this effect. Mycobacterial growth inhibition assays (MGIAs) represent one such clinically relevant approach. Using whole blood or peripheral blood mononuclear cells (PBMCs), MGIAs offer an unbiased measure of the vaccine-induced antigen-specific immune response, taking into account the ability of this response to function in its respective immune environment. MGIAs also have the potential to decrease the time and resources required to evaluate a new TB vaccine in preclinical studies.
The MGIA project brought together an international group of scientific experts to work on growth inhibition assays from 2010 to 2014. Participants included researchers from regions in which TB is endemic, such as Cape Town, South Africa, and areas in which TB is not endemic, such as the United Kingdom and Washington, DC, St. Louis, MO, New York, and Colorado in the United States.
GOALS OF THE MGIA PROJECT AND ASSAY SELECTION
The MGIA project was initially supported by a World Health Organization (WHO) working group and was viewed as the follow-up project to a whole-blood enzyme-linked immunosorbent assay (ELISA) that the WHO had recommended previously (3). The aim was to develop a functional assay that could be applied in all clinical trials of TB vaccine candidates, thus aiding the identification of correlates of immunity. Although several MGIAs had been described previously in the literature (recently reviewed in reference 4), little work had been done on qualifying an assay that could be transferred across laboratories, using a standardized reproducible method for vaccine assessment. This was a major goal of the project, which was funded by Aeras and the U.S. Food and Drug Administration (FDA). Prior to 2010, most MGIA development had focused on whole-blood systems and had shown promise (5–10). However, especially given the logistical challenges associated with real-time processing of fresh blood samples in clinical vaccine trials, efforts were made in this project to assess the potential of using cryopreserved PBMCs and to compare the outcomes with those from whole blood. The specific early goals were as follows: (i) to assess the variability, reproducibility, and transferability of four different MGIAs in naive, BCG-vaccinated, and BCG-revaccinated subjects; (ii) to compare PBMC and whole-blood MGIA responses induced by wild-type BCG and a new recombinant BCG vaccine; (iii) to determine the effect of M. tuberculosis infection status on inhibition of mycobacterial growth in a population where TB is endemic (South Africa); (iv) to test sera obtained from subjects enrolled in the enhanced BCG vaccine MGIA study for antibodies to capsular and cell surface constituents, including major polysaccharide antigens, and to compare the preimmunization and postimmunization antibody levels with MGIA outcomes; and (v) to explore other immune mechanisms underlying mycobacterial growth inhibition (e.g., by correlation with ELISpot and intracellular cytokine staining flow analyses of blood samples from the BCG study cohorts).
Although four previously reported MGIAs were considered for initial evaluation, a whole-blood assay based on the methods of Wallis et al. was selected as a focus for this project, using the Bactec mycobacteria growth indicator tube (MGIT) system to quantitate mycobacterial growth (5) (referred to here as the direct MGIA). The rationale for this decision included the relative simplicity of the assay and its use of standardized reagents and equipment that are readily available in many clinical TB laboratories. Furthermore, the assay had been used previously to demonstrate antimycobacterial activity of bactericidal drugs that correlated with sterilizing activity observed during in vivo therapy (5, 11). The Bactec MGIT system has several advantages over traditional CFU-based methods of mycobacterial quantification, as it utilizes oxygen depletion as a detection method that is sensitive not only to the number of viable bacteria but also to their rates of metabolism and growth. It is unaffected by clumping and does not require serial dilutions, providing an accurate computer-generated readout based on validated technology.
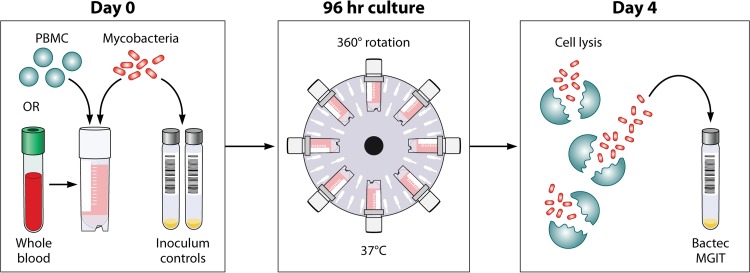
It was also agreed that an adaptation of this assay using cryopreserved PBMCs should be developed and evaluated for comparison with whole-blood results. It is widely accepted that the cellular immune response is central to protection against M. tuberculosis and, although innate whole-blood components such as neutrophils are known to play a role in the host response, they may be less relevant for vaccine-induced effects on adaptive immunity. Furthermore, the use of cryopreserved cells could aid in transferability of the assay to different trial sites, eliminating the need to evaluate samples in real time using fresh material. Rather, cells could be stored and analyzed in the future, which not only is logistically simpler but also would negate the need for a Bactec MGIT system at every site. Finally, the ability to use cryopreserved cells would enable additional retrospective studies of samples from historical clinical vaccine trials, for validation and exploratory work. A schematic diagram and a description of the method for the direct MGIA are provided in Fig. 1.
FIG 1.
Schematic of the MGIT mycobacterial growth inhibition assay method. Whole blood or cell culture medium containing the appropriate concentration of PBMCs (or mouse splenocytes in mouse studies) is inoculated with an equal volume of mycobacteria at a low MOI (∼1 CFU/10,000 PBMCs). Cultures are incubated in 2-ml tubes at 37°C for 96 h, with 360° rotation. Cells are then lysed to remove red blood cells and/or to release intracellular mycobacteria. The lysate is inoculated into Bactec MGITs. The tubes are placed in the Bactec 960 system and incubated at 37°C until positivity is detected by fluorescence. On day 0, direct-to-MGIT viability controls are set up by directly inoculating MGIT tubes with the same volume of mycobacteria as the samples. The TTP data are converted to log10 CFU values using a standard curve, and the final values are expressed as absolute CFU values or values relative to the control value, indicating the amount of growth inhibition that occurred during the culture period.
The studies involved in this project are presented chronologically; work began using samples from clinical studies (as the ultimate goal was development of a human assay), but the challenges encountered resulted in a decision to focus on preclinical adaptations in the latter stages. One of the greatest impediments to initial MGIA developmental efforts using human samples was the lack of availability of sufficiently large single whole-blood or PBMC samples from recently BCG-vaccinated volunteers; this was required for assay optimization and assessment of reproducibility between laboratories. Using animal samples such as mouse splenocytes, however, it is possible to overcome this issue, as 50 million to 100 million splenocytes may be isolated from each mouse. Furthermore, the ability to select age- and sex-matched naive inbred animals removes a number of potential sources of variability found in human clinical trials. Animal models also provide the opportunity for biological validation by correlating assay outcomes with protection from in vivo challenges with pathogenic M. tuberculosis (see below).
