Abstract
Intervertebral disk (IVD) degeneration is a natural progression of the aging process. Degenerative disk disease (DDD) is a pathologic condition associated with IVD that has been associated with chronic back pain. There are a variety of different mechanisms of DDD (genetic, mechanical, exposure). Each of these pathways leads to a final common result of unbalancing the anabolic and catabolic environment of the extracellular matrix in favor of catabolism. Attempts have been made to gain an understanding of the process of IVD degeneration with in Vitro studies. These models help our understanding of the disease process, but are limited as they do not come close to replicating the complexities that exist with an in Vivo model. Animal models have been developed to help us gain further understanding of the degenerative cascade of IVD degeneration In Vivo and test experimental treatment modalities to either prevent or reverse the process of DDD. Many modalities for treatment of DDD have been developed including therapeutic protein injections, stem cell injections, gene therapy, and tissue engineering. These interventions have had promising outcomes in animal models. Several of these modalities have been attempted in human trials, with early outcomes having promising results. Further, increasing our understanding of the degenerative process is essential to the development of new therapeutic interventions and the optimization of existing treatment protocols. Despite limited data, biological therapies are a promising treatment modality for DDD that could impact our future management of low back pain.
Keywords: Biologics, Disk degeneration, Disk repair, Gene therapy, Injectables, Intervertebral disk, Stem cells, Tissue engineering, Spine
ABBREVIATIONS
- IVD
intervertebral disk
- DDD
degenerative disk disease
- NP
nucleus pulposus
- AF
anulus fibrosus
- ECM
extra cellular matrix
- ROS
reactive oxygen species
- MSCs
mesenchymal stem cells
- TE-IVD
tissue engineered-whole implantable IVD
- TDR
total disk replacement
- hTERT
human telomerase reverse transcriptase
- ACAN
aggrecan
- THBS
thrombospondin
- CILP
cartilage intermediate layer protein
- ASPN
aspor
- TIMP
tissue inhibitor of metalloproteinase
- TGF
transforming growth factor
- MMPs
matrix metalloproteinases
- IGF
insulin-like growth factor
- FGF
fibroblast growth factor
- PDGF
platelet derived growth factor
- HSCs
hematopoetic stem cells
The intervertebral disk (IVD) is a fibrocartilaginous structure whose main function is to transmit compressible load between the vertebral bodies, while also providing flexibility. The IVD differs from other connective tissues in the body, in that it shows degenerative and aging conditions early in life.1 Age-associated maturation of IVDs, suggestive of degeneration, has been observed as early as age 11 and histological evidence of diminished blood supply to the vertebral endplates has been reported to start in the second decade of life with increasing prevalence with advancing age.1,2 IVD degeneration also involves structural failure and is commonly associated with chronic back pain.1-3 Back pain is one of the most common ailments with a point prevalence of between 12% and 30%, with around 10% of patients proceeding to develop chronic back pain.3 The direct medical cost of back pain has reached $253 billion dollars annually in the United States.4 There have also been effects on the psychological well-being of patients with significant increases in stress, depression, and anxiety after the onset of low back pain. Chronic back pain is a major health issue due to the debilitating nature of the symptomatology and the socioeconomic impact. There are a variety of reasons for back pain with disk degeneration only being one possible
cause. Not every therapy for back pain will focus on pathology at the IVD level. This paper will focus on therapies that are directly related to the IVD. Other conventional treatments for back pain include physical therapy, epidurals injections, surgical decompression, disk replacement, and fusion. These modalities for treatment will be covered in the degenerative thoracic and lumbar section of this focus issue. Significant efforts have been made to further understand the biological basis and clinical significance of IVD degeneration with the goal of preventing or reversing degenerative disk disease (DDD) and improving the clinical outcomes for patients afflicted with low back pain.
ANATOMY AND FUNCTION OF THE IVD
The IVD lies between the vertebral bodies and is composed of 3 main structures: the cartilaginous endplates, the nucleus pulposus (NP), and the anulus fibrosus (AF; Figure 1).5-8 The NP is composed of collagen II and elastin fibers which are embedded in an aggrecan-containing gel. The fixed charge density of the aggrecan molecules in the NP generates high osmotic pressures, which contributes to the highly hydrated nature of the NP, helps maintain IVD height, and distributes loading across the endplate.5,6 The cells in the NP have a low density, but are considered notochordal in origin prior to becoming chondrocyte like with aging.5 In the young individual, prior to the onset of disk degeneration, there is a distinct border between the gel-like nucleus and the concentric rings of the AF.7 The AF is comprised of 15 to 25 lamellae (concentric rings) with parallel collagen fibers within each lamellae and perpendicular collagen fibers between the adjacent lamellae which give rise to the tensile strength of the AF.7 The outer AF contains fibroblast-like, thin, elongated cells and the inner AF cells are more of a sphere and appear similar to articular chondrocytes. The AF functions to contain the NP and maintain pressurization of the NP under compressive loads. The cartilaginous endplate is a thin horizontal layer of hyaline cartilage which is avascular in the adult.7,8
FIGURE 1.
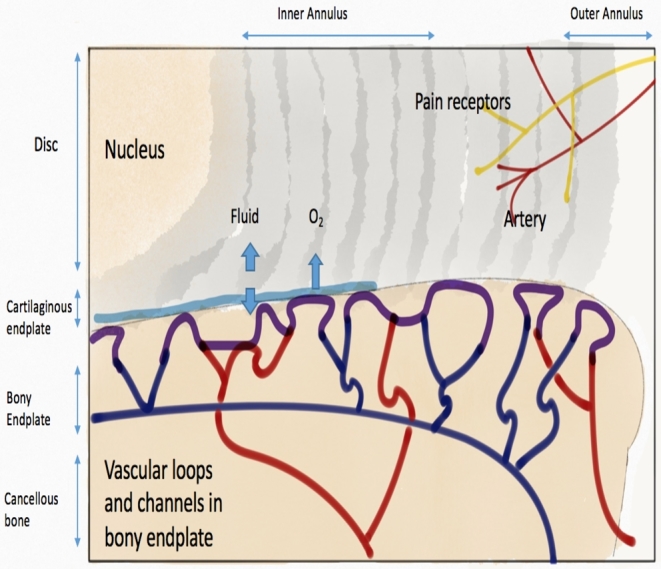
Anatomy of IVD with sectioned view showing blood supply and innervation of IVD. ©Maritza Dowdell, 2016.
