Abstract
Background
Serum erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) are important tests in the initial diagnosis of periprosthetic joint infection. Many surgeons also use these tests to determine if infection has resolved between stages of a 2-stage procedure, but little data exist regarding this practice.
Methods
A retrospective review of our institutional total joint databases was conducted to determine sensitivity, specificity, and predictive values of elevated ESR and/or CRP to diagnose persistent infection between stages.
Results
Among 16 knees and 5 hips, sensitivity was 50% for CRP, 75% for ESR, and 100% when combined. The negative predictive value of persistent infection was 100% when neither test was elevated.
Conclusions
Results of this study support the use of CRP and ESR as indicators of the resolution of periprosthetic joint infection between stages of 2-stage revision.
Keywords: Periprosthetic infection, 2-stage revision, Erythrocyte sedimentation rate, C-reactive protein
Introduction
Periprosthetic joint infection (PJI) is an important problem in hip and knee replacement surgery, as on average, between 1% and 2.5% [1], [2] of patients undergoing primary total joint replacement of the hip or knee will suffer a deep PJI. Well-developed, evidence-based algorithms are in place for the initial diagnosis of PJI [3], and there is significant evidence to support the use of erythrocyte sedimentation rate (ESR) [1], [2], [4], [5], [6], [7], [8] and C-reactive protein (CRP) [2], [4], [5], [6], [8], [9] as a part of these diagnostic criteria.
Following the initial PJI diagnosis, therapeutic options must be considered, including surgical revision and reimplantation (1 stage or 2 stage) or debridement with retention of some or all of the prosthetic components [10]. In North America, in the setting of chronic infection, 2-stage revision and reimplantation are most commonly performed [3]. In this procedure, the prosthetic components are removed, the joint is debrided, and an antibiotic eluting spacer is placed. A course of intravenous antibiotics is administered, and following resolution of infection, reimplantation with new components is usually undertaken. Unfortunately, there is no currently accepted algorithm to determine infection resolution between stages. Although well established as important tools to diagnose PJI, there is not yet reliable evidence that low ESR and/or CRP values can be used as an indicator of the elimination of infection or as a clinical marker for the timing of the second stage of surgical revision. The purpose of this study is to evaluate the usefulness of serum ESR and CRP levels in determining resolution of infection prior to reimplantation in 2-stage revision for treatment of PJI.
Material and methods
After institutional review board approval, a retrospective review of our institutional database was conducted to identify cases of PJI treated at our institution between July 1, 2004 and July 1, 2012. Following identification, patient records were accessed to evaluate for inclusion in the study. Inclusion criteria for this study were: patients with a history of plan for 2-stage revision and reimplantation following a diagnosis of periprosthetic infection of the hip or knee joint according to the retrospectively applied Musculoskeletal Infection Society (MSIS) guidelines [11] with 2-year follow-up. Specifically, records were screened for infection with: communicating sinus tract with the prosthesis, or a pathogen isolated by culture from 2 or more fluid or tissue samples from the joint, or by fulfilling 4/6 of the following: (1) elevated ESR and CRP, (2) elevated synovial white blood cell count, (3) elevated synovial polymorphonuclear percentage, (4) presence of purulence in the affected joint, (5) culture isolation of a microorganism from one tissue or fluid sample, and (6) greater than 5 neutrophils per high power field in 5 fields at histologic examination at ×400 magnification [11]. There were no exclusion criteria other than not meeting inclusion criteria. During the period described, 26 hips were identified that retrospectively met MSIS guidelines for infection. Of those patients, 19 were excluded because they were not primary total hip arthroplasty with a plan for 2-stage revision and 2 were excluded because they did not have an adequate follow-up period, leaving 5 hips included in the review. Over the same period, 55 knees were identified that met infection criteria, 39 were excluded because they were not primary total knee arthroplasty with plan for 2-stage revision, leaving 16 knees included. Overall, 21 consecutive 2-stage revisions for periprosthetic infection were identified that met inclusion criteria (16 knees, 5 hips).
In all cases, all components (and cement if present) were removed and an antibiotic-impregnated cement spacer was placed in the first stage of revision. All patients were treated with intravenous organism-specific antibiotics under consultation with infectious disease specialists. Patients were followed in clinic, and serial ESR and CRP measurements were obtained. The final decision regarding the second stage reimplantation occurred at the discretion of the treating surgeon when clinical, radiologic, and laboratory results were felt to be consistent with infection resolution.
For each patient that met inclusion criteria, relevant data were extracted from the medical record, including the ESR and CRP values before first-stage resection, as well as before the second stage reimplantation. An ESR greater than 30 mm/h and a CRP greater than 1 mg/dL were defined as elevated (positive) as suggested by the MSIS workgroup [11]. Persistent infection was defined as any clinical recurrence of PJI according to the aforementioned MSIS guidelines during a follow-up period of at least 2 years after reimplantation.