Participants in the BCG vaccination and revaccination study were recruited under a protocol approved by the Oxfordshire Research Ethics Committee. Written informed consent was obtained from all individuals prior to enrollment in the trial. Human studies performed at the South African Tuberculosis Vaccine Initiative (SATVI) were approved by the Human Research Ethics Committee of the University of Cape Town. All adult participants provided written informed consent prior to participation in the studies. In the case of children, written informed consent was provided by a parent or legal guardian. All of the work at Saint Louis University was approved by the Saint Louis University Institutional Review Board, and PBMCs from independent healthy BCG-vaccinated subjects were collected under a protocol approved by the Institutional Review Board of the Albert Einstein College of Medicine. All animal studies were approved by the appropriate institutions, which used IACUC-approved protocols and methods. At Public Health England, all animal procedures and the study design were approved by the Public Health England, Porton Down, Ethical Review Committee and were authorized under an appropriate U.K. Home Office project license.
EARLY OPTIMIZATION WORK
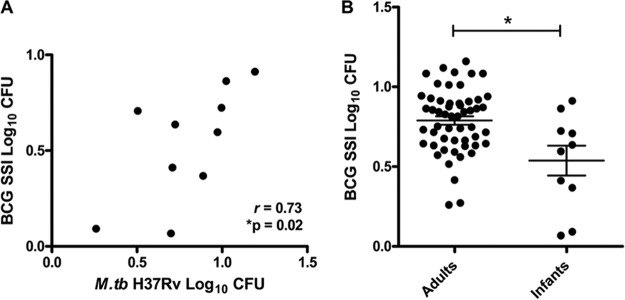
BCG was selected as a surrogate for M. tuberculosis in the majority of these studies to avoid the need for biosafety level 3 (BSL3) facilities. It was established previously that measures of growth inhibition of BCG correlated with those of M. tuberculosis H37Rv in a murine MGIA (12), and this was confirmed in the direct MGIA (Fig. 2A). Early experiments using the direct PBMC MGIA indicated high intra-assay variability between replicate cultures. This was addressed by considering various aspects of assay preparation, the culture period, and 96-hour processing. It was determined that the standard antibiotic supplement (penicillin and streptomycin) in the culture medium should not be used at any stage of sample processing after thawing, due to the effect of artificially reducing the inoculum (13). However, this altered the multiplicity of infection (MOI), such that the vaccine effect was overwhelmed. A smaller inoculum resulted in increased intra-assay variability. To maintain the same initial inoculum volume, a lower MOI was achieved by using greater cell concentrations; although this did improve reproducibility, a balance had to be reached, given the limiting factor of cell numbers in many clinical trials. Experiments altering the MOI in the direct splenocyte MGIA have been described (14). Once the optimal MOI was identified, variability in the inoculum itself was addressed by comparing different declumping methods, such as vortex-mixing, filtering, and sonicating. Vortex-mixing with glass beads resulted in low intra-assay variability while retaining high recovery of CFU. Other variables examined included BCG strain (14), culture rotation methods, and lysis agents. Variability between sites in whole-blood MGIA performance has yet to be defined but could result from factors such as differences in blood anticoagulants and inoculum strains.
FIG 2.
Direct whole-blood MGIA for South African infants. (A) Whole-blood samples from 10 South African infants were assessed using the direct whole-blood MGIA. There was a significant correlation between growth of BCG SSI and M. tuberculosis (M.tb) H37Rv (Spearman's r = 0.73; P = 0.02). (B) Whole-blood samples from 10 South African infants were compared with those from 53 South African adults using the direct whole-blood MGIA. There was a significant reduction in growth of BCG SSI in infants, compared with adults. *, P < 0.05, Mann-Whitney U test between groups.
HUMAN MGIA STUDIES
BCG vaccination and revaccination.
A clinical trial of BCG vaccination and revaccination in U.K. adults was conducted at the University of Oxford (13). The direct PBMC MGIA detected a significant improvement in mycobacterial growth inhibition following primary BCG vaccination, consistent with epidemiological data on the efficacy of BCG in this population. No improvement in mycobacterial growth inhibition was observed following BCG revaccination, using either the whole-blood or PBMC assay (13). This is in contrast to a previous study with U.S. volunteers, in which the direct whole-blood MGIA, as well as the primary lymphocyte MGIA (effector lymphocytes added to infected autologous monocytes [9]) and the secondary lymphocyte MGIA (using antigen-expanded T cells [6]), detected enhanced mycobacterial growth inhibition after BCG revaccination (7).
In the Oxford study, the direct PBMC MGIA demonstrated a stronger primary vaccine effect and greater reproducibility over repeated baseline bleeds, compared with whole blood (13), most likely due to the processing of longitudinal PBMC samples in one batch. This is not an option for fresh blood, which may suffer from batch effects, including those associated with different vials of mycobacterial inoculum. However, this was not the case in other studies, such as the AERAS-422 vaccine trial at Saint Louis University described below, in which the direct whole-blood MGIA proved less variable among subjects included in each vaccinated group, compared to primary, secondary, or titrated PBMC MGIAs (15).
An additional study was conducted in South Africa, comparing 10-week-old infants who were BCG vaccinated at birth with older adults. Infants demonstrated enhanced ability to inhibit growth of BCG SSI (but not M. tuberculosis H37Rv) in the direct whole-blood MGIA, compared with adults (Fig. 2B). These data suggest that BCG vaccination may induce preferential growth inhibition of BCG even in newborns.
Study of immune correlates of risk.
Recently, Fletcher et al. reported the findings of a study on the immune correlates of risk among BCG-vaccinated infants in South Africa (1). In that population, BCG-specific IFN-γ ELISpot responses and levels of Ag85A-specific IgG antibodies measured at 4 to 6 months of age were associated with reduced risk of developing TB disease over the next 3 years of life (1). However, the ability to control mycobacterial growth in the direct PBMC MGIA was not associated with reduced risk of TB disease. This could be due to the very low frequency of BCG-specific IFN-γ-secreting T cells (median of <50 spot-forming cells/1 million PBMCs) and the fact that small numbers of PBMCs were used in the assay (it has since been established that the direct MGIA is more robust when greater numbers of PBMCs are used). Furthermore, autologous serum was not used in the assay; therefore, the assay would not have measured the contribution of Ag85A-specific IgG, or any other antibody, to growth inhibition (1). The study was also performed prior to subsequent further optimization of the assay.
Comparison of wild-type BCG and a new recombinant BCG vaccine.
Hoft et al. performed the direct whole-blood MGIA as part of a clinical trial of AERAS-422, a live recombinant BCG expressing perfringolysin and three M. tuberculosis antigens, namely, 85A, 85B, and Rv3407 (15). Between November 2010 and August 2011, 24 volunteers were enrolled (AERAS-422, high dose, n = 8; AERAS-422, low dose, n = 8; Tice BCG, n = 8). High-dose AERAS-422 vaccination induced Ag85A- and Ag85B-specific lymphoproliferative responses and marked antimycobacterial activity detectable in the direct whole-blood MGIA (15). A systems biology approach using modular analysis identified positive correlations between postvaccination T cell gene expression and MGIA activity and negative correlations between postvaccination monocyte gene expression modules and MGIA activity. Unfortunately, the development of varicella-zoster virus reactivations occurred in two of eight vaccine recipients given the high dose of AERAS-422, resulting in discontinuation of AERAS-422 clinical development. However, these results demonstrated that the direct whole-blood MGIA could detect vaccine-induced increases in antimycobacterial immune activity (15).
Assessment of M. tuberculosis infection status.
