ABSTRACT
Bovine leukemia is classified into two types: enzootic bovine leukosis (EBL) and sporadic bovine leukosis (SBL). EBL is caused by infection with bovine leukemia virus (BLV), which induces persistent lymphocytosis and B-cell lymphoma in cattle after a long latent period. Although it has been demonstrated that BLV-associated lymphoma occurs predominantly in adult cattle of >3 to 5 years, suspicious cases of EBL onset in juvenile cattle were recently reported in Japan. To investigate the current status of bovine leukemia in Japan, we performed immunophenotypic analysis of samples from 50 cattle that were clinically diagnosed as having bovine leukemia. We classified the samples into five groups on the basis of the analysis and found two different types of EBL: classic EBL (cEBL), which has the familiar phenotype commonly known as EBL, and polyclonal EBL (pEBL), which exhibited neoplastic proliferation of polyclonal B cells. Moreover, there were several atypical EBL cases even in cEBL, including an early onset of EBL in juvenile cattle. A comparison of the cell marker expressions among cEBL, pEBL, and B-cell-type SBL (B-SBL) revealed characteristic patterns in B-cell leukemia, and these patterns could be clearly differentiated from those of healthy phenotypes, whereas it was difficult to discriminate between cEBL, pEBL, and B-SBL only by the expression patterns of cell markers. This study identified novel characteristics of bovine leukemia that should contribute to a better understanding of the mechanism underlying tumor development in BLV infection.
KEYWORDS: bovine leukemia, immunophenotyping, early onset EBL, expression patterns of B-cell markers
INTRODUCTION
Bovine leukemia is a fatal disorder in cattle that is characterized by neoplastic lymphocytosis and systemic lymphoma. There are two types of bovine leukemia based on their epidemiologies. Enzootic bovine leukosis (EBL) is caused by infection with a retrovirus called bovine leukemia virus (BLV), and sporadic bovine leukosis (SBL) is not a transmissible cancer and has an unknown etiology.
BLV belongs to the Deltaretrovirus genus of the Retroviridae family and commonly infects host B cells. During the infection, approximately 60% to 70% of BLV-infected cattle become asymptomatic carriers at what is called the aleukemic (AL) stage. However, after a few months to years of this asymptomatic period, nearly 30% of infected cattle develop persistent lymphocytosis (PL), and then <5% develop lymphoma, which is a lethal form of this disease (1, 2). The clinical condition in BLV-infected cattle is characterized by an increase in the number of circulating B lymphocytes (>10,000 cells/μl in peripheral blood), and it has been found that the lymphoma occurs predominantly in adult cattle >3 to 5 years old (2, 3). The virus is transmitted to a new animal through the transfer of BLV-positive cells in blood or milk and probably via blood-sucking insects (4). Moreover, BLV infection occurs from mother-to-child in utero or in the birth canal to a low or moderate extent (5, 6). Experimental transmissions of BLV have been reported in rabbits, rats, chickens, pigs, goats, and sheep; however, only sheep develop leukemia and thus are often used as a model of this disease (1, 7, 8).
SBL is further subdivided into juvenile, thymic, and cutaneous forms depending on the age and tumor-developing site (9). The juvenile form occurs in calves ≤2 years old (usually 6 months old) and typically shows as systemic lymphoma. The thymic form develops in calves from 6 months to 2 years old and is characterized by strong lymphoproliferation of thymic tissue. The cutaneous form has been found in cattle between 1 and 3 years old and shows as multifocal lymphoproliferation in the skin. However, there are several reports on atypical SBL cases, such as intermediate cases that involve an overlap of the juvenile and thymic forms and multicentric lymphadenopathy in adult cattle >3 years old that are negative for BLV (10, 11). Therefore, the classification of bovine leukemia remains inconsistent.
EBL is characterized by systemic B-cell lymphoma associated with BLV infection, whereas SBL includes tumors of both B-cell and T-cell origins. The diagnosis of bovine leukemia is based on the observation of lymphadenopathy through palpation and rectal examination during routine examination practices, but many clinical cases have been found in meat hygiene inspection centers after the cattle are slaughtered (12). The cell origin in the tumor-developing sites is determined by immunohistochemical analysis to confirm cell marker expression, and BLV association is usually determined by BLV antibody enzyme-linked immunosorbent assay or by detection of the virus genome by PCR. Quantitative analyses, such as flow cytometry and real-time PCR, are useful for the quantitative evaluation of the expression levels of cell markers and BLV provirus loads, but those methods are less frequently used clinically. The detection of monoclonality in B-cell proliferations using clonal rearrangement of the immunoglobulin heavy chain (IgH) gene is an effective way to diagnose B-cell lymphoma, and it is established not only for humans (13, 14) but also for dogs (15–18), cats (19), and pigs (20). In cattle, one study used a PCR-based IgH analysis to estimate the amount of founder clones in follicles of ileal Peyer's patches (21), but no study has investigated the diagnosis of bovine B-cell lymphoma using this method.
Although EBL has been eradicated in certain European countries (22–24), it is still prevalent worldwide, including in Japan where the numbers of EBL cases have increased recently. A nationwide survey in Japan conducted from 2009 to 2011 indicated a high seroprevalence of BLV in both dairy and beef cattle (40.9% and 28.7%, respectively) (25). Moreover, in a few scientific papers and domestic reports, EBL onset in juvenile calves in the field has been recently reported in Japan (26), even though EBL occurs predominantly in adult cattle.
In this study, we performed quantitative analyses and PCR-based IgH analysis to evaluate cell marker expression, BLV provirus loads, and B-cell clonality using clinical samples from cattle in Japan diagnosed as having bovine leukemia. Surprisingly, we not only found many cases of early onset EBL but also identified several atypical EBL types previously unreported, such as polyclonal B-cell lymphoma with high provirus loads or a lack of peripheral lymphocytosis in EBL cattle. Thus, in this paper, we report a novel characteristic of bovine leukemia that was recently identified in the field in Japan. Our finding should contribute to a deeper understanding of immunophenotypic features of bovine leukemia and perhaps of the mechanism underlying tumor development during disease progression after BLV infection.
RESULTS
Sample collection and phenotypic analysis of cattle with lymphoma.
To examine immunophenotypic features of bovine lymphoma, we collected 176 samples from 50 cattle that were clinically diagnosed as having bovine leukemia in livestock hygiene centers and meat hygiene inspection centers in Japan (Table 1). Because we first aimed to clarify the early onset of EBL, juvenile calves <3 years old that were positive for BLV were given priority for sample collection. The samples were subjected to three analyses: flow cytometry analysis for cell marker expression, PCR-based IgH analysis for B-cell clonality, and quantitative real-time PCR for BLV provirus loads. First, we determined the expression of the cell markers by evaluating not only the percentages of positive cells but also the numbers of cell populations (see Fig. S1 in the supplemental material), because single cell population results suggested that the samples were highly tumorigenic, since tumor cells should express similar patterns of cell markers, whereas normal cells showed several populations having different marker expressions; thus, these results were indicative of the extent of tumorigenesis. Second, amplification of the gene encoding the IgH region of interest was performed to investigate B-cell clonality, and the results of the amplification were divided into those of high or low clonality. A clear DNA band by electrophoresis indicated monoclonal or oligoclonal B-cell expansion (high clonality) (see Fig. S2, lanes 1 to 4), whereas a smear indicated the existence of polyclonal B cells (low clonality) (Fig. S2, lanes 5 to 9 and 11 to 14). In some cases, we found unclear results that were difficult to classify as high or low clonality (Fig. S2, lane 10). DNA sequencing of the amplicon indicated that the clear DNA band found in a high-clonality sample did not consist of two or three B-cell clones of similar clone sizes but of a single clone of B cells (data not shown). Third, BLV provirus loads were quantified as the copy numbers of the BLV Tax gene contained in 50 ng of genomic DNA.