Results
In this series, reimplantation of the joint took place an average of 207 days after removal of prosthesis (range of 96-483 days). Following revision and reimplantation, 4 of the 21 reimplanted joints suffered from persistent periprosthetic infection. No significant differences in age of the patients or time interval between stages were noted between patients that cleared infection and patients that were classified as persistently infected (Table 1). While 3 out of the 4 (75%) patients who experienced persistent infection were female, there was no significant gender difference identified in patients in whom the infection was successfully eradicated. No significant difference in organisms isolated was identified in this study, and Staphylococci species were most commonly identified in this series (Table 2).
Table 1.
Patient demographics.
| Category | Infection eradicated | Persistent infection |
|---|---|---|
| Average age | 65.6 (95% CI 61.3-70.0) | 67.8 (95% CI 58.8-76.7) |
| Males | 10 (59%) | 1 (25%) |
| Females | 7 (41%) | 3 (75%) |
| Interval between stages | 202 d (95% CI 155-251) | 228 d (95% CI 129-327) |
The distribution of demographics of the patients included in analysis is shown. There were no significant differences in age, gender, or timing of second stage of revision seen in our study.
Table 2.
Organisms identified in original PJI.
| Organism | Infection eradicated | Persistent infection |
|---|---|---|
| MRSA | 3 | 1 |
| OSSA | 3 | 2 |
| Coag. neg. Staph. | 2 | |
| Diphtherioids | 2 | |
| Propionibacterium | 1 | 1 |
| E. coli | 1 | |
| Candida spp. | 1 | |
| Culture negative | 2 | |
| No culture found | 2 |
There was not a single predominant organism in either group. Staph. species were found in 3/4 patients with persistent infection; however Staph. species were also found in patients without persistent infection, and there is no significant difference in organisms isolated.
The mean ESR value before reimplantation was 15.4 (95% confidence interval [CI] 9.6-21.2) in patients without clinical recurrence and 39.0 (95% CI 27.0-51.0) in patients with persistent infection defined by clinical recurrence. In an unpaired, 2-tailed, Student t test, the P value of this difference was .0015. The mean CRP value before reimplantation was 0.75 (95% CI 0.10-1.40) in patients without clinical recurrence and 2.92 (95% CI 1.59-4.26) in patients with persistent infection defined by clinical recurrence. The P value of this difference was .0063.
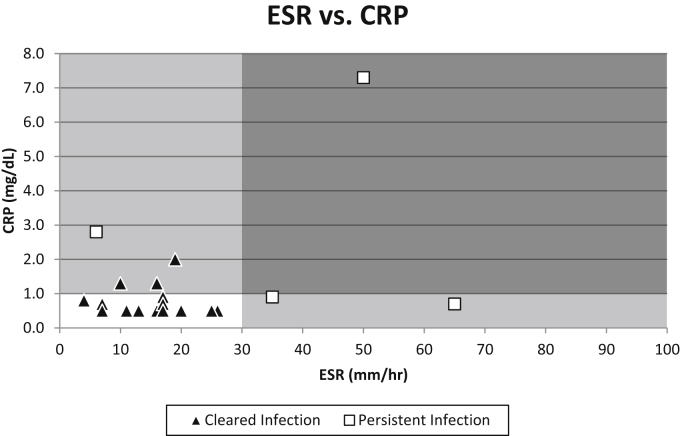
Of the 17 patients who were successfully treated, 14 (67%) had normalization of both ESR and CRP before reimplantation. Alternatively, 3 patients (18%) had isolated CRP elevation, no patient had isolated ESR elevation, and no patient had both values elevated. Of the total 21 patients, 4 patients experienced recurrent periprosthetic infection, indicating a failure to eradicate infection before reimplantation. Of these 4 patients between stages before reimplantation, 1 had an isolated elevation of CRP, 2 had isolated ESR elevation, and 1 patient had elevation of both ESR and CRP. Test characteristics for ESR, CRP, and the 2 values combined were determined (Table 3). The sensitivity to detect persistent infection of ESR alone was 75%, with specificity of 100%, positive predictive value (PPV) of 100%, and negative predictive value (NPV) of 94.4%. For CRP alone, sensitivity was 50%, specificity was 82%, PPV 40%, and NPV 87.5%. ESR and CRP combined, performed better than the tests individually. Combined sensitivity, specificity, PPV, and NPV were all 100%. Patients who experienced persistent infection were seen to have either a markedly higher value for ESR, CRP, or both (Fig. 1). All patients that experienced successful eradication of infection can be seen to be either within or near the cutoff values for both ESR and CRP.