NUTRITION OF IVD
Early in life, vascular channels transverse the cartilaginous endplates. These disappear by 5 years of age. The IVD blood supply arises from 2 separate capillary plexuses (Figure 1). The first supplies the outer AF and the other arises from the vertebral bodies and terminates in the bone–cartilage junction.2 The IVD is an avascular structure with some portions of the NP being located 8 mm from the nearest blood supply.2 Nutrition is driven by a diffusion gradient of glucose, oxygen, and other macromolecules. The cells within the NP are furthest from the blood supply leading to low oxygen tension leading to anaerobic metabolism within the NP. The microenvironment of the NP thus has a higher concentration of lactic acid and lower pH than other portions of the disk, which can negatively affect cell function.
NORMAL IVD AGING
The IVD undergoes age-related changes earlier in life than many other tissues resulting in histomorphologic and functional changes. While age-related changes of the IVD are normal, the process of disk degeneration is a distinct pathological condition that involves structural failure and can occur at an age-accelerated rate.1,7,9 The cartilaginous endplates have decreased permeability and vascular supply with advanced aging leading to alterations in the microenvironment of the IVD that favor catabolism.2 The overall proteoglycan content of the IVD declines with aging leading to a less hydrated IVD, which leads to altered biomechanical properties of the IVD. Collagen type II fibers are replaced with collagen type I fibers in the inner AF and NP.7,8,10 The NP begins to accumulate yellow pigmentation which also makes it less distinguishable from the AF.
MECHANISMS OF IVD DEGENERATION
Etiology of IVD Degeneration
IVD degeneration is attributed to a complex interplay between environmental and genetic factors. DDD is a process that includes a progressive decrease in disk nutrient supply and changes in extracellular matrix (ECM) composition, which weakens the tissue strength and alters the cell metabolism. A decrease in nutrient supply has been shown to negatively impact the IVD in its function to maintain the ECM. Nutrient supply has been found to be altered in the degenerative disk leading to decreased oxygen concentration and lower pH.11 Calcification of the endplates has also been shown to lead to a decreased blood supply. Inadequate nutrition inhibits the ability of the IVD to respond to increased load or injury. Structural damage is accrued over time further propagating the degenerative cycle.12 However, genetics may play a larger role in DDD than both inadequate nutrition and mechanical damage. Twin studies have shown that genetics may in fact contribute as much as 70% of an individual's risk for DDD.13,14 There are several categories of genes that contribute to DDD and they are grouped based on their function within the IVD. Polymorphisms within the Aggrecan (ACAN), COL1, COL9, COL11, FN, HAPLN1, Thrombospondin, Cartilage intermediate layer protein (CILP), and Asporin (ASPN) genes affect IVD structure.15 Polymorphisms in the genes for the catabolic MMP1, MMP2, MMP3, PARK2, and PSMB9 and anticatabolic tissue inhibitor of metalloproteinases (TIMPs) favor catabolism and can contribute to the degeneration of the IVD.15 These polymorphisms affect the delicate balance that exists between the anabolic and catabolic mediators that exists within the IVD. Anything that increases the inflammatory cascade contributes to the degenerative pathway of the IVD, by disrupting the balance between anabolism and catabolism of the IVD. Polymorphisms within the IL-1, IL-6, and COX 2 have been associated with DDD.16-18 COX-2 specifically has been thought to contribute to the pain cascade within the degenerative disk.18 Other genetic polymorphisms that have been found to contribute to the onset of DDD are those that code for vitamin D receptor (VDR) and GDF5.19-20 The polymorphisms in VDR, ACAN, COL9, ASPN, MMP3, IL1, and IL6 have been validated in more than 1 ethnic population and have the broadest association with disk degeneration making them logical targets of any intervention that uses gene therapy.15
Environmental factors are also felt to have an impact on the pathogenesis of DDD. It was previously thought that “wear and tear” or repetitive physical loading was a major risk factor for DDD, but twin studies indicated that this only played a minor role in disk degeneration21. Obesity has been implicated as a risk factor in the degenerative process. There is mixed epidemiological evidence with regard to the effect of obesity in the degenerative cascade. Recent literature indicates that a BMI > 25 kg/m2 was an independent risk factor for development of radiographic evidence of DDD and obesity at a young age was a strong risk factor for future increased in the number of degenerated disks.22 Another hypothesis states that cardiovascular disease and atherosclerosis that are associated with obesity are a homolog for the atherosclerosis of spinal vessels leading to the degenerative cascade. Other studies indicate that obesity is associated with significantly increased levels of IL-6 and proinflammatory cascades throughout the body which could contribute to the inflammatory mediated pathway of disk degeneration.23
Cigarette smoking is the only chemical exposure that has been associated with disk degeneration. Twin studies have shown a disconcordance in the amount of disk degeneration on MRI, with those that were exposed to cigarette smoke having higher rates of disk degeneration. Cigarette smoking is presumed to inhibit blood flow at the vertebral endplates. The prevailing theory is that there is an interplay between cigarette smoking and activation of muscarinic receptors located at the endplate.
Biochemical Factors
The most important early change to the IVD is the increased degradation of aggrecan and other aggregating proteoglycans.8 This biochemical change leads to loss of hydration of the disk which alters the biomechanics of the IVD, leading to accrual of structural damage to the IVD over time. Decreased hydration of the NP forces the AF to resist compression directly. The AF resists tensile forces much better than compressive forces. As the AF acts to resist compressive forces, it becomes stiff and weak and propagates the degenerative pathway. The IVD is characterized by a homeostasis of slow ECM breakdown and synthesis which is regulated by both anabolic (eg, Transforming Growth Factor (TGF), etc) and catabolic (Matrix Metalloproteinases (MMPs), ADAMTS, HRTA1, etc) agents, as well as inhibitors of the previously mentioned factors. Excessive stress can alter this homeostasis and contribute to a degenerative cascade. The imbalance in the anabolic and catabolic pathways often leads to an inflammatory reaction which further propagates the degenerative process. The catabolic environment of the IVD leads to matrix degradations products being formed, which in turn trigger the production of inflammatory mediators leading to further matrix degradation products.24 Previous literature evaluating DDD showed differences in the expression of catabolic enzymes between nondegenerate and degenerate disks, and this can be modulated by immobilization and mechanical overloading. The production of MMP1 and ADAMTS 4 was seen in low levels in the nondegenerate disks, indicating a role for these molecules in homeostasis, while MMP 3 and MMP 13 were undetectable in the nondegenerate disks. In the degenerative disks, production of MMPs 1, 3, 13 and ADAMTs 4 increased as the severity of the degeneration worsened (Figure 2). However, this was accompanied with an increase in some of the inhibitors of MMPs (TIMPs 1, 2) but not others (TIMPs 3).25 This study highlighted that while there was an increase in the expression of numerous MMPs with disk degeneration, the most clinically relevant finding was the lack of the increase in specific TIMPs (TIMPs 3). Increasing expressions of TIMPs and restoring the balance between anabolic and catabolic environments could be a possible therapeutic target for inhibition of disk degeneration.