TABLE 1.
Basic information of the cattle analysed in this study
| Category | No. of cattle or samples |
|
|---|---|---|
| With lymphoma (n = 50) | Without lymphoma (n = 7) | |
| Age (years) | ||
| 0–1 | 10 | 2 |
| 1–2 | 10 | 0 |
| 2–3 | 16 | 0 |
| ≥3 | 14 | 4 |
| No information | 0 | 1 |
| Breed | ||
| Holstein | 17 | 6 |
| Japanese Black | 29 | 0 |
| Crossbreed | 3 | 0 |
| No information | 1 | 1 |
| Sex | ||
| Male | 7 | 2 |
| Female | 39 | 5 |
| No information | 4 | 0 |
| BLV infection | ||
| Positive | 44 | 3 |
| Negative | 6 | 4 |
| Sampling sites | ||
| Total | 176 | 21 |
| Peripheral blood | 41 | 7 |
| Lymph node | 90 | 12 |
| Spleen | 16 | 1 |
| Thymus | 6 | 1 |
| Solid tumor in organ | 23 | 0 |
To confirm the validity of our analysis, the numbers of cell populations determined by the flow cytometry analysis were compared between B-cell clonality types. The percentages of single populations in B-cell-associated markers, CD5, IgM, WC4, CD21, and CD79a, were increased in high-clonality samples relative to those in low-clonality samples (Fig. 1A). Moreover, the provirus loads of the high-clonality samples were significantly higher than those of the low-clonality samples (Fig. 1B, left). Thus, these results indicated that the high-clonality samples exhibited a single pattern of cell marker expression and included large numbers of BLV-positive cells, whereas low-clonality samples showed multiple populations similar to normal cells and small numbers of provirus loads.
FIG 1.
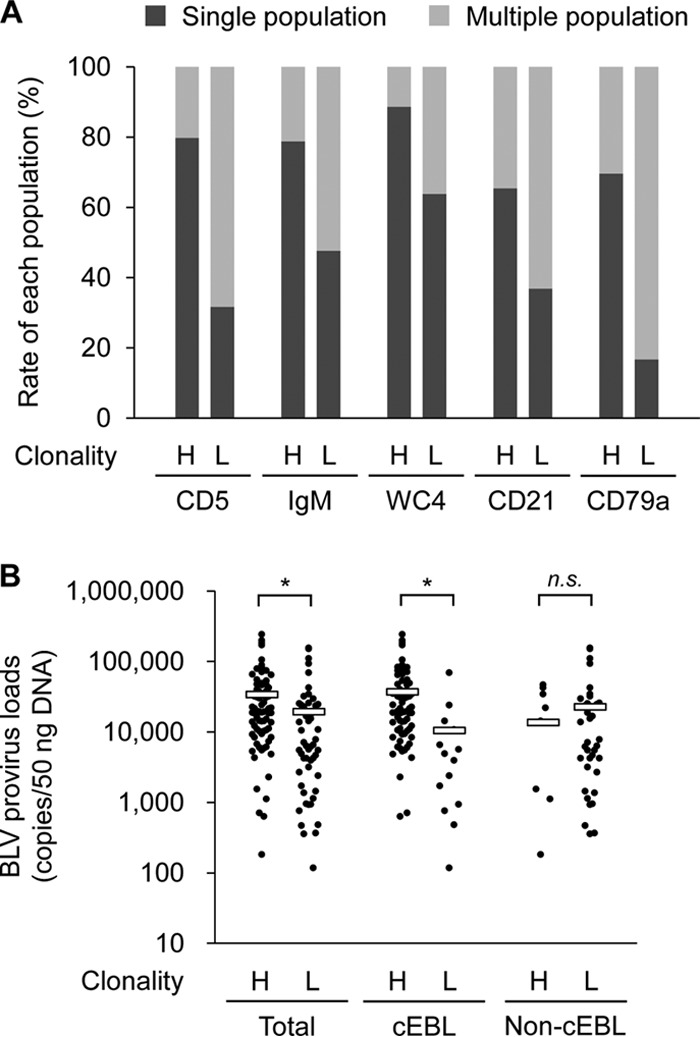
The relationship between population count in cell marker expression, B-cell clonality, and bovine leukemia virus (BLV) provirus loads. (A) The percentages of single (tumorigenic phenotype) or multiple (normal phenotype) cell populations for each cell marker were compared between high and low B-cell clonality. In all 176 samples from 50 cattle, 80 samples were of high clonality (H), 63 were of low clonality (L), and 33 were of unclear results or there were no data from flow cytometry analysis. (B) The relationship between B-cell clonality and BLV provirus loads. In all 176 samples from 50 cattle, 84 samples were of high clonality (H), 55 were of low clonality (L), and 37 were of unclear results or there were no data of provirus loads. The 50 cattle include 26 with classic enzootic bovine leukosis (cEBL) (H, n = 72; L, n = 15) and 24 non-cEBL cattle (H, n = 12; L, n = 40). *, P < 0.05 by Kruskal-Wallis test followed by Steel-Dwass test; n.s., not significant.
Classification and diagnosis of cattle with lymphoma.
All cattle with lymphoma were classified into five groups on the basis of the results of cell marker expression, B-cell clonality, and BLV provirus loads: classic EBL (cEBL), polyclonal EBL (pEBL), B-cell-type SBL (B-SBL), T-cell-type SBL (T-SBL), and nontypeable cases (Table 2). cEBL was defined as a monoclonal or oligoclonal B-cell lymphoma associated with BLV infection, whereas B-SBL was defined as B-cell lymphoma unrelated to BLV. The criteria of the association with BLV infection was set as ≥2,000 copies per 50 ng DNA (400 copies per 10 ng DNA), because according to a previous study, the average BLV copy number in whole blood from BLV-infected cattle without lymphoma was 330 copies per 10 ng DNA, whereas that of EBL cattle was 2,800 copies per 10 ng DNA (27). A novel type of EBL found in this study, pEBL, was similar to cEBL except for having low B-cell clonality, which suggested that pEBL was characterized by the neoplastic proliferation of polyclonal B cells. T-SBL was categorized as T-cell lymphoma regardless of BLV infection, and the nontypeable cases included non-T- or -B-cell tumors or simply a nonneoplastic lymphadenopathy (data not shown). Eventually, our diagnosis of cattle with lymphoma resulted in 52.0% with cEBL, 16.0% with pEBL, 8.0% with B-SBL, and 6.0% with T-SBL (Table 2). Although several samples derived from cEBL cattle showed low clonality, their provirus loads were significantly lower than those of high-clonality samples (Fig. 1B, middle and right). Thus, we speculate that these low-clonality samples were derived from immature sites of tumor development in cEBL cattle, containing large numbers of normal cells.
TABLE 2.
Classification and diagnosis of clinical samples suspected as bovine leukemia
| Diagnosis | Abbreviation | No. of cattle (%) | Cell type | B-cell clonality | BLV provirus loads (copies/50 ng DNA) |
|---|---|---|---|---|---|
| Classic EBL | cEBL | 2 (52.0) | B-cell | High | ≥2,000 |
| Polyclonal EBL | pEBL | 8 (16.0) | B-cell | Lowa | ≥2,000 |
| B-cell-type SBL | B-SBL | 4 (8.0) | B-cell | Higha | <2,000 |
| T-cell-type SBL | T-SBL | 3 (6.0) | T-cell | Lowa | Unrestricted |
| Nontypeable | 9 (18.0) | Did not correspond to any of the diagnoses | |||
These groups include samples that showed unclear results of clonality.