As described, there was a significant correlation in outcomes using BCG and M. tuberculosis in the direct whole-blood MGIA. However, no difference in the magnitude of growth inhibition was detected between healthy M. tuberculosis-infected and noninfected subjects, using either mycobacterial strain, in a region of South Africa in which TB is endemic (R. Baguma and T. J. Scriba, unpublished observations). This was in contrast to a previous study in which levels of growth of luminescent mycobacteria were significantly lower in blood from tuberculin skin test (TST)-positive subjects, compared with TST-negative subjects, in a region in which TB is not endemic (10). The discordance between these findings might have resulted from the cross-sectional designs, in which infection with M. tuberculosis was defined using a single IFN-γ release assay or TST, and it is not known whether volunteers from either study were recently or historically exposed to M. tuberculosis. Furthermore, despite being QuantiFERON negative, the South African volunteers had readily detectable T cell responses to BCG stimulation, suggesting high levels of immunological sensitization to mycobacteria (likely due to childhood BCG vaccination and/or exposure to environmental mycobacteria).
IMMUNE MECHANISMS OF GROWTH INHIBITION
One major goal of this project was to elucidate immune mechanisms underlying the mycobacterial growth inhibition observed. The influences of IFN-γ-producing T cells, antibodies, monocyte/lymphocyte (ML) ratios, and hemoglobin (Hb) were assessed.
IFN-γ-producing T cells.
The magnitude of mycobacterial growth inhibition following BCG vaccination in the Oxford study did not correlate with the antigen-specific IFN-γ ELISpot response, and a boosted IFN-γ ELISpot response observed following BCG revaccination was not reflected in enhanced MGIA activity (13). Among the South African volunteers, although QuantiFERON-TB Gold-positive individuals had greater levels of ESAT-6- and CFP-10-specific IFN-γ-secreting T cells, this did not translate into improved MGIA responses. These findings are consistent with previous observations that in vitro mycobacterial growth inhibition does not correlate with IFN-γ production (7, 8).
Antibodies.
Chen et al. demonstrated that IgG antibody responses to arabinomannan (AM), a mycobacterial envelope polysaccharide, increased significantly following BCG vaccination, using samples from the Oxford trial (16). Their studies suggested that such antibodies, particularly when targeting specific oligosaccharide epitopes, may play a functional role in mycobacterial growth inhibition. Phagocytosis and intracellular growth inhibition were significantly enhanced when BCG was opsonized with postvaccination sera, and the enhancements correlated with IgG titers to AM. Furthermore, increased phagolysosomal fusion in M. tuberculosis-infected macrophages was observed with postvaccination serum but not prevaccination serum, indicating that intracellular growth reduction was FcR mediated. Interestingly, the authors also found that the antibody response at 4 weeks after BCG vaccination correlated with mycobacterial growth inhibition in the direct PBMC MGIA (16). A functional role of antibodies to AM has since been further supported by murine immunization studies with AM conjugate vaccines (17). These data highlight the importance of assessing the humoral compartment in MGIAs and comparing PBMC- and whole-blood-based approaches to better assess the potential contributions of antibody-mediated responses.
Monocyte/lymphocyte ratio.
In blood samples from healthy volunteers, a greater proportion of monocytes with respect to lymphocytes (higher ML ratio) is associated with increased mycobacterial growth and increased probability of a type I IFN transcriptional signature (18). Altering the ML ratio in vitro affects the control of mycobacterial growth, with either very high or very low ML ratios being associated with poorer control in the direct PBMC MGIA (18). This is consistent with the observations that profoundly altered ML ratios were associated with increased risk of developing TB disease among non-HIV-exposed infants, HIV-exposed uninfected infants, HIV-positive adults starting antiretroviral therapy, and postpartum women (2, 19–21). Accordingly, Hoft et al. showed that antimycobacterial immune activity correlated positively with T cell expression signatures and negatively with monocyte expression signatures in the AERAS-422 study (15). Further studies with animal models may better define the subpopulations of monocytes and lymphocytes important for mycobacterial growth inhibition.
Hemoglobin.
Using samples from the Oxford BCG vaccination study, a positive correlation was observed between mean corpuscular Hb levels and mycobacterial growth in the direct whole-blood MGIA (22). Experimental addition of Hb or ferric iron to PBMCs from both humans and nonhuman primates (NHPs) resulted in increased mycobacterial growth, an effect reversed by addition of the iron chelator deferoxamine. Furthermore, expression of Hb-related genes correlated significantly with mycobacterial growth in whole blood and, to a lesser extent, in PBMCs (22). This association between Hb/iron levels and mycobacterial growth may in part explain differences in outcomes between whole-blood and PBMC MGIAs and should be taken into account when using these assays.
ANIMAL MGIA STUDIES
Given that early clinical studies indicate the need for more investigations before a human PBMC MGIA can be qualified, MGIA development using animal models of TB represents an important ongoing strategy. In addition to the benefits regarding sample volume and reduced heterogeneity described above, animal models have the important advantage that the MGIA outcomes could be correlated with protection from in vivo challenges with pathogenic M. tuberculosis, which would then biologically validate the assay as being capable of assessing effective antimycobacterial immunity. Where there is a need to test vaccine candidates for antigen dose, adjuvant dose, or antigen-adjuvant combinations, MGIAs could save time, animals, and funds. As the direct MGIA does not depend on any species-specific immune reagents, it can be easily adapted for use across a range of animal species.
In an effort to improve intersite reproducibility, the animal phase of the MGIA project utilized a single shared batch of frozen mycobacteria. The BCG batch was prepared at Aeras and tested for viability and reproducibility prior to distribution to project partners. Investigators also agreed that, for each experiment, the CFU of mycobacterial stocks would be determined and standard curves would be used to convert Bactec MGIT time-to-positivity (TTP) data to log10 CFU for the growth counts, for ease of data presentation and interpretation.
Mouse model.
A MGIA using infected macrophages cultured separately from mouse splenocytes, and then combined, previously demonstrated reproducible growth inhibition data at 7 days of incubation (23). Using this MGIA, immune splenocytes from mice vaccinated with five different TB vaccines limited mycobacterial growth in vitro, compared to naive controls. Importantly, the vaccine-induced MGIA correlated at a group level with protective immune responses induced in experimentally matched animals, using a mouse model of pulmonary TB (23).
More recent studies have focused on simplifying the mouse MGIA model. Marsay et al. showed that the direct MGIA using splenocytes has the ability to detect mycobacterial growth inhibition following BCG vaccination (24). Furthermore, investigators from the Center for Biologics Evaluation and Research (CBER), FDA, used the direct splenocyte MGIA to demonstrate reproducible in vitro protective responses from mice immunized with either BCG or a subunit vaccine, and they showed that the responses correlated on a group level with in vivo protection data from experimentally matched mice (25). They also confirmed that mycobacterial quantification using the Bactec MGIT system correlated strongly with plating of organ homogenates and CFU counting and is faster and easier to perform than the traditional CFU method (26).