FIGURE 2.
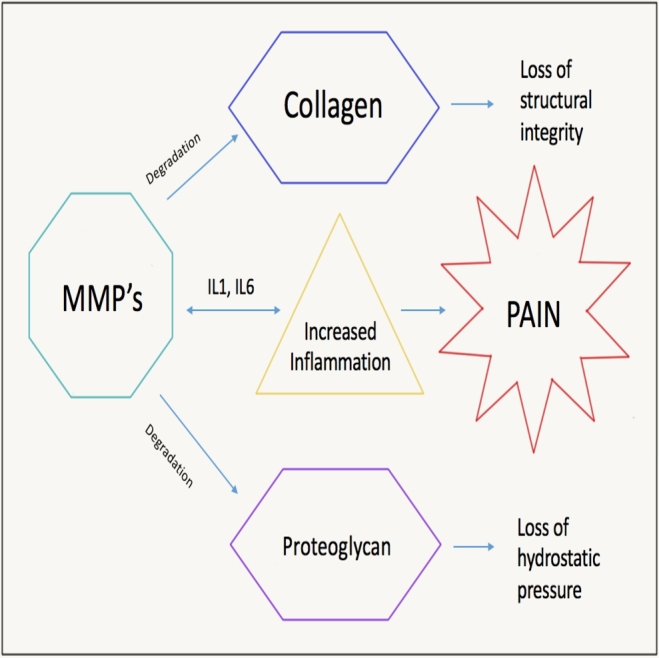
Inflammatory pathways leading to disk degeneration.
Cell Senescence
There is an association with IVD cell senescence and the development of DDD26,27. Cell senescence is defined as irreversible cell cycle arrest caused by a variety of external stimuli or telomere uncapping. Senescent cells show various morphological changes as they will aggregate in clusters, increase in size, and become flat and vacuolized. In addition, these cells will also fail to replicate in response to mitogenic stimuli and aberrantly secrete proinflammatory cytokines and matrix degradation proteases.28,29 The prevailing theory behind how cell senescence contributes to DDD is that senescent cells alter the balance of catabolic and anabolic pathways for ECM production in favor of catabolism.30 The exact trigger for disk cell senescence is not perfectly understood and is thought to be cause dependent.31
Telomere erosion is 1 contributing factor in IVD cell degeneration. During serial replication, the telomere length becomes increasingly shortened leading to incomplete replication of DNA. This activates the p53-p21-Rb signaling pathway that leads to senescence with replication. There is significant correlation between shortening of telomere length and advancing levels of DDD.32 Oxidative stress in the microenvironment of the IVD also triggers degeneration of the disk. NP cells are a major source of reactive oxygen species (ROS). Increasing levels of ROS have been associated with increasing levels of DDD, inhibition of proliferation of NP cells, and activation of the senescent signal pathways to induce cell cycle arrest of NP cells.33-35 Reducing nutrient supply and growth factors (Insulin-like Growth Factor (IGF-1), Fibroblast Growth Factor (FGF), Platelet Derived Growth Factor (PDGF)) at the level of the IVD has been shown to increase rates of disk cell senescence. Specifically, IGF-1 has been shown to prevent disk cell senescence induced by oxidative damage.34 The availability of growth factors to IVD cells is limited by endplate calcification.36-37 Most importantly, abnormal mechanical loading has also been implicated as the initial event culminating in upregulation of senescence-associated genes (such as p16, p27, RB, PTEN etc).38,39 In addition to activating the cell senescence pathway through the p53-p21-Rb pathway, mechanical stress has also shown to increase the production of ROS which leads to degeneration and senescence through a different pathway.40,41
Programmed Cell Death
Cell senescence is not the only pathway leading to a decrease in IVD cells, with an associated contribution to IVD degeneration. IVD cells also undergo programmed cell death through 1 of 3 apoptosis pathways (mitochondrial, death receptor, and endoplasmic reticulum pathways). The integrity of the ECM plays a vital role in maintaining the IVD. Degenerative processes within the ECM environment lead to increased catabolism causing multiple biological changes in the IVD cells leading to activation of the different apoptosis pathways. The different pathways of IVD cell apoptosis tend to be active at different levels of degeneration of the IVD. The endoplasmic reticulum pathway is most important during mild stages of IVD degeneration, the death receptor pathway is most prominent in mild/moderate stages of IVD degeneration, and the mitochondrial pathway is most prominent in severe stages of degeneration. Potential future therapeutic interventions can be applied toward decreasing cellular apoptosis. It would be important to identify the apoptotic pathway that is most likely to be active prior to implementing any therapeutic interventions. Inhibitors of caspases have been hypothesized to decrease the amount of degeneration and cellular apoptosis of the IVD.42
Understanding the pathogenesis of degenerative disk disease can help us target specific pathways in the degenerative cascade with the goal of prevention or reversal of the disease. There are a multitude of therapeutic options that could potentially be harnessed with the goal of preventing DDD including interrupting the inflammatory cascade, increasing anabolism of the ECM, preventing cellular apoptosis or senescence, or gene therapy.