Difference in susceptibility to cEBL onset by breed and age.
To examine the relationship between the onset of bovine leukemia and the background information of cattle, the breeds and ages of the cattle were compared for each type of lymphoma. In the Japanese Black breed, 69.0% of the cattle were diagnosed with cEBL, followed by 13.8% with pEBL, and 3.4% each with B-SBL and T-SBL (Fig. 2, left). By contrast, in Holsteins, the percentage with cEBL was 23.5%, whereas the percentages with pEBL, B-SBL, and T-SBL were 17.6%, 17.6%, and 5.9%, respectively. These data suggested that the Japanese Black cattle are more susceptible to EBL onset than the Holstein cattle and, conversely, that Holstein cattle might be susceptible to SBL onset. Regarding the ages of the cattle, B-SBL and T-SBL were mainly found in juvenile calves, and pEBL seemed to occur in cattle >1 year old (Fig. 2, right). As we suspected, many cEBL cases were found in juvenile cattle <3 years old, and surprisingly, the frequency of an early onset of cEBL was equal to that in adult cattle. Thus, we found that most juvenile cattle that were clinically diagnosed as EBL were classified as having cEBL; hence, early onset of EBL truly occurred in Japanese cattle.
FIG 2.
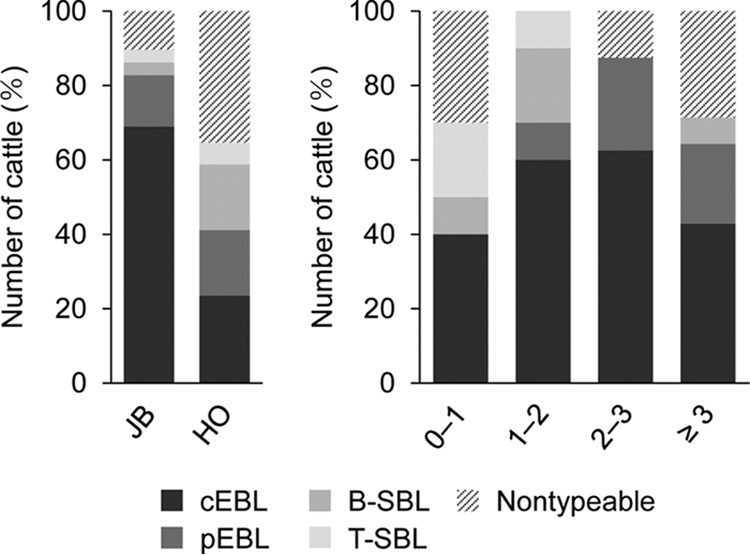
Breed and age of the cattle with lymphomas. The percentages and numbers of each type of lymphoma were compared according to the breed (left) and age (right) of the cattle. JB, Japanese Black; HO, Holstein. Numbers on the x axis on the right indicate age in years.
Lack of lymphocytosis in peripheral blood of cEBL cattle.
Another remarkable point is that there were nonnegligible numbers of cEBL cattle that did not show lymphocytosis, defined as lymphocyte counts >10,000 cells per 1 μl blood (Fig. 3A), which was not caused by a failure in counting of lymphocytes by an automated hemocytometer, because the numbers of lymphocytes strongly correlated with those of white blood cells (WBCs) (Fig. 3B). Furthermore, compared with those from the cattle that showed lymphocytosis, the peripheral blood mononuclear cells (PBMCs) from the cattle lacking lymphocytosis exhibited an immature tumor phenotype, which was defined as comprising multiple cell populations and low B-cell clonality (Table 3). The other sampling sites from these cattle without lymphocytosis, including lymph nodes and solid tumors in organs, were highly tumorigenic; for example, CE5 and CE6 in cEBL3 and CE37 in cEBL22 (see Data Set S1). Therefore, these results suggested the possibility that certain BLV-infected cattle developed B-cell lymphoma without going through a PL stage.
FIG 3.
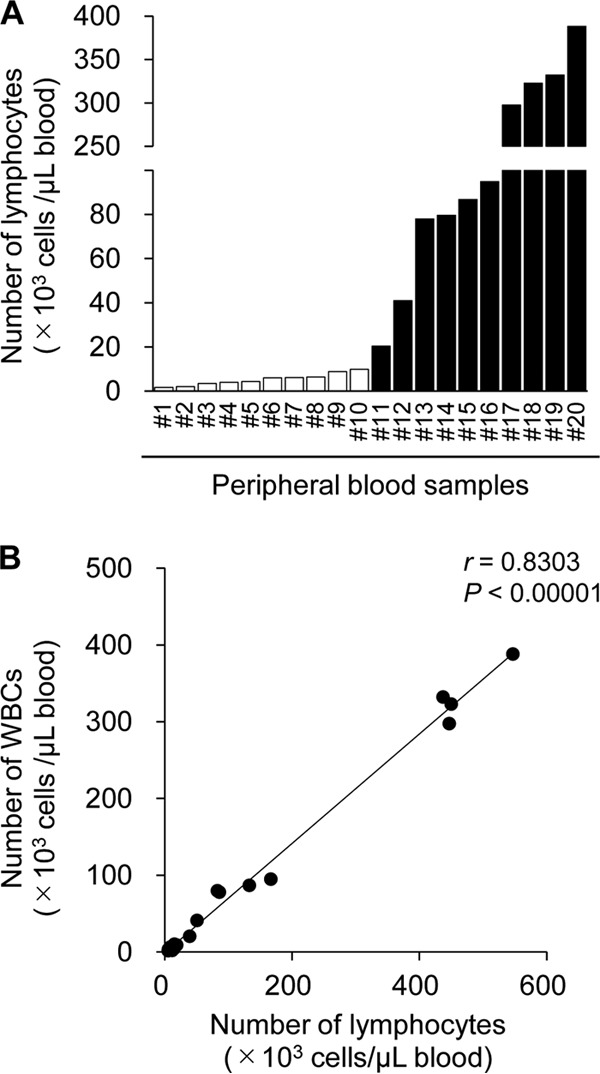
Lack of lymphocytosis in cattle with classic enzootic bovine leukosis (cEBL). (A) The numbers of lymphocytes in peripheral blood samples from cEBL cattle are shown (n = 20). Open bars indicate that the lymphocyte count was <10,000 cells per 1 μl of blood. (B) Correlation between the numbers of whole blood cells and lymphocytes in peripheral blood samples from cEBL cattle (n = 20).
TABLE 3.
Relationship between lymphocytosis and tumorigenesis in peripheral blood
| Lymphocytosis | No. of cases where tumorigenesis in peripheral blood was: |
|||
|---|---|---|---|---|
| Observed | Not observed | Not analysed | Total | |
| Observed | 10 | 0 | 0 | 10 |
| Not observed | 2 | 6 | 2 | 10 |
| Not analysed | 2 | 1 | 3 | 6 |
| Total | 14 | 7 | 5 | 26 |
Immunophenotyping of bovine B-cell lymphoma.