BCG gives a robust and reproducible protective effect against challenge with M. tuberculosis in C57BL/6 mice; therefore, this model has been used for further optimization of the mouse direct MGIA. Zelmer et al. sought to enhance the sensitivity of the direct splenocyte MGIA and found that detection of vaccine-induced inhibition could be improved by decreasing the MOI (27). It was also shown that the capacity to detect mycobacterial growth inhibition in BCG-vaccinated mice was time sensitive, with growth inhibition being detected only at the peak of the BCG immune response (approximately 6 weeks in C57BL/6 mice) (14). As there is no amplification of the antigen-specific immune response in the direct MGIA, it is only the ability of the cells resident in the spleen at the time of harvest that can be assessed for antimycobacterial capacity (14). Since BCG is a live replicating mycobacterium, there can be variations in the development of the peak of the immune response and variations in the persistence of BCG vaccine in lungs and spleen; this can affect the ability to reproducibly select the optimal time point for measuring vaccine responses (28, 29). However, selecting the time point for the peak immune response may be a lesser challenge in assessing a subunit vaccine, and the ability of MGIAs to assess the effectiveness of subunit vaccines has shown early promise (23).
Guinea pig model.
Investigators at Colorado State University and Public Health England are in the process of adapting the direct MGIA for use with a guinea pig model. Early indications are that PBMCs provide more robust information than does whole blood, and further studies with vaccine candidates to examine the utility of PBMC MGIAs in the guinea pig model are in progress.
NHP model.
The direct whole-blood MGIA has been adapted for use with NHP samples at the University of Oxford, in collaboration with Sharpe and colleagues at Public Health England. In a study of seven rhesus macaques, the assay demonstrated strong enhancement of mycobacterial growth inhibition following BCG vaccination, which was still significant following correction for changes in hemoglobin levels (22). Work is currently ongoing to optimize the NHP direct PBMC MGIA and to correlate the outcomes with protection from in vivo BCG and M. tuberculosis challenges, on an individual animal basis. Reproduction of the MGIA in NHPs could help to inform human studies and to provide biological validation of the assay.
CONCLUSIONS
The MGIA represents a functional assay for assessing TB vaccine activity, which has the potential to correlate with vaccine-induced immune protection. To date, the direct whole-blood MGIA has demonstrated the most consistent effects across a range of different clinical vaccine studies in humans. However, because this assay must be performed within hours after blood drawing, it requires laboratory infrastructure close to the clinical site, making it difficult to assess in many vaccine trials, particularly in countries in which TB is endemic. Cryopreserved PBMCs are thus the preferred specimen type for logistical reasons, and the direct PBMC MGIA is currently undergoing further optimization and harmonization across laboratories as part of the European Research Infrastructures for Poverty Related Diseases collaborative infrastructure program (H. McShane and R. Tanner, personal communication). Use of MGIAs in animal models of TB provides an opportunity for biological validation with respect to protection from pathogenic in vivo challenges and could be more time- and cost-effective. Animal MGIAs could also reduce the number of virulent M. tuberculosis challenge experiments required to identify promising vaccine candidates for progression to clinical trials (30).
The results presented indicate that the MGIA has promise as a functional assay for the assessment of candidate TB vaccines. It also provides a tractable model for investigating the mechanisms involved in antimycobacterial immunity. For example, findings from this project have highlighted the importance of both lymphoid and myeloid cell-mediated immunity and antibody-mediated immunity in these assays. It would be interesting to consider how exposure of an individual to other mycobacterial species or infections (such as helminths) would affect the ability to control mycobacterial growth in vitro, and work to this end is under way. The influence of regulatory immune mechanisms and suppressors, which would be represented in unbiased samples such as whole blood and PBMCs, should also be explored.
In this report, we have summarized the numerous accomplishments of this project. Many parameters of a standardized MGIA that can be routinely performed in laboratories with access to Bactec MGIT equipment have been established. However, MGIAs are technically demanding, and it is clear that further work is required, particularly to reduce the variability intrinsic to functional assays. Use of cryopreserved PBMCs remains a challenge, as does the goal of qualifying this assay for use in human studies in particular. Utilization of the direct MGIA in animal models (especially mice and NHPs) for assessment of new TB vaccines seems more valuable at this time. A number of explorations of MGIAs as functional TB tests should be performed in the future, and some of these goals are as follows: (i) to demonstrate that the direct PBMC MGIA meets requirements as a qualified assay; (ii) to determine whether there are significant differences when BCG or M. tuberculosis strains are used for in vitro infection; (iii) to evaluate differences when MGIAs are used in countries in which TB is endemic or not endemic; (iv) to determine whether MGIAs can be used in M. tuberculosis-infected populations; (v) to identify factors in PBMCs and whole blood that mediate and influence growth inhibition; (vi) to evaluate the performance of the PBMC MGIT assay in trials of novel TB vaccine candidates; (vii) to determine whether the test is reproducible at different sites; and (viii) to determine whether the direct whole-blood MGIA can be further adapted or refined for use in vaccine or TB studies. If MGIAs were validated and shown to correlate reproducibly and consistently with efficacy demonstrated in clinical trials of candidate vaccines for TB, they would hold great potential. Much as neutralizing antibodies are currently used to assess the efficacy of certain other vaccines, MGIA results could be applied for regulatory purposes, as a surrogate marker of efficacy.
ACKNOWLEDGMENTS
We thank Robert S. Wallis for serving as a consultant on the MGIA project. We thank Megan Fitzgerald, Veerabadran Dheenadhalayan, and Nathalie Cadieux of Aeras for the production and distribution of the BCG standard for the MGIA. We also thank Lewis Schrager and Barry Walker for critical comments on the manuscript. We are indebted to Thomas Evans, former Chief Executive Officer of Aeras, for his support of the MGIA project. I (M.J.B.) thank Pere-Joan Cardona for the portrait. We thank Patrick Lane for providing graphical enhancement of Fig. 1.
We thank the U.S. FDA (FDA grant IU18FD004012-01) and Aeras for financial support. The research leading to some of these results has also received funding from the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement 312661 (European Research Infrastructures for Poverty Related Diseases), the European Union Horizon 2020 research and innovation program under grant agreement 643381, the Universities Federation for Animal Welfare, the Wellcome Trust, and the Bill and Melinda Gates Foundation.
We declare that we have no competing interests.
M.J.B., R.T., S.M., T.J.S., J.M.A., D.H., A.I., S.S., A.W., D.F.H., H.M., and H.A.F. conceived and designed the experiments, R.T., T.J.S., J.M.A., A.Z., D.H., A.I., S.S., A.W., A.P.-N., M.E., E.S., and D.F.H. performed the experiments, and M.J.B., R.T., S.M., T.J.S., J.M.A., D.H., A.I., D.F.H., H.M., and H.A.F. wrote the paper.
Biographies