ANIMAL MODELS FOR DISK DEGENERATION
Due to the increasing disease burden of lower back pain, animal models have been developed to further elucidate understanding and improve existing treatment strategies for this condition. In Vitro systems can be helpful in the understanding of specific pathways and components of IVD degeneration. However, an in Vivo animal model more faithfully replicates the inherently complex process of DDD. Several animal models have been developed (including mice, rats, rabbits, dogs, goats, sheep, and primates) using a variety of different mechanisms to simulate disk degeneration (genetic alterations, mechanical loading, or induced structural damage to the IVD).43,44 Animal models cannot perfectly replicate degeneration of the human IVD for a variety of reasons, but can help us further our understanding of the process beyond what is possible with in Vitro models. Many of the species studied, with the exception of goat and sheep, have notochordal cells that are present into adulthood, which complicates investigations into therapies with cellular regenerative therapies.45,46 Disk size also impacts the degenerative potential seen. The IVD relies on diffusion for the nutritional requirements of the NP. The IVD disk of humans is much larger than of the common animal models that are seen which could hasten the degenerative cascade in humans. Disk geometry has also been studied with the goal of selecting an animal IVD model that most closely replicates the human IVD. The mouse lumbar IVD most closely matches the human IVD.47 The majority of animal models of DDD are quadrupeds as bipedal models are limited in use due to ethical concerns. The forces seen by the IVD in quadrupeds are hypothesized to be up to 4 times larger due to the complexity of stabilizing a horizontally aligned spine vs a vertically balanced spine.46 Understanding the differences and similarities between animal models and the human IVD can allow us to implement interventions in the animal model with the goal of translating these therapies a clinical benefit in the future. There are many different avenues for biological intervention at the level of the IVD based on the amount of degeneration present (Figure 3). The amount of degeneration that is present in the IVD provides an insight into the biology of the disk at that time. In earlier stages of degeneration, biomolecular interventions could have an effect to rebalance the anabolic and catabolic pathways in the degenerative cascade. In intermediate stages of degeneration, cell implantation can be used to repopulate the disk. In advanced stages of degeneration, tissue engineering constructs that mimic the native disk would have to be employed for biological reconstruction. Each of these methods has been attempted in small and large animal preclinical trials. More recently, functional behaviors are being assessed in animal models of IVD degeneration to better identify relationships between painful behaviors and DDD.47
FIGURE 3.
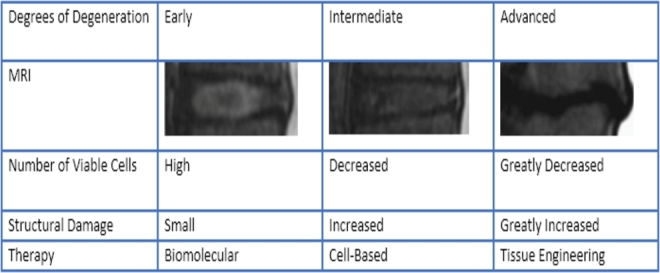
Therapeutic strategies based on level of disk degeneration.
Therapeutic Protein Injections
Protein solutions can be injected directly into IVD to stimulate cell growth and/or anabolic responses with the goal of reversing the degenerative cascade and preventing further disk degeneration.48 The IVD has previously been shown to respond to exogenous growth factors.49,50 Specific growth factors that have been shown to stimulate the growth of both bone and cartilage are the bone morphogenic proteins (OP-1, BMP-14) and members of the transforming growth factor-β. An in Vivo rabbit study showed an injection of intradiscal OP-1 induced an increase in proteoglycan content of the NP and increase of disk height.51 This study has been repeated and also been shown to improve MRI findings of disk degeneration as well.52-53 Rat models have shown an anabolic response of the IVD when injections of OP-1 were performed, with a restoration of normal disk morphology.51,54 Sheep models of DDD have shown that BMP-13 injection prevents loss of hydration of the NP.55 Negative effects have also been seen with injection of “therapeutic proteins”, as injection of BMP-2 in a rabbit model has been shown to exacerbate degeneration of the IVD.56 The limits to protein injections are the short durations of its therapeutic benefits. This promising potential treatment for disk degeneration could be improved with the development of a slow release carrier vehicle or gene-based delivery to increase to duration of benefit seen.
Gene-based Therapy
Gene therapy is based on inducing changes to intradiscal gene expression. These genes are delivered through a vector and are either injected directly into the cell or transduced into cells via a viral vector. Traditionally used viral vectors are a retroviral vector, adenovirus, adeno-associated virus, and baclovirus.57 Nonviral vectors are in development, but to this point have not approached viral vectors in terms of their efficacy. The biggest drawback to using a retroviral vector is the potential for insertional mutagenesis leading to potential malignancies. An adenovirus vector has the drawback of the being highly immunogenic leading to major immune response against the foreign transgene-encoded proteins which has the potential to limit the efficacy of this technique. Viral vectors for gene transposition also carry a major expense in their preparation in addition to the still unknown risk to patients. Further development of nonviral transmission agents could mitigate cost and increase the safety of this technique for treating DDD significantly. One of the nonviral transmission agents in development is microbubbles delivered via sonoporation. This technique uses microbubbles to carry plasmid DNA, which encodes for the proteins of interest, and delivers these microbubbles into to the cell with the use of sonoporation (or ultrasound-induced transient holes on the cell surface). Another strategy for intradiscal gene therapy would be to focus less on upregulating the anabolic cascade which carries a significant energy expenditure and focus more on downregulation of gene expression that are harmful to the physiological balance of the disk.57 Promising targets for gene therapy have included LMP-1 (regulation of BMP-7), disintegrin, MMPs, TIMPs, and chondrocyte-specific transcription factors (Ad-Sox9).58-63 In rat models, plasmid DNA was mixed with microbubbles as a delivery system and transfected genes were still expressed up to 24 weeks from treatment in the cultured IVD. In a rabbit model, increased LMP-1 led to increased expression of PG, BMP-2, and BMP-7. In a separate rabbit model, increased expression of TIMPs was associated with delayed degeneration and increased expression of Ad-Sox9 which led to retained chondrocytic appearance and normalization of the ECM.63
Cell Therapy
Therapeutic protein injections and gene-based treatment modalities have decreased efficacy with more advanced stages of disk degeneration due to the decreased number of cells available within the IVD to respond to these signals.64 Cell-based therapy is a good treatment strategy in mid-stage degeneration to increase the number of IVD cells present with the disk. Many studies have proven that both autologous and allogenic disk cells survive within the disk. An injured canine model established that NP chondrocyte implantation contributed to ECM regeneration and prevention of further disk degeneration.65 Mesenchymal stem cells (MSCs) can differentiate into all lineages of mesenchymal tissue, including the chondrogenic lineage and IVD-cell-specific phenotypes. This makes MSCs a potentially ideal option for repair of IVD as they are easy to obtain and once they differentiate, they would be able to produce proteoglycans and collagen for the disk ECM. Differentiation of MSCs to IVD cells is dependent on growth factors, oxygen tension, as well as a variety of other conditions. In addition, adipose-derived stem cells also show promise for IVD tissue engineering.66 While there is some conflicting data, MSCs have generally been superior to differentiated disk cells in regeneration of the disk morphology after injection both in Vivo and in Vitro. Porcine studies have shown improved outcomes in disk repair when using articular chondrocytes vs MSCs with the hypothesis that well-differentiated cells are more apt to survive the ischemia of the disk environment.67 Other studies in rabbit models have shown equivalent outcomes between the 2 cell lineages. MSCs can be an ideal substitute for IVD cells due to their better accessibility and success of this treatment option.67 Another study shows that a combination of both of these cell lines leads to improved performance and survivability of implanted cells.68 Both of these cells lines have great potential for repair of the degenerated disk.