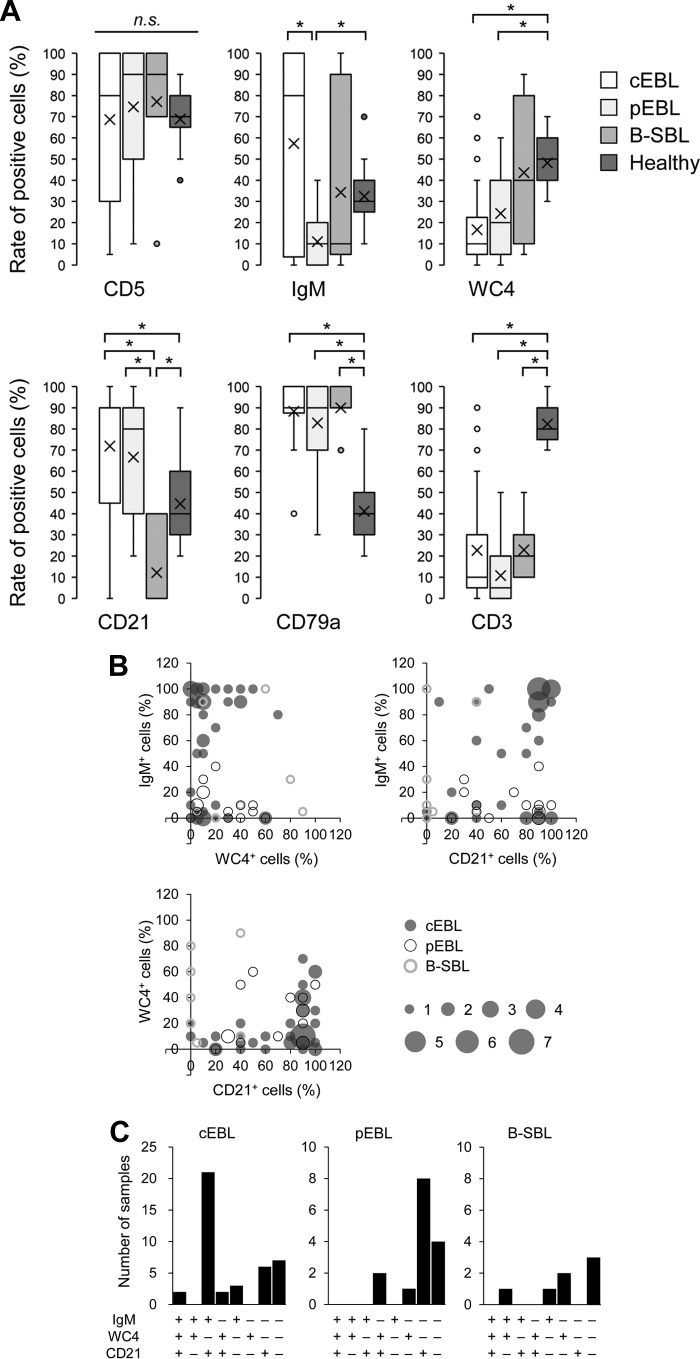
Our diagnosis of three types of B-cell lymphomas mainly depended on B-cell clonality and provirus loads, not on individual cell markers. To detect differences in expression patterns, the expression levels of each cell marker in cEBL were compared with those from the other B-cell lymphomas and healthy controls. The B-cell lymphomas were all clearly distinguished from a healthy phenotype by their high CD79a expression and low CD3 expression (Fig. 4A). Moreover, the expressions of WC4 in cEBL and pEBL were significantly lower than those in healthy controls, whereas the expression of CD21 was quite low in B-SBL compared with that in all other groups. The difference between cEBL and pEBL was determined by the significantly low levels of IgM expression in pEBL; however, cEBL still appeared to be divided into two populations: a major IgM+ group (IgM positive cells ≥ 50%) and a minor IgM− group (IgM positive cells < 50%). The expression patterns of three B-cell markers, IgM, WC4, and CD21, were distinctly different among the three types of B-cell lymphomas. To clarify their immunophenotypic character, dual and triple expressions of these cell markers were compared with each other (Fig. 4B and C). The typical pattern of cell marker expression in cEBL was IgM+, WC4−, and CD21+; by contrast, that of pEBL was IgM−, WC4−, and CD21+. There was no characteristic pattern in B-SBL except for low CD21 expression, although certain samples of B-SBL highly expressed WC4 (see Data Set S1, samples BS2 and BS3). A comparison of BLV provirus loads between cEBL and pEBL showed no difference (see Fig. S3A), and in agreement with this result, there was no significant difference in the expressions of viral protein gp51 (Fig. S3B). Moreover, the quantified provirus loads did not correlate with the expression levels of gp51 (Fig. S3C). Taken together, the three types of B-cell lymphomas, cEBL, pEBL, and B-SBL, showed different patterns of cell marker expression, which suggests marker expression could be applied as a tool for the diagnosis of bovine B-cell lymphoma.
FIG 4.
Immunophenotyping based on expression pattern of cell markers between three B-cell lymphomas and healthy controls. (A) The expression levels of six cell markers in samples with classic enzootic bovine leukosis ([cEBL] n = 42), polyclonal EBL ([pEBL] n = 15), B-cell-type sporadic bovine leukosis ([B-SBL] n = 7), and healthy controls (n = 17) are shown as box-and-whisker plots. Each box indicates the median and lower and upper quartiles and whiskers indicate lower and upper extremes. The “x” marks indicate the averages, and dots represent outliers that are much greater than normal or much less than normal. *, P < 0.05 by Kruskal-Wallis test followed by Steel-Dwass test. (B) Dual expressions of IgM, WC4, and CD21 in cEBL, pEBL, and B-SBL. Each axis indicates the percentages of positive cells, and the bubble size indicates the number of samples which showed identical expression patterns. (C) Triple expression of IgM, WC4, and CD21 in cEBL, pEBL, and B-SBL. +, cell marker expression ≥ 50%; −, cell-marker expression < 50%.
In this study, many juvenile cattle had developed cEBL (Fig. 2), so we attempted to characterize the juvenile cEBL by immunophenotyping. However, compared with those in adult cEBL cattle >3 years old, there was no clear difference in the expression levels of the cell markers (see Fig. S4A). Similarly, we did not find any correlation between BLV provirus loads and cattle age (Fig. S4B) or any difference in the expressions of viral protein gp51 (Fig. S4C). Therefore, these results suggested that the phenotypes of cEBL in juvenile cattle were similar to those of adult cattle; thus, the process for developing B-cell lymphoma might be similar in both generations.
Discriminant analysis between B-cell lymphoma and healthy controls.
To further characterize the three B-cell lymphomas on the basis of the expression levels of the six cell markers, a linear discriminant analysis was performed to discriminate between lymphomas and healthy controls. It was remarkable that the discriminant analysis correctly classified the three lymphomas and healthy controls with high sensitivity and specificity and an accuracy of >97.6% (Fig. 5A, top). Particularly, no classification error occurred between pEBL and controls or between B-SBL and controls. By contrast, a clear discrimination could not be obtained between cEBL and other types of B-cell lymphomas. Above all, the discrimination scores of cEBL and pEBL overlapped widely with each other, which was indicated by poor sensitivity (87.8%), specificity (61.5%), and accuracy (81.5%) (Fig. 5A, bottom). By contrast, the discrimination between pEBL and B-SBL was clear and showed correct classifications in 100% of both samples. Next, a multiple discriminant analysis was used to visualize the differences in cell marker expression among three B-cell lymphomas and healthy controls (Fig. 5B). This analysis showed independent clustering of marker expression in healthy controls from that of B-cell lymphoma, which indicated that cattle with B-cell lymphoma could be distinguished from healthy cattle by the expression patterns of the cell markers. Furthermore, pEBL and B-SBL were obviously distributed in two separate areas. However, the boundary between these two lymphomas and cEBL was unclear. Thus, the discriminant model based on cell marker expression was useful for discriminating between healthy cattle and cattle with B-cell lymphoma or between those with pEBL and B-SBL but remained insufficient to correctly classify the three types of B-cell lymphomas.
FIG 5.

