Abstract
Introduction:
One of the most important goals of periodontitis therapy is the elimination of deep periodontal pockets. In regenerative periodontal therapy, different types of bone grafts, membranes, growth factors, etc. are used to improve regeneration of lost periodontal tissue.
The aim of this study was to evaluate the effect of surgical therapy supported by the use of bone replacement material in the treatment of deep intrabony pockets, compared to surgical treatment (flap surgery) without the use of bone replacement in advanced periodontitis.
Methods and materials:
The study included 50 patients of both sexes with advanced periodontitis, divided into two groups. After initial periodontal therapy was performed, plaque index (PI), papillary bleeding index (PBI) were verified, and depth of periodontal pockets was measured in both groups. One group (group 1) of the patients underwent surgical therapy, open flap surgery, while the other group (group 2) underwent the same surgical treatment method (open flap surgery), during which bone defects were filled with bone replacement material.
Results:
The results showed that both group 1 and group 2 experienced improvements after periodontal surgical therapy. In group 1, there are no statistically significant changes in all three plaque index measurements (PI), while there has been a significant reduction in PI in group 2 following the surgery. For the PBI index, it was determined that there were statistically significant changes in values in group 1, both after surgical procedures and six months later, as well as in group 2. Statistical analysis of the results of the probing depth of pockets has shown that there are significant changes in the measurement of the depth of periodontal pocket one month after the surgery, as well as six months later, meaning that there has been a significant reduction in the depth of the periodontal pocket one month following the surgery as well as six months later, for both groups. However, we did not determine a statistically significant difference in the probing depth of pockets between these two groups.
Conclusion:
Six months after a surgical therapy, clinical parameters showed a reduction of the probing depth of the periodontal pocket in both examined groups. The use of bone replacement did not yield significantly better results in reducing the depth of probing compared to the standard flap surgery. We believe that future research should focus on testing the effectiveness of new regenerative methods and materials (bone replacements with various properties, membranes, and surgical methods) that will result in better treatment results with predictable outcomes.
Keywords: periodontal regeneration, intrabony defect, bone replacement
1. INTRODUCTION
Periodontitis is characterized by an infection of all structures around the tooth, including the periodontal ligament, cementum, bone, and soft tissue. During the development of periodontal disease, complex and irreversible mechanisms of alveolar bone resorption occur (1). One of the most important goals of periodontal therapy is the elimination of deep periodontal pockets. Periodontal therapy includes removal of supragingival and subgingival plaque, individual approach to education and motivation for oral hygiene, scraping and root polishing, and periodontal surgical therapy. The primary goal of periodontal surgery is to remove necrotic cementum and necrotic epithelial pocket tissue through open access (under visual control–flap surgery). Most periodontal surgical procedures lead to the elimination or reduction of soft tissue of the periodontal pocket, and the creation of a new epithelial attachment (2). In recent years, the use of regenerative procedures has become a common method for recovering the lost support structures of the periodontium.
Guided tissue/bone regeneration (GTR/GBR) is a surgical procedure aimed at the regeneration of periodontal tissues, which can overcome some of the constraints of conventional therapy, i.e. open flap surgery. Various forms of treatment include the use of bone substitutes of different origins (autotransplants, allografts, and alloplastic materials) (3, 4). Studies have shown that autogenous, humane, demineralized, dry-frozen bone is osteoconductive and osteoinductive, and as such has yielded clinically best results (5), but researchers often encountered the problem of its deficiency. Unlike autogenous bones, xenogeneic and alloplastic materials are commercially available in unlimited quantities. Thus, there is a limited need for intra or extraoral bone graft donor sites (6, 7). According to some authors (8, 9), the use of bone replacement materials (guided bone regeneration) has shown better results than open flap surgery alone, including the improved height of epithelial attachment and reduced probing depth. Development of a new generation of fully synthetic, biologically active bone replacements fosters and promotes new clinical trials.
The aim of this study was to evaluate the effect of surgical periodontal therapy supported by the use of bone replacement material in the treatment of deep intrabony pockets, compared to surgical treatment (flap surgery) without the use of bone replacement in advanced periodontitis.
2. MATERIALS AND METHODS
This study included 50 adult subjects of both sexes, with advanced periodontitis, who had at least one real pocket measuring 5mm or deeper, in at least two quadrants. All respondents gave consent to the treatment of periodontitis which, following the initial periodontal therapy, included surgical therapy (open flap surgery with or without bone replacement).
Clinical parameters of plaque index (PI), papillary bleeding index (PBI), and probing pocket depth (PPD) were recorded at baseline, as well as one month and six months post-treatment. Following the standard methods of preparation, periodontal surgical treatment via open flap surgery was performed in 25 patients (group 1), and via open flap surgery with addition of Maxresorb® (Bottis dental) was carried out in 25 patients (group 2).
3. RESULTS
During periodontal surgical therapy, a total of 204 teeth were treated. For the sake of easier discussion of the results of surgical treatment, we divided the teeth into three groups (1-3 single-rooted teeth, 4-5 double-rooted teeth, and 6-7 three-rooted teeth) (Diagram 1).
Diagram 1.