Tissue Engineering
Once advanced degeneration and severe loss of cellularity is present within the IVD, there is little potential for reversal of this damage with cell-based implantation or therapeutic protein injections alone. Functional substitutes for damaged disk tissues must be introduced in the form of a scaffold, and physical conditioning of cells using either mechanical or electrical stimuli should also be performed.69-71 Advancements in tissue engineering has enabled the constructions of tissue engineered-whole implantable IVD (TE-IVD). Both NP and AF composites replace the severely degenerated disk. This has been shown to engraft into the disk space in a rat tail model, where the TE-IVD exhibited similar properties to the native disk in biomechanical and biochemical testing.72-73 Total disk replacement (TDR) was also performed in the canine cervical spine (axial loading closely matches human cervical spine) and the TE-IVDs engrafted to the host tissue and partially maintained disk height. When the TE-IVDs are used in combination with the protein and gene-based therapies, further clinical improvements are seen. A canine model of TDR with TE-IVDs loaded either with or without human telomerase reverse transcriptase (hTERT) gene in the NP cells showed an antidegenerative effect in the with hTERT group.74
Annular Repair
Injury to the AF can generate the catabolic cascade. However, the delivery of many of the therapeutic proteins or cell-based therapies involves puncturing the AF. Injury to the AF with a small needle puncture has been previously established to cause acceleration of disk degeneration in a 10-year follow-up of patients undergoing discography.75,76 It would not be ideal to iatrogenically cause further degeneration of the IVD while attempting to either halt or reverse the degenerative cascade. AF repair has previously been attempted with suturing and anuloplasty techniques. Both of these techniques failed to improve anular strength in both sheep and porcine models.77-78 To date no anular repair techniques have proven successful on an in Vivo model, which has led to the development of more robust in Vitro herniation models with aggressive cyclic loading and systematic screening processes. New modalities for anular repair are being studied and developed. Anular repair techniques have great potential to work in conjunction with other treatment modalities in DDD including drug and cell delivery.79
Clinical Trials and Treatment of DDD
There have been a limited number of clinical trials for biological treatment of IVD disease. Therapies which have proven benefit in preclinical trials are in varying phases of being attempted in clinical trials to prove their efficacy and safety for human use. There have been several trials of injections of stem cells into degenerated disk. The injections of hematopoietic stem cells for discogenic back pain failed to yield any improvement in the level of low back pain after 1-year follow up.80 Injection of IVD cells, in the EuroDISC study, 3 months after discectomy, showed reduction in back pain on 2-year follow-up compared to discectomy alone.81 In addition, disk height was maintained, and there was less adjacent segment disease in the patients who underwent autologous disk chondrocyte transplantation plus discectomy group vs the discectomy alone group.81 Other clinical trials have also shown some benefit to injections of MSCs into the disk with improvement of pain and MRI findings.82,83 Injection of allogenic juvenile chondrocytes into the IVD of patients with single-level DD has also been investigated with early results showing improvement in pain in a majority of patients and improved findings in 10/13 patients on MRI at 6 months.84 However, despite these encouraging preliminary results, there are many obstacles that remain prior to the clinical use of cell-based therapies. The IVD harsh microenvironment had led many to be skeptical that biological and cell-based therapy will make an impact on the process of disk degeneration. In NP cells, hypoxia inducible factor is stable in an oxygen-independent fashion, which allows these cells to survive the harsh microenvironment.85 It is unknown if implanted cells acquire the properties of NP cells to adapt to the avascular environment that is present. Future goals of research in cell-based therapies could be to identify the correct cells for implantation (Hematopoetic Stem Cells (HSCs), Mesenchymel Stems Cells (MSCs), chondrocytes, notochordal cells, adult IVD disk cells, etc) and development of new tools to diagnose IVD degeneration needing cell therapy. There have been preliminary results showing a possible benefit of an intradiscal PRP injection, with 47% of patients achieving a 50% improvement in pain via the visual analog scoring system.86 There are several nonpublished clinical trials examining the effect of GDF-5 and OP-1 on disk repair. Mesenchymal lineage adult stem cells implantation has shown promising results as 42% of patients undergoing treatment had a 50% reduction in pain compared to 13% for placebo, but this is also unpublished data.87 There are no clinical in Vivo trials that have been performed in humans with regard to gene therapy. In Vitro studies have shown decrease in MMP expression in IVD cells after introductions of IL-1 receptor antagonist via an adenovirus vector.88 Another in Vitro study transfected human cells with a combination of TGF-β1, IGF-1, BMP-2 and noted proteoglycan synthesis was 4.7 times that of control cells.89 Prior to any in Vivo or ex vivo (with subsequent cell implantation) studies with viral vectors, there must be questions answered with regard to viral vector safety or whether it would be safer to optimize the less effective but ultimately less or nonvirulent vectors such as the ultrasound microbubble technique previously described.
Summary and Future Therapies
A thorough understanding of the mechanisms for disk degeneration is necessary to design treatment strategies for biological healing and repair of the IVD. The amount of degeneration present within the IVD could dictate the appropriate intervention as different treatment strategies could have differing efficacies at different points along the degenerative cascade. The process of IVD degeneration is complex and multifactorial, and thus the solutions for reversing this process will be equally if not more complex and likely involve multiple solutions depending on the disease phenotype and progression. Future studies should advance the number in Vivo human studies with all of the available treatment modalities. There should also be continued elucidation of intracellular signaling pathways to allow for more targeted regulation of IVD cells. As our ability to understand the process of IVD degeneration grows, new modalities of treatment will be identified and developed, and current modalities of treatment will be optimized. These new treatments will require rigorous testing that can progress to human studies to evaluate their efficacy and impact on reversing IVD degeneration. Biological therapies remain a promising possibility to address the complex processes of painful disk degeneration.