Discriminant analysis of classic enzootic bovine leukosis (cEBL), polyclonal EBL (pEBL), B-cell-type sporadic bovine leukosis (B-SBL), and healthy controls. (A) Discriminant scores giving a classification performance of B-cell lymphomas and controls based on a linear discriminant analysis with the expression of six cell markers: CD5, IgM, WC4, CD21, CD79a, and CD3. (B) Scatter plots of multiple discriminant analyses to visualize the clustering of cell marker expression from each B-cell lymphoma and from healthy controls. First, second, and third linear discriminants are shown as LD1, LD2, and LD3, respectively. cEBL, n = 41; pEBL, n = 13; B-SBL, n = 7; healthy control, n = 17.
DISCUSSION
The diagnosis of EBL requires verification that the samples exhibit neoplastic proliferation of B cells and that BLV infection is associated with tumor development. In this study, we confirmed cell origins by using the expression levels of cell markers, BLV association by the amount of provirus, and tumor maturation of B cells by the clonal rearrangement of the IgH gene. Our examination classified the cattle clinically diagnosed as having bovine leukemia into five groups and revealed a novel type of EBL, pEBL (Table 2). Furthermore, we found several atypical EBL cases, including the onset of EBL in juvenile cattle and EBL lacking peripheral lymphocytosis (Fig. 2 and 3). Immunophenotyping of three B-cell lymphomas, that is, cEBL, pEBL, and B-SBL, made their characteristics clear, which was sufficient to enable their discrimination from healthy phenotypes (Fig. 5). However, a discriminant analysis between cEBL and pEBL or between cEBL and B-SBL did not show clear classification, which suggested that it was difficult to discriminate bovine B-cell lymphomas only by cell marker expression. Taken together, the combination of the analyses we performed in this study, that is, cell marker expression, population number, B-cell clonality, and BLV provirus loads, was useful for correctly diagnosing the type of bovine leukemia.
BLV spreads within the host by two distinct processes (2, 28). First, the virus replicates actively and infects a new target during the initial period of the infection (known as the infectious or replicative cycle). Then, the developed host immune response limits the infection of new target cells, and thus the cells whose provirus is inserted in genomic transcribed regions, not promoter regions, are selected. Therefore, the second process for viral replication depends on the proliferation and expansion of infected lymphocytes (also known as the mitotic cycle). In experimental infections in cattle, BLV transmission shifted from the infection of new targets to clonal expansion during the 2 months after inoculation, and negative selection by the host immune response eliminated 97% of the clones detected at seroconversion (28). Because of these processes for viral replication, the analysis of B-cell clonality was an effective method for distinguishing lymphomas from the early stage of BLV infection. Furthermore, our results indicate that it was possible to classify the samples from PL cattle, which were probably in the mitotic cycle, as having low clonality (Fig. S2 in the supplemental material). Thus, PCR-based IgH analysis has the potential to be a powerful tool for the simple diagnosis of B-cell lymphoma.
We cannot deny the possibility that cattle with pEBL were only in the PL stage because of the low B-cell clonality determined in the IgH analysis. However, a flow cytometric analysis of the samples from the cattle with pEBL indicated a single cell population that had shared cell markers with similar expression levels, and a solid mass was found in several organs in the gross pathological analysis (see Data Set S1). Therefore, pEBL phenotypes might be an intermediate state between the PL stage and the cEBL stage. By contrast, cEBL lacking peripheral lymphocytosis indicated the possibility for the direct development of B-cell lymphoma in cattle in the AL stage. Van der Maaten and Miller have described that, although cattle with persistent lymphocytosis have a high risk of developing tumors, the PL stage is not a requisite step in development of lymphoma (29). In addition, in one review on BLV infection, the authors described that tumors can occur directly in infected animals without lymphocytosis, whereas that was not clearly delineated prior to this work (2). Although the mechanism for the development of pEBL or nonlymphocytosis EBL has not been elucidated in detail, disease progression in BLV infection might be more complicated than previously assumed.
In this study, we determined the value of BLV provirus loads, 2,000 copies per 50 ng DNA, as the set point for BLV association with tumor development. However, there are still some doubts about the adequacy of this value as a borderline between cEBL and B-SBL. According to a previous study, the average copy number of the BLV gene in whole blood of BLV-infected cattle that did not show lymphoma was 330 copies per 10 ng DNA, but the maximum copy number in these cattle was 2,600 copies, which was nearly equal to the average copy number from EBL cattle (2,800 copies) (27). Thus, it appears to be difficult to distinguish B-SBL showing high BLV provirus loads from cEBL. A possible solution to enable distinguishing between cEBL and BLV-positive B-SBL is to use an inverse PCR method that identifies the clonality of integration sites of the BLV genome in the host, since cEBL consists of monoclonal expansion of cells in a B-cell clone which hold identical integration sites, whereas integration sites in each BLV-infected B cell in B-SBL are diverse, although this method takes longer to obtain the results. An establishment of simpler methods to analyze integration sites of BLV would be critical for the correct diagnosis of bovine leukemia.
It has been demonstrated that it takes a long time for B-cell lymphoma to develop during the disease progression of BLV infection; thus, EBL generally occurs in adult cattle >3 to 5 years old (2, 3). Previously, one report indicated that an early onset of EBL at <3 years of age was found in Japan, although the diagnosis of EBL was performed simply by detecting the env gene (26). Our results strongly support this finding, because a total of 20 juvenile calves were diagnosed as having cEBL (Fig. 2). The mechanism for an early onset of cEBL remains unclear, but there are several possible related factors, such as genomic viral mutation, host susceptibility, and infection during the fetal period. First, a mutation of the virus genome can affect the incubation period until tumor development, because in a previous study, the percentage of EBL cases associated with the L233-Tax protein was significantly higher than that associated with the P233-Tax in young cattle (26). Moreover, a mutation in an N-linked envelope glycosylation site (N230E) resulted in high provirus loads during experimental infection in sheep, which led to an accelerated pathogenesis and shortening of the incubation period (30). Second, it has been reported that certain alleles of the major histocompatibility complex class II DRB3 gene are involved in susceptibility and resistance against BLV infection (31, 32). Moreover, genomic diversity of the DRB3 gene varies between cattle breeds, and the BoLA-DRB3*1601 allele associated with susceptibility to a high BLV provirus load was frequent in Japanese Blacks but infrequent in Holsteins (33–35). Because our results showed a strong susceptibility to cEBL onset in Japanese Black cattle, this breed might be a key factor in understanding the mechanism for the development of bovine leukemia. Third, it is possible that early infection during the fetal period can become a cause of early onset EBL. In this study, there were several cEBL cattle <6 months old; especially, one of them was only 1 month old, which suggested vertical transmission of BLV in utero or in the birth canal (Data Set S1). An experimental infection with BLV in sheep demonstrated that splenectomized sheep, which lack an efficient immune response against viral replication, failed to control the progressive accumulation of infected cells, resulting in an accelerated onset of leukemia (36). Thus, the impairment of a BLV-specific immune response, such as immune tolerance induced by mother-to-child transmission, might be involved in the onset of cEBL in juvenile calves.
Previously, it was reported that CD5, which is a marker usually expressed in mature T cells, was highly expressed in B cells from BLV-infected cattle, and this CD5+ population in B cells expanded during PL (37). According to studies in humans and mice, mature B cells are divided into three types: B-1a cells (CD5+ CD11b+), B-1b cells (CD5− CD11b+), and B-2 cells (conventional B-cell, CD5− CD11b−) (38). B-1a and B-1b cells belong to the B-1 cell family and can be self-productive in maintaining their numbers in the body; thus, they have a longer life than that of conventional B cells. Because of this long life in the body, it has been considered that BLV can infect cattle during their entire life. Our data indicated that CD5+ lymphomas were predominant in cattle with cEBL and pEBL (Fig. 4), which suggested that the B-1 cell family was more common as a tumor origin, although CD5− lymphomas were found in this study and in previous reports (39).