Table 3.
Test characteristics for ESR and CRP in identifying persistent infection.
| “Positive” test | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) |
|---|---|---|---|---|
| CRP elevated | 50.0 | 82.4 | 40.0 | 87.5 |
| ESR elevated | 75.0 | 100 | 100 | 94.4 |
| Both values elevated | 25.0 | 100 | 100 | 85.0 |
| Either value elevated | 100 | 82.4 | 57.1 | 100 |
Shown in this table are the sensitivity, specificity, and both positive and negative predictive values for the tests alone and combined. Results for specificity and negative predictive value are more compelling due to higher patient numbers. In our study, using the criteria of both tests positive (as a rule-in test), we obtained a specificity and PPV of 100%, while using the criteria of either test positive (as a rule-out test), we obtained sensitivity and NPV of 100%.
Figure 1.
In this figure, patients who cleared their infection without clinical recurrence are notated by triangle markers, while patients who experienced persistent infection are notated by square markers. Clinical cutoff values for ESR (30 mm/h) and CRP (1 mg/dL) are demonstrated by the shaded areas of the figure. It is clear that patients with persistent infection demonstrated either dramatically elevated ESR, CRP, or both.
Discussion
The most notable result in our study is the NPV of ESR and CRP when used as a combined test. Of the 14 patients who experienced normalization of both markers before the second stage reimplantation of their prosthesis, none experienced clinical recurrence, yielding an NPV of 100%. This finding is consistent with expectations, as ESR and CRP are considered to be sensitive markers of inflammation. Ultimately, this study does support the hypothesis that if ESR and CRP are both within normal limits before reimplantation, infection has likely been eradicated.
An interesting observation was the high PPVs in our study, especially of ESR and of the 2 tests combined. We believe that this finding may be questionable because many patients in our study had ESR values in the high 20s and were therefore borderline elevated according to MSK Infection Society guidelines. It is difficult to draw firm conclusions regarding sensitivity and PPV in this study because of the small sample size for patients who experienced persistent infection. Further research with larger numbers of patients would facilitate statistical analysis, to include receiver operating characteristic curves on ESR and CRP between stages of 2-stage revision in both the hip and the knee, to ascertain optimal target values for these laboratories before reimplantation.
As in all retrospective reviews, there are obvious limitations to consider. The retrospective nature introduces a potential for bias in analysis, and the small sample size inherent in this relatively infrequent surgery at this institution limits the strength of conclusions drawn. Low numbers of persistently infected patients, especially, do not allow the use of a receiver operating characteristic curve to identify optimum cutoff values. The majority of cases in this series predate publication of MSK Infection Society guidelines, which could create challenges obtaining standardized data from charts; however, at our institution, surgeons regularly obtained the data required for classification even before guideline publication.
While ESR and CRP have been extensively studied as tools for diagnosis of PJI, there remains a relative paucity of published literature studying ESR and CRP between stages of 2-stage revision. Our search of the literature yielded 3 previous studies [12], [13], [14] which examined similar questions. One interesting prior study by Shukla et al [12] suggested the utility of ESR and CRP to determine resolution of infection between stages of 2-stage revision for PJI was limited based on evidence that the specificity was too low to be useful, especially in patients with other inflammatory conditions such as rheumatoid arthritis, lupus, etc. Two other studies (Ghanem et al [13] and Kusuma et al [14]), both concluded that ESR and CRP were not useful based on test characteristics and receiver operating characteristic curves. Neither of these studies found a difference in mean ESR or CRP between patients with or without persistent infection. Kusuma et al reported optimal ESR and CRP cutoff values of 43.5 mm/h and 17.75 mg/L, respectively. Based on those cutoff values, the sensitivities and NPVs were 0.67 and 0.05 for ESR and 0.17 and 0.07 for CRP. Unfortunately, both of these studies suffered from low patient numbers and were underpowered to detect a true difference. Like prior studies, our study does not support a high specificity for these serum tests. In contrast with existing literature, however, our data suggest that they are potentially useful on the merit of their sensitivity and NPV, particularly in patients who had normal inflammatory markers at baseline. Our data also suggest that there is a true difference in ESR and CRP values during this period between patients who have cleared infection, and those who are persistently infected, though unfortunately is unable to precisely identify cutoff values due to low patient numbers.