Disclosure
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
REFERENCES
- 1. Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002, 27:2631–2644. [DOI] [PubMed] [Google Scholar]
- 2. Benneker LM, Heini PF, Alini M, Anderson SE, Ito K. 2004 Young Investigator Award Winner: vertebral endplate marrow contact channel occlusions and intervertebral disc degeneration. Spine. 2005, 30, 2:167–173. [DOI] [PubMed] [Google Scholar]
- 3. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it?, Spine. 2006, 31, 18:2151–2161. [DOI] [PubMed] [Google Scholar]
- 4. United States bone and joint initiative. In: Rosemont IL, et al., The Burden of Musculoskeletal Diseases in the United States (BMUS). 3rd ed 2014. Available at: http://www.boneandjointburden.org. Accessed on July 23, 2016. [Google Scholar]
- 5. Purmessur D, Cornejo MC, Cho SK, Hecht AC, Iatridis JC. Notochordal cell-derived therapeutic strategies for discogenic back pain. Global Spine J. 2013, 3, 3:201–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Samartzis D, Cheung KM. Lumbar intervertebral disk degeneration. Orthop Clin North Am. 2011, 42, 4:xi–xii. [DOI] [PubMed] [Google Scholar]
- 7. Westrick E, Sowa G, Kang J. The intervertebral disc: normal, aging, and pathologic. In: Herkowitz HN, Garfin SR, Eismont FJ et al., Rothman-Simeone the Spine. 6th ed Saunders, Philadelphia: 2011:97–128. [Google Scholar]
- 8. Roughley PJ. Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 2004, 29, 23:2691–2699. [DOI] [PubMed] [Google Scholar]
- 9. Miller JA, Schmatz C, Schultz AB, Lumbar disc degeneration: correlation with age, sex, and spine level in 600 autopsy specimens. Spine. 1988, 13:173–178. [PubMed] [Google Scholar]
- 10. Vo N, Hartman R, Patil P et al. , Molecular mechanisms of biological aging in intervertebral discs. J Orthop Res. 2016, 34, 8:1289–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nachemson A, Lewin T, Maroudas A, Freeman MA. In vitro diffusion of dye through the end-plates and the annulus fibrosus of human lumbar inter-vertebral discs. Acta Orthop Scand. 1970, 41, 6:589–607. [DOI] [PubMed] [Google Scholar]
- 12. Holm S, Holm AK, Ekström L, Karladani A, Hansson T. Experimental disc degeneration due to endplate injury. J Spinal Disord Tech. 2004, 17, 1:64–71. [DOI] [PubMed] [Google Scholar]
- 13. Livshits G, Popham M, Malkin I et al. , Lumbar disc degeneration and genetic factors are the main risk factors for low back pain in women: the UK Twin Spine Study. Ann Rheum Dis. 2011, 70, 10:1740–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kalichman L, Hunter DJ. The genetics of intervertebral disc degeneration. Familial predisposition and heritability estimation. Joint Bone Spine. 2008, 75, 4:383–387. [DOI] [PubMed] [Google Scholar]
- 15. Mayer JE, Iatridis JC, Chan D, Qureshi SA, Gottesman O, Hecht AC. Genetic polymorphisms associated with intervertebral disc degeneration. Spine J. 2013, 13, 3:299–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996, 21, 2:218–224. [DOI] [PubMed] [Google Scholar]
- 17. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014, 10, 1:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miyamoto H, Saura R, Doita M, Kurosaka M, Mizuno K. The role of cyclooxygenase-2 in lumbar disc herniation. Spine. 2002, 27, 22:2477–2483. [DOI] [PubMed] [Google Scholar]
- 19. Williams FM, Popham M, Hart DJ et al. , GDF5 single-nucleotide polymorphism rs143383 is associated with lumbar disc degeneration in Northern European women. Arthritis Rheum. 2011, 63, 3:708–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Eser B, Cora T, Eser O et al. , Association of the polymorphisms of vitamin D receptor and aggrecan genes with degenerative disc disease. Genet Test Mol Biomarkers. 2010, 14, 3:313–317. [DOI] [PubMed] [Google Scholar]
- 21. Videman T, Sarna S, Battié MC et al. , The long-term effects of physical loading and exercise lifestyles on back-related symptoms, disability, and spinal pathology among men. Spine. 1995, 20, 6:699–709. [DOI] [PubMed] [Google Scholar]
- 22. Liuke M, Solovieva S, Lamminen A et al. , Disc degeneration of the lumbar spine in relation to overweight. Int J Obes. 2005, 29, 8:903–908. [DOI] [PubMed] [Google Scholar]
- 23. Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006, 83, 2:461S–465S. [DOI] [PubMed] [Google Scholar]
- 24. Sakai D, Grad S. Advancing the cellular and molecular therapy for intervertebral disc disease. Adv Drug Deliv Rev. 2015, 84:159–171. [DOI] [PubMed] [Google Scholar]
- 25. Le maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004, 204, 1:47–54. [DOI] [PubMed] [Google Scholar]
- 26. Kepler CK, Ponnappan RK, Tannoury CA et al. , The molecular basis of intervertebral disc degeneration, Spine J. 2013, 13:318–330. [DOI] [PubMed] [Google Scholar]
- 27. Risbud MV, Shapiro IM. Role of cytokines in intervertebral disc degeneration: pain and disc content. Nat Rev Rheumatol. 2014, 10, 1:44–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Acosta JC, O’loghlen A, Banito A et al. , Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell. 2008, 133, 6:1006–1018. [DOI] [PubMed] [Google Scholar]
- 29. Acosta JC, Banito A, Wuestefeld T et al. , A complex secretory program orchestrated by the inflammasome controls paracrine senescence. Nat Cell Biol. 2013, 15, 8:978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gruber HE, Ingram JA, Davis DE, Hanley EN. Increased cell senescence is associated with decreased cell proliferation in vivo in the degenerating human annulus. Spine J. 2009, 9, 3:210–215. [DOI] [PubMed] [Google Scholar]
- 31. Ben-porath I, Weinberg RA. The signals and pathways activating cellular senescence. Int J Biochem Cell Biol. 2005, 37, 5:961–976. [DOI] [PubMed] [Google Scholar]
- 32. Le maitre CL, Freemont AJ, Hoyland JA. Accelerated cellular senescence in degenerate intervertebral discs: a possible role in the pathogenesis of intervertebral disc degeneration. Arthritis Res Ther. 2007, 9, 3:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dimozi A, Mavrogonatou E, Sklirou A, Kletsas D. Oxidative stress inhibits the proliferation, induces premature senescence and promotes a catabolic phenotype in human nucleus pulposus intervertebral disc cells. Eur Cell Mater. 2015, 30:89–102. [DOI] [PubMed] [Google Scholar]