Dr. Michael J. Brennan. I was the first one in my family to go to college. I owe a great deal to my parents and sister, who sacrificed much so that I could take the time needed to become a scientist. To have experienced a career I love, I have been exceptionally fortunate. Throughout, I have held different positions at the National Institutes of Health and at the World Health Organization and I eventually retired from the U.S. Food and Drug Administration. Now near the end of my career, I am employed by the nonprofit organization Aeras and have learned many new things about the manufacturing and clinical investigation of vaccines, especially those for tuberculosis. All over, I have travelled to six continents, participated in many valuable conferences, and had meaningful and insightful conversations with hundreds of scientists. Perhaps those conversations have been the best part of all.

Dr. Rachel Tanner is a postdoctoral scientist at the Jenner Institute, University of Oxford. She obtained a B.A. (Hons) in Biological Sciences at the University of Oxford and developed an early interest in vaccine immunology, taking up a Research Assistant post at the Centre for HIV/AIDS Vaccine Immunology. Dr. Tanner received her D.Phil. in Clinical Medicine at the Jenner Institute, University of Oxford, in 2015. Her Ph.D. project was concerned with the development and optimization of a MGIA across multiple species, and she continues to work on such functional assays as part of her postdoctoral research. Dr. Tanner's broader interests are in immune correlates and the host immune response to TB vaccination.