The expression levels of the B-cell markers were beneficial for the characterization of B-cell lymphoma. It appears that CD79a is a valid cell marker to confirm a B-cell origin of samples for the diagnosis of cattle with lymphoma, although it is difficult to discriminate each type of B-cell lymphoma. In this study, cattle with cEBL were divided into two populations on the basis of their IgM expression, whereas all pEBL cases were IgM− phenotypes (Fig. 4). Moreover, both EBLs were characterized by high expression of CD21 and low expression of WC4 (CD19 homolog). We previously reported that IgMlow B cells did not express virus protein, whereas IgMhigh B cells highly expressed virus protein (40). Moreover, this report showed that treatment with anti-WC4 antibody increased the percentages of gp51+ cells in vitro. In humans, the CD19 molecule is one of the most reliable biomarkers for normal and neoplastic B cells and is involved in the modulation of B-cell receptor (BCR) signaling as a complex with a complement receptor, CD21 (41). Thus, there is a possibility that the expression levels of IgM, WC4, and CD21 in the samples from EBL cattle are involved in the modification of BCR signaling, which can affect the expression of virus protein. Although several B-cell lymphomas with diminished expression of CD19 have been reported in humans (42, 43), the significance of reduced CD19 expression in EBL cattle remains unknown.
EBL causes a large economic loss because it is a lethal disorder in cattle. Currently, there is no effective vaccine against BLV infection, and so potential biomarkers for the prediction of EBL onset are actively being investigated. However, common definitions for the classification of EBL and discrimination from other bovine lymphomas have not been established; thus, the classification of clinical samples has been performed according to the original criteria used in individual papers. Here, we identified novel criteria for the classification of bovine leukemia that are based on immunophenotypic features, which should be useful for obtaining more reliable clinical information on EBL onset. To elucidate the mechanisms underlying tumor development and to establish effective prediction methods for EBL onset, further analysis is required for a clear classification of bovine leukemia.
MATERIALS AND METHODS
Blood and tissue samples.
Peripheral blood and tissue, such as the spleen, lymph nodes, and solid tumors in several organs, were collected from cattle with lymphoma at livestock hygiene centers and meat hygiene inspection centers in Japan. Bovine blood samples from BLV-infected or uninfected cattle were obtained from several farmers, and BLV infection was diagnosed at the Hokkaido University Veterinary Teaching Hospital (Sapporo, Japan), as previously described (44). PBMCs were purified by density gradient centrifugation (Percoll; GE Healthcare, Little Chalfont, UK). Tumor samples were cut with scissors into small pieces, and the single-cell suspension was collected and washed twice with phosphate-buffered saline. Genomic DNA was extracted from 1 × 106 to 5 × 106 PBMCs or single cells from tissues using a Wizard genomic DNA purification kit (Promega, Madison, WI, USA).
Cell marker expression.
Cells were stained with antibodies specific to markers of T cells or B cells, as described previously (40, 45). Briefly, double staining was performed using anti-IgM (IL-A30; Bio-Rad, Hercules, CA, USA) prelabeled with Zenon Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, MA, USA) and the following antibodies: anti-CD5 (CACT105A; WSU Monoclonal Antibody Center, Pullman, WA, USA), anti-WC4 (CC55, CD19 like; Bio-Rad), anti-CD21 (GB25A; WSU Monoclonal Antibody Center), and anti-CD3 (MM1A; WSU Monoclonal Antibody Center). Alexa Fluor 647-conjugated anti-mouse IgG (Thermo Fisher Scientific) was used for the detection of antibody binding (anti-CD5, anti-WC4, anti-CD21, and anti-CD3). By contrast, cells were stained with anti-CD79a (HM57; Bio-Rad) and anti-BLV-gp51 (BLV1; WSU Monoclonal Antibody Center) prelabeled with Zenon Alexa Fluor 647 (Thermo Fisher Scientific) after treatment with FOXP3 Fix/Perm buffer (BioLegend, San Diego, CA, USA) and FOXP3 Perm buffer (BioLegend). To induce BLV antigen expression, cells were cultivated overnight in RPMI medium (Invitrogen, Carlsbad, CA, USA) containing 10% heat-inactivated fetal calf serum (Thermo Fisher Scientific), 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Thermo Fisher Scientific) before incubating with anti-BLV-gp51. Binding of the antibodies was detected using FACS Verse (BD Biosciences, San Jose, CA, USA) and FCS Express 4 (De Novo Software, Glendale, CA, USA).
B-cell clonality.
Genomic DNA extracted from blood and tissue samples was used as a template for PCR-based IgH analyses. The gene encoding the IgH region of interest was amplified using the following primer pairs: VH primer, 5′-AGC TCG AGA TGA ACC CAC TGT G-3′, and JH primer, 5′-AGA CTA GTG AAG ACT CTC GGG TGT G-3′, for the first-cycle PCR and CDR3 fw2 primer, 5′-C(G/T)G AGG AC(A/T) CGG CCA CAT A-3′, and JH primer for the second-cycle PCR (46). The amplification was performed in a reaction mixture containing 3 μl of 10× Ex Taq Buffer (TaKaRa Bio, Otsu, Japan), 2.4 μl of a 2.5 mM deoxynucleoside triphosphate (dNTP) mixture (TaKaRa Bio), 0.15 μl of TaKaRa Ex Taq (TaKaRa Bio), and 1 μl each of primers in ≤30 μl in double-distilled water. The PCR condition of the first or second cycle was as follows: one cycle at 96°C for 2 min, followed by a three-step procedure consisting of 20 s at 96°C, 30 s at 61°C, and 45 s at 72°C for 35 cycles (first-cycle PCR) or 20 s at 96°C, 30 s at 56°C, and 20 s at 72°C for 35 cycles (second-cycle PCR). The amplicons were confirmed by electrophoresis in an ethidium bromide-stained 3% Tris-borate-EDTA agarose gel. To confirm the identity of the amplicon in PCR-based IgH analysis, one amplified product was purified using the FastGene gel/PCR extraction kit (Nippon Genetics, Tokyo, Japan), was cloned into the pGEM-T Easy vector (Promega), and was sequenced using the CEQ 2000 DNA analysis system (Beckman Coulter, Fullerton, CA, USA).
BLV provirus loads.
The BLV Tax gene was amplified using DNA extracted from the blood and tissue samples of cattle with lymphomas. We performed the amplification in reaction mixtures containing 5 μl of a 2× Cycleave PCR mix (TaKaRa Bio), 0.5 μl of probe/primer mix for BLV (TaKaRa Bio), 1 μl of a DNA template, and 3.5 μl of PCR-grade water (TaKaRa Bio) using a real-time PCR system (LightCycler 480 system II; Roche Diagnostics, Mannheim, Germany), according to the manufacturer's instructions. Serial dilutions of the BLV-positive control (TaKaRa Bio) were used for generating calibration curves to determine the provirus loads. Each result is expressed as the number of BLV copies per 50 ng of genomic DNA determined using a NanoDrop 8000 spectrophotometer (Thermo Fisher Scientific).
Statistical analysis.
Differences between groups were examined for statistical significance using the Wilcoxon rank sum test. The Kruskal-Wallis tests followed by the Steel-Dwass tests were performed for multiple group comparisons. A P value of <0.05 was considered to indicate statistical significance. Linear discriminant analyses were performed using the data of cell marker expression to look for linear combinations of quantitative variables. The discrimination was derived by maximizing the separation of the groups in the data. To visualize the diversity of the data, a multiple discriminant analysis was performed, and the results are presented as scatter plots using three principal components of the scores.
Supplementary Material
ACKNOWLEDGMENTS
This research was partially supported by JSPS KAKENHI, Research Project for Improving Animal Disease Prevention Technologies to Combat Antimicrobial Resistance 2017-2021 FY, and by grants from the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry, Japan (grant no. 26058B to S.K.) and the NARO, Bio-oriented Technology Research Advancement Institution (a special scheme project on regional developing strategy; grant no. 16817557 to S.K.).