- 34. Gruber HE, Hoelscher GL, Ingram JA, Bethea S, Hanley EN. IGF-1 rescues human intervertebral annulus cells from in vitro stress-induced premature senescence. Growth Factors. 2008, 26, 4:220–225. [DOI] [PubMed] [Google Scholar]
- 35. Park JS, Park JB, Park IJ, Park EY. Accelerated premature stress-induced senescence of young annulus fibrosus cells of rats by high glucose-induced oxidative stress. Int Orthop. 2014, 38, 6:1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004, 13, 8:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jiang L, Zhang X, Zheng X et al. , Apoptosis, senescence, and autophagy in rat nucleus pulposus cells: Implications for diabetic intervertebral disc degeneration. J Orthop Res, 2013, 31:692–702. [DOI] [PubMed] [Google Scholar]
- 38. Adams MA, Roughley PJ. What is intervertebral disc degeneration, and what causes it?. Spine. 2006, 31:2151–2161. [DOI] [PubMed] [Google Scholar]
- 39. Xing QJ, Liang QQ, Bian Q et al. , Leg amputation accelerates senescence of rat lumbar intervertebral discs. Spine. 2010, 35:E1253–E1261. [DOI] [PubMed] [Google Scholar]
- 40. Martin JA, Brown TD, Heiner AD, Buckwalter JA. Chondrocyte senescence, joint loading and osteoarthritis. Clin Orthop. 2004, 427, suppl:S96–S103. [DOI] [PubMed] [Google Scholar]
- 41. Feng C, Liu H, Yang M, Zhang Y, Huang B, Zhou Y. Disc cell senescence in intervertebral disc degeneration: causes and molecular pathways. Cell Cycle. 2016, 15, 13:1674–1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ding F, Shao ZW, Xiong LM. Cell death in intervertebral disc degeneration. Apoptosis. 2013, 18, 7:777–785. [DOI] [PubMed] [Google Scholar]
- 43. Erwin WM, DeSouza L, Funabashi M et al. , The biological basis of degenerative disc disease: proteomic and biomechanical analysis of the canine intervertebral disc. Arthritis Res Ther. 2015, 17:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bergknut N, Rutges JP, Kranenburg HJ et al. , The dog as an animal model for intervertebral disc degeneration?, Spine. 2012, 37, 5:351–358. [DOI] [PubMed] [Google Scholar]
- 45. Aguiar DJ, Johnson SL, Oegema TR. Notochordal cells interact with nucleus pulposus cells: Regulation of proteoglycan synthesis. Exp Cell Res. 1999, 246, 1:129–137. [DOI] [PubMed] [Google Scholar]
- 46. Alini M, Eisenstein SM, Ito K et al. , Are animal models useful for studying human disc disorders/degeneration?, Eur Spine J. 2008, 17, 1:2–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Lai A, Moon A, Purmessur D et al. , Annular puncture with tumor necrosis factor-alpha injection enhances painful behavior with disc degeneration in vivo. Spine J. 2016, 16, 3:420–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cao P, Jiang L, Zhuang C et al. , Intradiscal injection therapy for degenerative chronic discogenic low back pain with end plate Modic changes. The Spine Journal. 2011, 11:100–106. [DOI] [PubMed] [Google Scholar]
- 49. Moriguchi Y, Alimi M, Khair T et al. , Biological treatment approaches for degenerative disk disease: a literature review of in vivo animal and clinical data. Global Spine J. 2016, 6, 5:497–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Thompson JP, Oegema TR, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991, 16, 3:253–260. [DOI] [PubMed] [Google Scholar]
- 51. Imai Y, Okuma M, An HS et al. , Restoration of disc height loss by recombinant human osteogenic protein-1 injection into intervertebral discs undergoing degeneration induced by an intradiscal injection of chondroitinase ABC. Spine. 2007, 32, 11:1197–1205. [DOI] [PubMed] [Google Scholar]
- 52. Walsh AJ, Bradford DS, Lotz JC. In vivo growth factor treatment of degenerated intervertebral discs. Spine. 2004, 29, 2:156–163. [DOI] [PubMed] [Google Scholar]
- 53. Masuda K, Imai Y, Okuma M et al. , Osteogenic protein-1 injection into a degenerated disc induces the restoration of disc height and structural changes in the rabbit anular puncture model. Spine. 2006, 31, 7:742–754. [DOI] [PubMed] [Google Scholar]
- 54. Chubinskaya S, Kawakami M, Rappoport L, Matsumoto T, Migita N, Rueger DC. Anti-catabolic effect of OP-1 in chronically compressed intervertebral discs. J Orthop Res. 2007, 25, 4:517–530. [DOI] [PubMed] [Google Scholar]
- 55. Wei A, Williams LA, Bhargav D et al. , BMP13 prevents the effects of annular injury in an ovine model. Int J Biol Sci. 2009, 5, 5:388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Huang KY, Yan JJ, Hsieh CC, Chang MS, Lin RM. The in vivo biological effects of intradiscal recombinant human bone morphogenetic protein-2 on the injured intervertebral disc: an animal experiment. Spine. 2007, 32, 11:1174–1180. [DOI] [PubMed] [Google Scholar]
- 57. Nishida K, Suzuki T, Kakutani K et al. , Gene therapy approach for disc degeneration and associated spinal disorders. Eur Spine J. 2008, 17, suppl 4:459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nishida K, Doita M, Takada T et al. , Sustained transgene expression in intervertebral disc cells in vivo mediated by micro bubble enhanced ultrasound gene therapy. Spine. 2006, 31, 13:1415–1419. [DOI] [PubMed] [Google Scholar]
- 59. Seki S, Asanuma-Abe Y, Masuda K et al. , Effect of small interference RNA (siRNA) for ADAMTS5 on intervertebral disc degeneration in the rabbit anular needle-puncture model. Arthritis Res Ther. 2009, 11, 6:R166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang YH, Zhao CQ, Jiang LS, Dai LY. Lentiviral shRNA silencing of CHOP inhibits apoptosis induced by cyclic stretch in rat annular cells and attenuates disc degeneration in the rats. Apoptosis. 2011, 16, 6:594–605. [DOI] [PubMed] [Google Scholar]
- 61. Leckie SK, Bechara BP, Hartman RA et al. , Injection of AAV2-BMP2 and AAV2-TIMP1 into the nucleus pulposus slows the course of intervertebral disc degeneration in an in vivo rabbit model. Spine J. 2012, 12, 1:7–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paul R, Haydon RC, Cheng H et al. , Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003, 28, 8:755–763. [PMC free article] [PubMed] [Google Scholar]
- 63. Bae WC, Masuda K. Emerging technologies for molecular therapy for intervertebral disk degeneration. Orthop Clin North Am. 2011, 42, 4:585–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sakai D, Andersson GB. Stem cell therapy for intervertebral disc regeneration: obstacles and solutions. Nat Rev Rheumatol. 2015, 11, 4:243–256. [DOI] [PubMed] [Google Scholar]