Dr. Sheldon Morris is a retired scientist who worked in the Laboratory of Mycobacterial Diseases and Cellular Immunology at the CBER, FDA, from 1986 to 2015. He served as Laboratory Chief from 2001 to 2015. Dr. Morris' activities included the regulatory evaluation of tuberculosis and malaria vaccines and scientific research involving the development of new tuberculosis vaccines and novel assays to support vaccine development. During his tenure at the CBER, Dr. Morris authored or coauthored more than 100 publications on mycobacterial disease research and was invited to give 52 scientific presentations. In addition, he was a member of the editorial board for Antimicrobial Agents and Chemotherapy and served as an ad hoc reviewer for 13 other journals. Importantly, Dr. Morris was selected to participate in 11 National Institutes of Health grant review panels and three Wellcome Trust grant reviews and was a scientific advisor to the World Health Organization during four meetings.

Dr. Thomas J. Scriba trained in Biological Sciences at Stellenbosch University, South Africa, and in T Cell Immunology at the University of Oxford, United Kingdom. He returned to South Africa for postdoctoral training in Paediatric and Clinical Immunology in Tuberculosis and Vaccinology at the Institute of Infectious Disease and Molecular Medicine, University of Cape Town. Dr. Scriba is Associate Professor at the University of Cape Town, South Africa, and is Deputy Director of Immunology at the South African Tuberculosis Vaccine Initiative. His research interests include the clinical immunology of tuberculosis and the development of novel prevention strategies against tuberculosis.

Dr. Jacqueline M. Achkar is a physician scientist and Associate Professor in the Department of Medicine (Division of Infectious Diseases) and the Department of Microbiology & Immunology at the Albert Einstein College of Medicine, Bronx, New York. She received her medical diploma and doctoral degree from the Free University of Berlin, Germany, and her master of science degree in Clinical Research Methods from the Albert Einstein College of Medicine, and she completed her medical residency and Infectious Disease Fellowship at the New York University School of Medicine. Dr. Achkar leads a successful translational research program in the field of tuberculosis serology and biomarker discovery, which includes the identification of human antibody targets and the study of antibody functions in the defense against TB.

Dr. Andrea Zelmer received her degree in Biology in 2002 and a Ph.D. in Infection Biology in 2005 from the Technical University Braunschweig, Germany. She subsequently moved to London, United Kingdom, to start a postdoctoral position at the UCL School of Pharmacy, to work on the treatment and pathogenesis of Escherichia coli K1 infection in neonates. In 2008, Dr. Zelmer moved to the London School of Hygiene & Tropical Medicine to develop imaging technologies and mouse models for testing of new drugs and vaccines against tuberculosis. She is now Assistant Professor at the London School of Hygiene & Tropical Medicine. Over the past three years, Dr. Zelmer has been focusing on developing clinically relevant animal models for vaccine testing and studying the immune response to vaccination.