We thank Enago for the English language review.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/CVI.00067-17.
REFERENCES
- 1.Gillet N, Florins A, Boxus M, Burteau C, Nigro A, Vandermeers F, Balon H, Bouzar AB, Defoiche J, Burny A, Reichert M, Kettmann R, Willems L. 2007. Mechanisms of leukemogenesis induced by bovine leukemia virus: prospects for novel anti-retroviral therapies in human. Retrovirology 4:18. doi: 10.1186/1742-4690-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutiérrez G, Rodríguez SM, de Brogniez A, Gillet N, Golime R, Burny A, Jaworski JP, Alvarez I, Vagnoni L, Trono K, Willems L. 2014. Vaccination against δ-retroviruses: the bovine leukemia virus paradigm. Viruses 6:2416–2427. doi: 10.3390/v6062416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsutsui T, Kobayashi S, Hayama Y, Yamamoto T. 2016. Fraction of bovine leukemia virus-infected dairy cattle developing enzootic bovine leucosis. Prev Vet Med 124:96–101. doi: 10.1016/j.prevetmed.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 4.Ooshiro M, Konnai S, Katagiri Y, Afuso M, Arakaki N, Tsuha O, Murata S, Ohashi K. 2013. Horizontal transmission of bovine leukemia virus from lymphocytotic cattle, and beneficial effects of insect vector control. Vet Rec 173:527. doi: 10.1136/vr.101833. [DOI] [PubMed] [Google Scholar]
- 5.Rodríguez SM, Florins A, Gillet N, de Brogniez A, Sánchez-Alcaraz MT, Boxus M, Boulanger F, Gutiérrez G, Trono K, Alvarez I, Vagnoni L, Willems L. 2011. Preventive and therapeutic strategies for bovine leukemia virus: lessons for HTLV. Viruses 3:1210–1248. doi: 10.3390/v3071210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mekata H, Sekiguchi S, Konnai S, Kirino Y, Honkawa K, Nonaka N, Horii Y, Norimine J. 2015. Evaluation of the natural perinatal transmission of bovine leukaemia virus. Vet Rec 176:254. doi: 10.1136/vr.102464. [DOI] [PubMed] [Google Scholar]
- 7.Lairmore MD. 2014. Animal models of bovine leukemia virus and human T-lymphotrophic virus type-1: insights in transmission and pathogenesis. Annu Rev Anim Biosci 2:189–208. doi: 10.1146/annurev-animal-022513-114117. [DOI] [PubMed] [Google Scholar]
- 8.Merimi M, Ozkan Y, Cleuter Y, Griebel P, Burny A, Martiat P, Van den Broeke A. 2009. Epigenetics and leukemia: unraveling oncogenic processes in the BLV ovine model. Front Biosci (Schol Ed.) 1:154–163. doi: 10.2741/s15. [DOI] [PubMed] [Google Scholar]
- 9.Grimshaw WT, Wiseman A, Petrie L, Selman IE. 1979. Bovine leucosis (lymphosarcoma): a clinical study of 60 pathologically confirmed cases. Vet Rec 105:267–272. doi: 10.1136/vr.105.12.267. [DOI] [PubMed] [Google Scholar]
- 10.Asahina M, Kimura K, Murakami K, Ajito T, Wu D, Goryo M, Aida Y, Davis WC, Okada K. 1995. Phenotypic analysis of neoplastic cells from calf, thymic, and intermediate forms of bovine leukosis. Vet Pathol 32:683–691. doi: 10.1177/030098589503200610. [DOI] [PubMed] [Google Scholar]
- 11.Grünberg W, Eisenberg SW. 2013. Atypical form of sporadic bovine leukosis (SBL) in the Netherlands. Vet Rec 173:398. doi: 10.1136/vr.101885. [DOI] [PubMed] [Google Scholar]
- 12.Bartlett PC, Sordillo LM, Byrem TM, Norby B, Grooms DL, Swenson CL, Zalucha J, Erskine RJ. 2014. Options for the control of bovine leukemia virus in dairy cattle. J Am Vet Med Assoc 244:914–922. doi: 10.2460/javma.244.8.914. [DOI] [PubMed] [Google Scholar]
- 13.Pan LX, Diss TC, Peng HZ, Isaacson PG. 1994. Clonality analysis of defined B-cell populations in archival tissue sections using microdissection and the polymerase chain reaction. Histopathology 24:323–327. doi: 10.1111/j.1365-2559.1994.tb00532.x. [DOI] [PubMed] [Google Scholar]
- 14.Ilyas M, Jalal H, Linton C, Rooney N. 1995. The use of the polymerase chain reaction in the diagnosis of B-cell lymphomas from formalin-fixed paraffin-embedded tissue. Histopathology 26:333–338. doi: 10.1111/j.1365-2559.1995.tb00194.x. [DOI] [PubMed] [Google Scholar]
- 15.Valli VE, Vernau W, de Lorimier LP, Graham PS, Moore PF. 2006. Canine indolent nodular lymphoma. Vet Pathol 43:241–256. doi: 10.1354/vp.43-3-241. [DOI] [PubMed] [Google Scholar]
- 16.Burnett RC, Vernau W, Modiano JF, Olver CS, Moore PF, Avery AC. 2003. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol 40:32–41. doi: 10.1354/vp.40-1-32. [DOI] [PubMed] [Google Scholar]
- 17.Tamura K, Yagihara H, Isotani M, Ono K, Washizu T, Bonkobara M. 2006. Development of the polymerase chain reaction assay based on the canine genome database for detection of monoclonality in B cell lymphoma. Vet Immunol Immunopathol 110:163–167. doi: 10.1016/j.vetimm.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Rütgen BC, Hammer SE, Gerner W, Christian M, de Arespacochaga AG, Willmann M, Kleiter M, Schwendenwein I, Saalmüller A. 2010. Establishment and characterization of a novel canine B-cell line derived from a spontaneously occurring diffuse large cell lymphoma. Leuk Res 34:932–938. doi: 10.1016/j.leukres.2010.01.021. [DOI] [PubMed] [Google Scholar]
- 19.Werner JA, Woo JC, Vernau W, Graham PS, Grahn RA, Lyons LA, Moore PF. 2005. Characterization of feline immunoglobulin heavy chain variable region genes for the molecular diagnosis of B-cell neoplasia. Vet Pathol 42:596–607. doi: 10.1354/vp.42-5-596. [DOI] [PubMed] [Google Scholar]
- 20.Sinkora M, Sun J, Sinkorová J, Christenson RK, Ford SP, Butler JE. 2003. Antibody repertoire development in fetal and neonatal piglets. VI. B cell lymphogenesis occurs at multiple sites with differences in the frequency of in-frame rearrangements. J Immunol 170:1781–1788. doi: 10.4049/jimmunol.170.4.1781. [DOI] [PubMed] [Google Scholar]
- 21.Niku M, Pessa-Morikawa T, Andersson LC, Iivanainen A. 2002. Oligoclonal Peyer's patch follicles in the terminal small intestine of cattle. Dev Comp Immunol 26:689–695. doi: 10.1016/S0145-305X(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 22.Nuotio L, Rusanen H, Sihvonen L, Neuvonen E. 2003. Eradication of enzootic bovine leukosis from Finland. Prev Vet Med 59:43–49. doi: 10.1016/S0167-5877(03)00057-6. [DOI] [PubMed] [Google Scholar]
- 23.Acaite J, Tamosiunas V, Lukauskas K, Milius J, Pieskus J. 2007. The eradication experience of enzootic bovine leukosis from Lithuania. Prev Vet Med 82:83–89. doi: 10.1016/j.prevetmed.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 24.Maresca C, Costarelli S, Dettori A, Felici A, Iscaro C, Feliziani F. 2015. Enzootic bovine leukosis: report of eradication and surveillance measures in Italy over an 8-year period (2005-2012). Prev Vet Med 119:222–226. doi: 10.1016/j.prevetmed.2015.02.024. [DOI] [PubMed] [Google Scholar]