Conclusions
Despite their limitations, serum ESR and CRP continue to have utility as “rule-out” tests in the interim period between stages of a 2-stage revision. Though a clear cutoff remains difficult to identify, this study indicates that a clear difference exists in both ESR and CRP values between patients who clear infection and those in whom infection persists. Additionally, in patients in whom both serum values return to normal levels, the likelihood of persistent infection is low. More information in larger studies is needed to identify optimal cutoff values and timing of serum testing to improve how these tests inform clinical decisions, but based on the data in this study, these authors conclude that it is a reasonable practice at this time for surgeons to continue to follow ESR and CRP throughout revision and reimplantation for PJI. At our institution, we continue to obtain serum ESR and CRP values for patients between stages of 2-stage revision for PJI to help guide decision-making.
Acknowledgments
The authors thank the NIH, who partially supported this work under short-term research grant #T35DK007386-33, as well as the NC-Tracs Institute, who provided assistance with database searches and data review.
Footnotes
One or more of the authors of this paper have disclosed potential or pertinent conflicts of interest, which may include receipt of payment, either direct or indirect, institutional support, or association with an entity in the biomedical field which may be perceived to have potential conflict of interest with this work. For full disclosure statements refer to http://dx.doi.org/10.1016/j.artd.2016.08.002.
Appendix A. Supplementary data
References
- 1.Lentino J.R. Prosthetic joint infections: bane of orthopedists, challenge for infectious disease specialists. Clin Infect Dis. 2003;36:1157. doi: 10.1086/374554. [DOI] [PubMed] [Google Scholar]
- 2.Schinsky M.F., Della Valle C.J., Sporer S.M., Paprosky W.G. Perioperative testing for joint infection in patients undergoing revision total hip arthroplasty. J Bone Joint Surg Am. 2008;90(9):1869. doi: 10.2106/JBJS.G.01255. [DOI] [PubMed] [Google Scholar]
- 3.Parvizi J., Della Valle C.J. AAOS Clinical Practice Guideline: diagnosis and treatment of periprosthetic joint infections of the hip and knee. J Am Acad Orthop Surg. 2010;18:771. doi: 10.5435/00124635-201012000-00007. [DOI] [PubMed] [Google Scholar]
- 4.Bottner F., Wegner A., Winkelmann W. Interleukin- 6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89(1):94. doi: 10.1302/0301-620X.89B1.17485. [DOI] [PubMed] [Google Scholar]
- 5.Della Valle C.J., Sporer S.M., Jacobs J.J. Preoperative testing for sepsis before revision total knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):90. doi: 10.1016/j.arth.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 6.Greidanus N.V., Masri B.A., Garbuz D.S. Use of erythrocyte sedimentation rate and C-reactive protein level to diagnose infection before revision total knee arthroplasty. A prospective evaluation. J Bone Joint Surg Am. 2007;89(7):1409. doi: 10.2106/JBJS.D.02602. [DOI] [PubMed] [Google Scholar]
- 7.Kamme C., Lindberg L. Aerobic and anaerobic bacteria in deep infections after total hip arthroplasty: differential diagnosis between infectious and non-infectious loosening. Clin Orthop Relat Res. 1981;(154):201. [PubMed] [Google Scholar]
- 8.Savarino L., Baldini N., Tarabusi C., Pellacani A., Giunti A. Diagnosis of infection after total hip replacement. J Biomed Mater Res B Appl Biomater. 2004;70(1):139. doi: 10.1002/jbm.b.30030. [DOI] [PubMed] [Google Scholar]
- 9.Fink B., Makowiak C., Fuerst M. The value of synovial biopsy, joint aspiration and C-reactive protein in the diagnosis of late peri-prosthetic infection of total knee replacements. J Bone Joint Surg Br. 2008;90(7):874. doi: 10.1302/0301-620X.90B7.20417. [DOI] [PubMed] [Google Scholar]
- 10.Cuckler J.M. The infected total knee: management options. J Arthroplasty. 2007;20(4 suppl 2):33. doi: 10.1016/j.arth.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Workgroup convened by the Musculoskeletal Infection Society New definition for periprosthetic joint infection. J Arthroplasty. 2011;26(8):1136. doi: 10.1016/j.arth.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 12.Shukla S.K., Ward J.P., Jacofsky M.C. Perioperative testing for persistent sepsis following resection arthroplasty of the hip for periprosthetic infection. J Arthroplasty. 2010;25(6 suppl 1):87. doi: 10.1016/j.arth.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 13.Ghanem E., Azzam K., Seeley M., Joshi A., Parvizi J. Staged revision for knee arthroplasty infection: What is the role of serologic tests before reimplantation? Clin Orthop Relat Res. 2009;467:1699. doi: 10.1007/s11999-009-0742-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kusuma S.K., Ward J., Jacofsky M., Sporer S.M., Della Valle C.J. What is the role of serological testing between stages of two-stage reconstruction of the infected prosthetic knee? Clin Orthop Relat Res. 2011;469:1002. doi: 10.1007/s11999-010-1619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.