Dr. David A. Hokey is the Senior Director of Human Immunology at Aeras in Rockville, Maryland. He received his Ph.D. in Immunology in 2005 from the University of Pittsburgh, where he worked primarily on dendritic cell-based tumor vaccines. He then performed his postdoctoral research under the guidance of Dr. David Weiner at the University of Pennsylvania, focusing on DNA vaccines for HIV, cancer, and influenza, with an emphasis on primate immunology and polychromatic flow cytometry. Dr. Hokey's current work at Aeras involves evaluation of immune responses to TB vaccine candidates in preclinical and clinical settings. His research interests include evaluation of novel vaccine platforms and adjuvants for manipulation of immune responses, as well as assay optimization, and he has worked in this area for 18 years.

Dr. Angelo Izzo received his B.Sc. (Hons) and Ph.D. at the University of Adelaide, South Australia. He then undertook postgraduate training at the Universita di Milano, Instituto di Virologia, Milan, Italy, and returned to the University of Adelaide as a Research Assistant. Dr. Izzo later took up a postdoctoral fellowship at the Trudeau Institute, Saranac Lake, New York, followed by the University of New Mexico School of Medicine, Albuquerque, New Mexico. He then became Assistant Professor at Midwestern University Department of Microbiology, Illinois, and is now Assistant Professor at Colorado State University, Fort Collins, Colorado. Dr. Izzo has over 25 years of experience investigating the immune response to pulmonary Mycobacterium tuberculosis infection in animal models. He has obtained extensive experience in understanding vaccine-induced immunology and immunopathogenesis of pulmonary tuberculosis using animal models.

Dr. Sally Sharpe is a Scientific Leader at Public Health England, Porton Down. She received a B.Sc. from London University in 1984 and then took up a Research Assistant post at the Centre of Applied Microbiology and Research at Porton Down, where she was trained in T cell immunology and flow cytometry. She worked within a team developing nonhuman primate models of HIV and was awarded a Ph.D. (Open University, 1997) for research into T cell responses to simian immunodeficiency virus. Since 2003, she has led work to develop refined macaque models of tuberculosis for the evaluation of new interventions, at the Health Protection Agency and subsequently at Public Health England at the Porton Down site.

Dr. Ann Rawkins (née Williams) obtained her B.Sc. at the University of Wales in Cardiff and began her career as a Research Assistant studying bacterial pathogenesis at the Centre of Applied Microbiology and Research at Porton Down, in 1985. She conducted her postgraduate studies on the pathogenic mechanisms of Legionella pneumophila and was awarded a Ph.D. in 1995. After a brief period investigating Salmonella infection of poultry, she began M. tuberculosis research, developing aerosol-challenge in vivo models for pathogenesis studies and subsequently for the evaluation of interventions, predominantly vaccines. The vaccine evaluation team that she established is internationally renowned and collaborates with the majority of TB vaccine developers, and she has been involved in multiple EU-funded consortia. She is currently a Scientific Leader providing scientific direction to the TB research program at the Public Health England laboratories at Porton Down, Salisbury, United Kingdom. She has published widely under the name of Williams.

Dr. Adam Penn-Nicholson is a Research Officer/Scientist at the South African Tuberculosis Vaccine Initiative at the University of Cape Town, South Africa. He previously worked as a postdoctoral fellow at the University of Cape Town. Dr. Penn-Nicholson received his Ph.D. at Case Western Reserve University, in Cleveland, Ohio. Dr. Penn-Nicholson's main focus is the discovery of blood-based biomarkers that prospectively predict TB disease risk and understanding the biology involved in progression from latent M. tuberculosis infection to active TB disease. He also provides scientific oversight on immunology for several clinical TB vaccine trials currently being conducted at the SATVI.

Mr. Mzwandile Erasmus has a B.Sc. from the University of Cape Town and joined the South African Tuberculosis Vaccine Initiative shortly after graduating in 2007. He is currently a Senior Laboratory Technologist and has been involved in multiple clinical and research studies conducted at the SATVI.

Dr. Elena Stylianou has a B.Sc. and a M.Sc. in Biochemistry from Imperial College, London, and a Ph.D. in Immunology from St. George's, University of London. She has since been at the Jenner Institute, University of Oxford, training as a postdoctoral immunologist. Dr. Stylianou has always found the complexity of the interactions between Mycobacterium tuberculosis and the human host fascinating, and both her Ph.D. and postdoctoral studies focused on preclinical vaccine research against tuberculosis. The key areas of her research include mucosal and systemic delivery of viral vectors, immune responses after vaccination, and evaluation of vaccine protective efficacy using M. tuberculosis infection models.

Dr. Daniel F. Hoft is the Director of the Division of Infectious Diseases, Allergy & Immunology at Saint Louis University. Dr. Hoft was trained in Infectious Diseases at the University of Iowa, where he received an NIH Physician Science Training Award, allowing him to complete a Ph.D. in Microbiology/Immunology. He came to Saint Louis University in 1992 and has received continuous NIH funding since his arrival. In 2006, he was named the Director of the new Division of Immunobiology. In 2010, the Division of Immunobiology, the Division of Infectious Diseases, and the Division of Allergy & Immunology were joined into the new Division of Infectious Diseases, Allergy & Immunology, with Dr. Hoft as Director. In 2014, he became the Principal Investigator of the Saint Louis University Vaccine & Treatment Evaluation Unit. The capacity to induce protective immune memory by vaccination is a powerful public health tool, and Dr. Hoft has been working in this area for 32 years.

Prof. Helen McShane is currently Professor of Vaccinology at the University of Oxford, Wellcome Senior Clinical Research Fellow, Deputy Head of Department for the Nuffield Department of Medicine, and honorary consultant physician in HIV medicine. Prof. McShane obtained an intercalated B.Sc. in 1988, followed by a degree in medicine in 1991 (both from the University of London). In 1997, she was awarded an MRC Clinical Training Fellowship to undertake a Ph.D. with Adrian Hill in Oxford and was later awarded a Ph.D. in 2001 (University of London). In 2001, she was awarded a Wellcome Clinician Scientist Fellowship, allowing her to complete her clinical training, and subsequently was awarded a C.C.S.T. in HIV and GU Medicine in 2003. In 2005, she was awarded a Wellcome Senior Clinical Research Fellowship; this fellowship was renewed in 2010. Professor McShane has led the TB vaccine research group at the University of Oxford for 16 years.