- 25.Murakami K, Kobayashi S, Konishi M, Kameyama K, Tsutsui T. 2013. Nationwide survey of bovine leukemia virus infection among dairy and beef breeding cattle in Japan from 2009-2011. J Vet Med Sci 75:1123–1126. doi: 10.1292/jvms.12-0374. [DOI] [PubMed] [Google Scholar]
- 26.Inoue E, Matsumura K, Soma N, Hirasawa S, Wakimoto M, Arakaki Y, Yoshida T, Osawa Y, Okazaki K. 2013. L233P mutation of the Tax protein strongly correlated with leukemogenicity of bovine leukemia virus. Vet Microbiol 167:364–371. doi: 10.1016/j.vetmic.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Somura Y, Sugiyama E, Fujikawa H, Murakami K. 2014. Comparison of the copy numbers of bovine leukemia virus in the lymph nodes of cattle with enzootic bovine leukosis and cattle with latent infection. Arch Virol 159:2693–2697. doi: 10.1007/s00705-014-2137-9. [DOI] [PubMed] [Google Scholar]
- 28.Gillet NA, Gutiérrez G, Rodriguez SM, de Brogniez A, Renotte N, Alvarez I, Trono K, Willems L. 2013. Massive depletion of bovine leukemia virus proviral clones located in genomic transcriptionally active sites during primary infection. PLoS Pathog 9:e1003687. doi: 10.1371/journal.ppat.1003687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van der Maaten MJ, Miller JM. 1990. Bovine leukosis virus, p 419–429. In Dinter Z, Morein B (ed), Virus infections of ruminants, vol 3 Elsevier Science Publisher BV, Amsterdam, Netherlands. [Google Scholar]
- 30.de Brogniez A, Bouzar AB, Jacques JR, Cosse JP, Gillet N, Callebaut I, Reichert M, Willems L. 2015. Mutation of a single envelope N-linked glycosylation site enhances the pathogenicity of bovine leukemia virus. J Virol 89:8945–8956. doi: 10.1128/JVI.00261-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forletti A, Juliarena MA, Ceriani C, Amadio AF, Esteban E, Gutiérrez SE. 2013. Identification of cattle carrying alleles associated with resistance and susceptibility to the bovine leukemia virus progression by real-time PCR. Res Vet Sci 95:991–995. doi: 10.1016/j.rvsc.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 32.Miyasaka T, Takeshima SN, Jimba M, Matsumoto Y, Kobayashi N, Matsuhashi T, Sentsui H, Aida Y. 2013. Identification of bovine leukocyte antigen class II haplotypes associated with variations in bovine leukemia virus proviral load in Japanese Black cattle. Tissue Antigens 81:72–82. doi: 10.1111/tan.12041. [DOI] [PubMed] [Google Scholar]
- 33.Miyasaka T, Takeshima SN, Matsumoto Y, Kobayashi N, Matsuhashi T, Miyazaki Y, Tanabe Y, Ishibashi K, Sentsui H, Aida Y. 2011. The diversity of bovine MHC class II DRB3 and DQA1 alleles in different herds of Japanese Black and Holstein cattle in Japan. Gene 472:42–49. doi: 10.1016/j.gene.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 34.Miyasaka T, Takeshima SN, Sentsui H, Aida Y. 2012. Identification and diversity of bovine major histocompatibility complex class II haplotypes in Japanese Black and Holstein cattle in Japan. J Dairy Sci 95:420–431. doi: 10.3168/jds.2011-4621. [DOI] [PubMed] [Google Scholar]
- 35.Takeshima S, Saitou N, Morita M, Inoko H, Aida Y. 2003. The diversity of bovine MHC class II DRB3 genes in Japanese Black, Japanese Shorthorn, Jersey and Holstein cattle in Japan. Gene 316:111–118. doi: 10.1016/S0378-1119(03)00744-3. [DOI] [PubMed] [Google Scholar]
- 36.Florins A, Reichert M, Asquith B, Bouzar AB, Jean G, François C, Jasik A, Burny A, Kettmann R, Willems L. 2009. Earlier onset of δ-retrovirus-induced leukemia after splenectomy. PLoS One 4:e6943. doi: 10.1371/journal.pone.0006943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Depelchin A, Letesson JJ, Lostrie-Trussart N, Mammerickx M, Portetelle D, Burny A. 1989. Bovine leukemia virus (BLV)-infected B-cells express a marker similar to the CD5 T cell marker. Immunol Lett 20:69–76. doi: 10.1016/0165-2478(89)90071-0. [DOI] [PubMed] [Google Scholar]
- 38.Kantor AB, Stall AM, Adams S, Herzenberg LA, Herzenberg LA. 1992. Differential development of progenitor activity for three B-cell lineages. Proc Natl Acad Sci U S A 89:3320–3324. doi: 10.1073/pnas.89.8.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yin SA, Makara M, Pan Y, Ishiguro H, Ikeda M, Numakunai S, Goryo M, Okada K. 2003. Relation between phenotype tumor cells and clinicopathology in bovine leukosis. J Vet Med Sci 65:599–606. doi: 10.1292/jvms.65.599. [DOI] [PubMed] [Google Scholar]
- 40.Ikebuchi R, Konnai S, Okagawa T, Nishimori A, Nakahara A, Murata S, Ohashi K. 2014. Differences in cellular function and viral protein expression between IgMhigh and IgMlow B-cells in bovine leukemia virus-infected cattle. J Gen Virol 95:1832–1842. doi: 10.1099/vir.0.065011-0. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Wei G, Liu D. 2012. CD19: a biomarker for B cell development, lymphoma diagnosis and therapy. Exp Hematol Oncol 1:36. doi: 10.1186/2162-3619-1-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang W, Agrawal N, Patel J, Edinger A, Osei E, Thut D, Powers J, Meyerson H. 2005. Diminished expression of CD19 in B-cell lymphomas. Cytometry B Clin Cytom 63:28–35. doi: 10.1002/cyto.b.20030. [DOI] [PubMed] [Google Scholar]
- 43.Masir N, Marafioti T, Jones M, Natkunam Y, Rüdiger T, Hansmann ML, Mason DY. 2006. Loss of CD19 expression in B-cell neoplasms. Histopathology 48:239–246. doi: 10.1111/j.1365-2559.2005.02317.x. [DOI] [PubMed] [Google Scholar]
- 44.Ikebuchi R, Konnai S, Shirai T, Sunden Y, Murata S, Onuma M, Ohashi K. 2011. Increase of cells expressing PD-L1 in bovine leukemia virus infection and enhancement of anti-viral immune responses in vitro via PD-L1 blockade. Vet Res 42:103. doi: 10.1186/1297-9716-42-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishimori A, Konnai S, Ikebuchi R, Okagawa T, Nakahara A, Murata S, Ohashi K. 2016. Direct polymerase chain reaction from blood and tissue samples for rapid diagnosis of bovine leukemia virus infection. J Vet Med Sci 78:791–796. doi: 10.1292/jvms.15-0577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saini SS, Allore B, Jacobs RM, Kaushik A. 1999. Exceptionally long CDR3H region with multiple cysteine residues in functional bovine IgM antibodies. Eur J Immunol 29:2420–2426. doi:. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.