- 65. Ganey T, Libera J, Moos V et al. , Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003, 28, 23:2609–2620. [DOI] [PubMed] [Google Scholar]
- 66. Clarke LE, Mcconnell JC, Sherratt MJ, Derby B, Richardson SM, Hoyland JA. Growth differentiation factor 6 and transforming growth factor-beta differentially mediate mesenchymal stem cell differentiation, composition, and micromechanical properties of nucleus pulposus constructs. Arthritis Res Ther. 2014, 16, 2:R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Acosta FL Jr, Metz L, Adkisson HD et al. , Porcine intervertebral disc repair using allogeneic juvenile articular chondrocytes or mesenchymal stem cells. Tissue Eng Part A. 2011, 17, 23-24:3045–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Allon AA, Aurouer N, Yoo BB, Liebenberg EC, Buser Z, Lotz JC. Structured coculture of stem cells and disc cells prevent disc degeneration in a rat model. Spine J. 2010, 10, 12:1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hudson KD, Alimi M, Grunert P, Härtl R, Bonassar LJ. Recent advances in biological therapies for disc degeneration: tissue engineering of the annulus fibrosus, nucleus pulposus and whole intervertebral discs. Curr Opin Biotechnol. 2013, 24, 5:872–879. [DOI] [PubMed] [Google Scholar]
- 70. Iwashina T, Mochida J, Sakai D et al. , Feasibility of using a human nucleus pulposus cell line as a cell source in cell transplantation therapy for intervertebral disc degeneration. Spine. 2006, 31, 11:1177–1186. [DOI] [PubMed] [Google Scholar]
- 71. Zhang Y, Drapeau S, Howard SA, Thonar EJ, Anderson DG. Transplantation of goat bone marrow stromal cells to the degenerating intervertebral disc in a goat disc injury model. Spine. 2011, 36, 5:372–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gebhard H, Bowles R, Dyke J et al. , Total disc replacement using a tissue-engineered intervertebral disc in vivo: new animal model and initial results. Evid Based Spine Care J. 2010, 1, 2:62–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Martin JT, Kim DH, Milby AH et al. , In vivo performance of an acellular disc-like angle ply structure (DAPS) for total disc replacement in a small animal model. J Orthop Res. 2016doi:10.1002/jor.23310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xin H, Zhang C, Wang D et al. Tissue-engineered allograft intervertebral disc transplantation for the treatment of degenerative disc disease: experimental study in a beagle model. Tissue Eng Part A. 2013;19(1–2):143–151. [DOI] [PubMed] [Google Scholar]
- 75. Guterl CC, See EY, Blanquer SB et al. , Challenges and strategies in the repair of ruptured annulus fibrosus. Eur Cell Mater. 2013, 25:1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Vadalà G, De Strobel F, Bernardini M, Denaro L, D’Avella D, Denaro V. The transpedicular approach for the study of intervertebral disc regeneration strategies: in vivo characterization. Eur Spine J. 2013, 22, suppl 6:S972–S978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ahlgren BD, Lui W, Herkowitz HN, Panjabi MM, Guiboux JP. Effect of anular repair on the healing strength of the intervertebral disc: a sheep model. Spine. 2000, 25, 17:2165–2170. [DOI] [PubMed] [Google Scholar]
- 78. Bailey A, Araghi A, Blumenthal S, Huffmon GV, Anular Repair Clinical Study Group Prospective, multicenter, randomized, controlled study of anular repair in lumbar discectomy: two-year follow-up. Spine. 2013, 38, 14:1161–1169. [DOI] [PubMed] [Google Scholar]
- 79. Likhitpanichkul M, Kim Y, Torre OM et al. , Fibrin-genipin annulus fibrosus sealant as a delivery system for anti-TNFα drug. Spine J. 2015, 15, 9:2045–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Haufe SM., Mork AR., Intradiscal injection of hematopoietic stem cells in an attempt to rejuvenate the intervertebral discs. Stem Cells Dev. 2006, 15:136–137. [DOI] [PubMed] [Google Scholar]
- 81. Meisel HJ, Ganey T, Hutton WC, Libera J, Minkus Y, Alasevic O, Clinical experience in cell-based therapeutics: intervention and outcome. Eur Spine J. 2006, 15, suppl 3:S397–S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Meisel HJ, et al. , Clinical experience in cell-based therapeutics: disc chondrocyte transplantation: a treatment for degenerated or damaged intervertebral disc. Biomol Eng. 2007, 24:5–21. [DOI] [PubMed] [Google Scholar]
- 83. Yoshikawa T, Ueda Y, Miyazaki K, Koizumi M, Takakura Y., Disc regeneration therapy using marrow mesenchymal cell transplantation: a report of two case studies. Spine. 2010, 35:E475–E480. [DOI] [PubMed] [Google Scholar]
- 84. Orozco L, et al. , Intervertebral disc repair by autologous mesenchymal bone marrow cells: a pilot study. Transplantation. 2011, 92:822–828. [DOI] [PubMed] [Google Scholar]
- 85. Agrawal A, et al. , Normoxic stabilization of HIF-1α drives glycolytic metabolism and regulates aggrecan gene expression in nucleus pulposus cells of the rat intervertebral disk. Am J Physiol Cell Physiol. 2007, 293:C621–C631. [DOI] [PubMed] [Google Scholar]
- 86. Levi D, Horn S, Tyszko S, Levin J, Hecht-leavitt C, Walko E. Intradiscal platelet-rich plasma injection for chronic discogenic low back pain: preliminary results from a prospective trial. Pain Med. 2016, 17, 6:1010–1022. [DOI] [PubMed] [Google Scholar]
- 87. Brown R. Safety and preliminary efficacy study of mesenchymal precursor cells (MPCs) in subjects with lumbar back pain. Available at: https://clinicaltrials.gov/ct2/show/NCT01290367?term=mesenchymalþprecursorþcellsþinþsubjectsþwithþchronicþdiscogenicþlumbarþbackþpain&rank=1. Accessed August 1, 2016. [Google Scholar]
- 88. Le Maitre CL, Hoyland JA, Freemont AJ. Interleukin-1 receptor antagonist delivered directly and by gene therapy inhibits matrix degradation in the intact degenerate human intervertebral disc: An in situ zymographic and gene therapy study. Arthritis Res Ther. 2007, 9, 4:R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Moon SH, Nishida K, Gilbertson LG et al. , Biologic response of human intervertebral disc cells to gene therapy cocktail. Spine. 2008, 33, 17:1850–1855. [DOI] [PMC free article] [PubMed] [Google Scholar]