Dr. Helen A. Fletcher is Associate Professor of Immunology and Deputy Director of the TB Centre at the London School of Hygiene & Tropical Medicine. Dr. Fletcher has worked in the field of TB immunology for 17 years, at University College London, the University of Oxford, and the London School of Hygiene & Tropical Medicine. She has used transcriptomics and cellular immunology to identify correlates of TB disease risk, including increased monocyte/lymphocyte ratios and increased CD4+ T cell activation in South African infants at risk of developing TB disease. Dr. Fletcher has published more than 90 papers, and her current work at the London School of Hygiene & Tropical Medicine continues to focus on immune correlates of risk and the host response to TB vaccination.
REFERENCES
- 1.Fletcher HA, Snowden MA, Landry B, Rida W, Satti I, Harris SA, Matsumiya M, Tanner R, O'Shea MK, Dheenadhayalan V, Bogardus L, Stockdale L, Marsay L, Chomka A, Harrington-Kandt R, Manjaly-Thomas ZR, Naranbhai V, Stylianou E, Darboe F, Penn-Nicholson A, Nemes E, Hatheril M, Hussey G, Mahomed H, Tameris M, McClain JB, Evans TG, Hanekom WA, Scriba TJ, McShane H. 2016. T-cell activation is an immune correlate of risk in BCG vaccinated infants. Nat Commun 7:11290. doi: 10.1038/ncomms11290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fletcher HA, Filali-Mouhim A, Nemes E, Hawkridge A, Keyser A, Njikan S, Hatherill M, Scriba TJ, Abel B, Kagina BM, Veldsman A, Agudelo NM, Kaplan G, Hussey GD, Sekaly RP, Hanekom WA. 2016. Human newborn bacille Calmette-Guérin vaccination and risk of tuberculosis disease: a case-control study. BMC Med 14:76. doi: 10.1186/s12916-016-0617-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanekom WA, Dockrell HM, Ottenhoff TH, Doherty TM, Fletcher H, McShane H, Weichold FF, Hoft DF, Parida SK, Fruth UJ. 2008. Immunological outcomes of new tuberculosis vaccine trials: WHO panel recommendations. PLoS Med 5:e145. doi: 10.1371/journal.pmed.0050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanner R, O'Shea MK, Fletcher HA, McShane H. 2016. In vitro mycobacterial growth inhibition assays: a tool for the assessment of protective immunity and evaluation of tuberculosis vaccine efficacy. Vaccine 34:4656–4665. doi: 10.1016/j.vaccine.2016.07.058. [DOI] [PubMed] [Google Scholar]
- 5.Wallis RS, Palaci M, Vinhas S, Hise AG, Ribeiro FC, Landen K, Cheon SH, Song HY, Phillips M, Dietze R, Ellner JJ. 2001. A whole blood bactericidal assay for tuberculosis. J Infect Dis 183:1300–1303. doi: 10.1086/319679. [DOI] [PubMed] [Google Scholar]
- 6.Worku S, Hoft DF. 2000. In vitro measurement of protective mycobacterial immunity: antigen-specific expansion of T cells capable of inhibiting intracellular growth of bacille Calmette-Guérin. Clin Infect Dis 30(Suppl 3):S257–S261. doi: 10.1086/313887. [DOI] [PubMed] [Google Scholar]
- 7.Hoft DF, Worku S, Kampmann B, Whalen CC, Ellner JJ, Hirsch CS, Brown RB, Larkin R, Li Q, Yun H, Silver RF. 2002. Investigation of the relationships between immune-mediated inhibition of mycobacterial growth and other potential surrogate markers of protective Mycobacterium tuberculosis immunity. J Infect Dis 186:1448–1457. doi: 10.1086/344359. [DOI] [PubMed] [Google Scholar]
- 8.Kampmann B, Tena GN, Mzazi S, Eley B, Young DB, Levin M. 2004. Novel human in vitro system for evaluating antimycobacterial vaccines. Infect Immun 72:6401–6407. doi: 10.1128/IAI.72.11.6401-6407.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver RF, Li Q, Boom WH, Ellner JJ. 1998. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: requirement for CD4+ T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol 160:2408–2417. [PubMed] [Google Scholar]
- 10.Kampmann B, Gaora PO, Snewin VA, Gares MP, Young DB, Levin M. 2000. Evaluation of human antimycobacterial immunity using recombinant reporter mycobacteria. J Infect Dis 182:895–901. doi: 10.1086/315766. [DOI] [PubMed] [Google Scholar]
- 11.Cheon SH, Kampmann B, Hise AG, Phillips M, Song HY, Landen K, Li Q, Larkin R, Ellner JJ, Silver RF, Hoft DF, Wallis RS. 2002. Bactericidal activity in whole blood as a potential surrogate marker of immunity after vaccination against tuberculosis. Clin Diagn Lab Immunol 9:901–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kolibab K, Parra M, Yang AL, Perera LP, Derrick SC, Morris SL. 2009. A practical in vitro growth inhibition assay for the evaluation of TB vaccines. Vaccine 28:317–322. doi: 10.1016/j.vaccine.2009.10.047. [DOI] [PubMed] [Google Scholar]
- 13.Fletcher HA, Tanner R, Wallis RS, Meyer J, Manjaly ZR, Harris S, Satti I, Silver RF, Hoft D, Kampmann B, Walker KB, Dockrell HM, Fruth U, Barker L, Brennan MJ, McShane H. 2013. Inhibition of mycobacterial growth in vitro following primary but not secondary vaccination with Mycobacterium bovis BCG. Clin Vaccine Immunol 20:1683–1689. doi: 10.1128/CVI.00427-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zelmer A, Tanner R, Stylianou E, Damelang T, Morris S, Izzo A, Williams A, Sharpe S, Pepponi I, Walker B, Hokey DA, McShane H, Brennan M, Fletcher H. 2016. A new tool for tuberculosis vaccine screening: ex vivo mycobacterial growth inhibition assay indicates BCG-mediated protection in a murine model of tuberculosis. BMC Infect Dis 16:412. doi: 10.1186/s12879-016-1751-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoft DF, Blazevic A, Selimovic A, Turan A, Tennant J, Abate G, Fulkerson J, Zak DE, Walker R, McClain B, Sadoff J, Scott J, Shepherd B, Ishmukhamedov J, Hokey DA, Dheenadhayalan V, Shankar S, Amon L, Navarro G, Podyminogin R, Aderem A, Barker L, Brennan M, Wallis RS, Gershon AA, Gershon MD, Steinberg S. 2016. Safety and immunogenicity of the recombinant BCG vaccine AERAS-422 in healthy BCG-naïve adults: a randomized, active-controlled, first-in-human phase 1 trial. EBioMedicine 7:278–286. doi: 10.1016/j.ebiom.2016.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen T, Blanc C, Eder AZ, Prados-Rosales R, Souza AC, Kim RS, Glatman-Freedman A, Joe M, Bai Y, Lowary TL, Tanner R, Brennan MJ, Fletcher HA, McShane H, Casadevall A, Achkar JM. 2016. Association of human antibodies to arabinomannan with enhanced mycobacterial opsonophagocytosis and intracellular growth reduction. J Infect Dis 214:300–310. doi: 10.1093/infdis/jiw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prados-Rosales R, Carreño L, Cheng T, Blanc C, Weinrick B, Malek A, Lowary TL, Baena A, Joe M, Bai Y, Kalscheuer R, Batista-Gonzalez A, Saavedra NA, Sampedro L, Tomás J, Anguita J, Hung SC, Tripathi A, Xu J, Glatman-Freedman A, Jacobs WR, Chan J, Porcelli SA, Achkar JM, Casadevall A. 2017. Enhanced control of Mycobacterium tuberculosis extrapulmonary dissemination in mice by an arabinomannan-protein conjugate vaccine. PLoS Pathog 13:e1006250. doi: 10.1371/journal.ppat.1006250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naranbhai V, Fletcher HA, Tanner R, O'Shea MK, McShane H, Fairfax BP, Knight JC, Hill AV. 2015. Distinct transcriptional and anti-mycobacterial profiles of peripheral blood monocytes dependent on the ratio of monocytes:lymphocytes. EBioMedicine 2:1619–1626. doi: 10.1016/j.ebiom.2015.09.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Naranbhai V, Moodley D, Chipato T, Stranix-Chibanda L, Nakabaiito C, Kamateeka M, Musoke P, Manji K, George K, Emel LM, Richardson P, Andrew P, Fowler M, Fletcher H, McShane H, Coovadia HM, Hill AV. 2014. The association between the ratio of monocytes:lymphocytes and risk of tuberculosis among HIV-infected postpartum women. J Acquir Immune Defic Syndr 67:573–575. doi: 10.1097/QAI.0000000000000353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naranbhai V, Kim S, Fletcher H, Cotton MF, Violari A, Mitchell C, Nachman S, McSherry G, McShane H, Hill AV, Madhi SA. 2014. The association between the ratio of monocytes:lymphocytes at age 3 months and risk of tuberculosis (TB) in the first two years of life. BMC Med 12:120. doi: 10.1186/s12916-014-0120-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Naranbhai V, Hill AV, Abdool Karim SS, Naidoo K, Abdool Karim Q, Warimwe GM, McShane H, Fletcher H. 2014. Ratio of monocytes to lymphocytes in peripheral blood identifies adults at risk of incident tuberculosis among HIV-infected adults initiating antiretroviral therapy. J Infect Dis 209:500–509. doi: 10.1093/infdis/jit494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tanner R, O'Shea MK, White AD, Müller J, Harrington-Kandt R, Matsumiya M, Dennis MJ, Parizotto EA, Harris S, Stylianou E, Naranbhai V, Bettencourt P, Drakesmith H, Sharpe S, Fletcher HA, McShane H. 2017. The influence of haemoglobin and iron on in vitro mycobacterial growth inhibition assays. Sci Rep 7:43478. doi: 10.1038/srep43478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parra M, Yang AL, Lim J, Kolibab K, Derrick S, Cadieux N, Perera LP, Jacobs WR, Brennan M, Morris SL. 2009. Development of a murine mycobacterial growth inhibition assay for evaluating vaccines against Mycobacterium tuberculosis. Clin Vaccine Immunol 16:1025–1032. doi: 10.1128/CVI.00067-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marsay L, Matsumiya M, Tanner R, Poyntz H, Griffiths KL, Stylianou E, Marsh PD, Williams A, Sharpe S, Fletcher H, McShane H. 2013. Mycobacterial growth inhibition in murine splenocytes as a surrogate for protection against Mycobacterium tuberculosis (M. tb). Tuberculosis (Edinb) 93:551–557. doi: 10.1016/j.tube.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 25.Yang AL, Schmidt TE, Stibitz S, Derrick SC, Morris SL, Parra M. 2016. A simplified mycobacterial growth inhibition assay (MGIA) using direct infection of mouse splenocytes and the MGIT system. J Microbiol Methods 131:7–9. doi: 10.1016/j.mimet.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Kolibab K, Yang A, Parra M, Derrick SC, Morris SL. 2014. Time to detection of Mycobacterium tuberculosis using the MGIT 320 system correlates with colony counting in preclinical testing of new vaccines. Clin Vaccine Immunol 21:453–455. doi: 10.1128/CVI.00742-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zelmer A, Tanner R, Stylianou E, Morris S, Izzo A, Williams A, Sharpe S, Pepponi I, Walker B, Hokey D, McShane H, Brennan M, Fletcher H. 2015. Ex vivo mycobacterial growth inhibition assay (MGIA) for tuberculosis vaccine testing: a protocol for mouse splenocytes. BioRxiv doi: 10.1101/020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaveh DA, Garcia-Pelayo MC, Hogarth PJ. 2014. Persistent BCG bacilli perpetuate CD4 T effector memory and optimal protection against tuberculosis. Vaccine 32:6911–6918. doi: 10.1016/j.vaccine.2014.10.041. [DOI] [PubMed] [Google Scholar]
- 29.Olsen AW, Brandt L, Agger EM, van Pinxteren LA, Andersen P. 2004. The influence of remaining live BCG organisms in vaccinated mice on the maintenance of immunity to tuberculosis. Scand J Immunol 60:273–277. doi: 10.1111/j.0300-9475.2004.01471.x. [DOI] [PubMed] [Google Scholar]
- 30.Tanner R, McShane H. 2017. Replacing, reducing and refining the use of animals in tuberculosis vaccine research. ALTEX 34:157–166. [DOI] [PubMed] [Google Scholar]