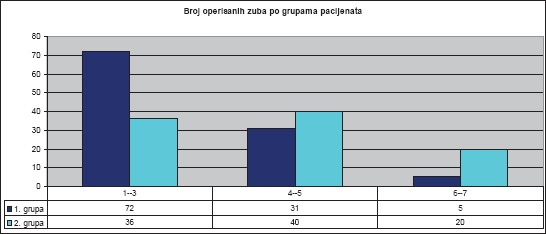
Number of treated teeth by groups of patients and tooth groups. Total number of treated teeth was 204.
We compared the mean PI values for both groups of patients before surgical therapy, one month after surgery, and six months after. PI values decreased after one month, as well as after six months (Diagram 2).
Diagram 2.
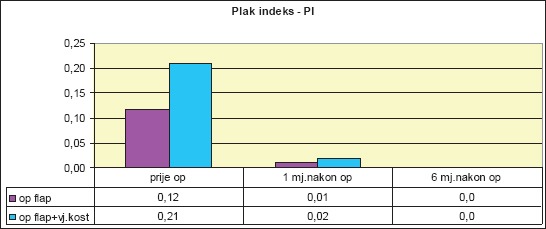
Mean values of plaque index (PI) per patient group
We compared the mean PBI values for both groups of patients before surgical therapy, one month after surgery, and six months after (Diagram 3).
Diagram 3.
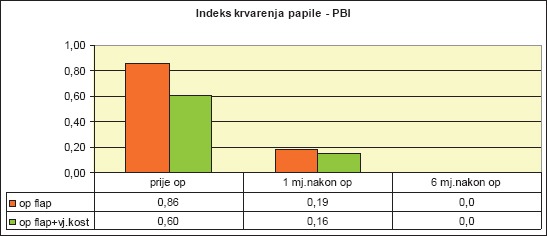
Mean values of papillary bleeding index (PBI) by patient groups
As the diagram clearly shows, the parameters differ before the surgical therapy and after the performed treatment. In order to determine the significance of the PBI difference in all three measurements, we used the independent-samples t-test:
Independent-samples t-test found that PBI for patients within group 1 significantly decreased, both one month after surgery (t(24) = 5.42, p <.001***), as well as after six months (t(24) = 2.14, p = .04*).
Independent-samples t-test for Group 2 also found that PBI significantly decreased, both one month after surgery (t(24) = 3.67, p <.001***), as well as after six months (t(24) = 1.44, p = .16).
Diagram 4 shows that PPDs for both groups differ before and after therapy. Level of significance of this difference was determined by independent-samples t-test.
Diagram 4.
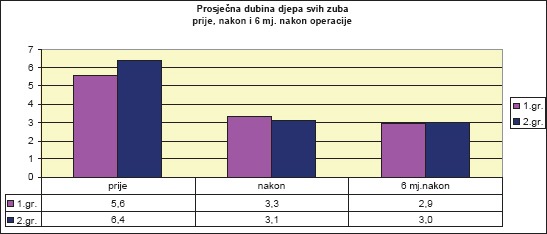
Mean values of probing pocket depth (PPD) by patient groups prior to surgery, one month, and six months after therapy
Observing the mean value of the PPD of intercanine teeth (1-3), the independent-samples t-test for group 1 determined a significant reduction one month after the operation (t(23) = 11.9, p <.001***), as well as after six months (t(23) = 5.18, p <.001***).
Independent-samples t-test for group 2 found that the mean value of the PPD of intercanine teeth (1-3) was significantly reduced after the operation (t(16) = 18.349, p <.001***).
Independent-samples t-test for Group 1 found that the mean PPD score in premolars (4-5) was significantly reduced after surgery (t(19) = 10.151, p <.001***), as well as after six months (t(19) = 3.511, p <.002**).
Independent-samples t-test determined that there was a significant difference in the mean value of PPD for premolars (4-5) in group 2, before and after surgery (t(21) = 21.710, p <.001 ***).
Independent-samples t-test determined that the mean PPD value in molars (6-7) within group 1 was significantly reduced after surgery (t(3) = 5.745, p <.01**).
Independent-samples t-test determined that the mean value of PPD in molars (6-7) within group 2 was significantly reduced after surgery (t(14) = 12.965, p <.001 ***).
4. DISCUSSION
In the last twenty years, periodontal therapy has progressed significantly, from formerly used resective methods, to principles of preservation and regeneration of periodontal tissues which are used today.
Bembi et al. found no significant difference in the results of treatment with two materials, NovaBone Putty–CMF, and Frios® Algipore®, followed by clinical parameters of probe depth and clinical attachment level. X-Ray monitoring has shown better results with NovaBone Putty, and they also believe that treatment results depend on good oral hygiene, which also ensures the lack of inflammation of periodontal tissue in the postoperative period (10). We agree that oral hygiene is of great importance, both during the preparation for surgical therapy, and in the postoperative period. In our study, gingival inflammation was monitored by the papillary bleeding index (PBI), before and after treatment. For group 1, the mean PBI score decreased significantly both one month after the operation (t(24) = 5.42, p <.001***), as well as after six months (t(24) = 2.14, p = .04*). Similarly, for the group 2, the mean value of PBI decreased significantly both one month after the operation (t(24) = 3.67, p <.001***), as well as after six months (t(24) = 1.44, p = .16).
Numerous bone transplants were used in various clinical trials and with different degrees of success (11, 12, 13). In our study, we used Maxresorb® which has an excellent homogeneous biphasic composition of 60% hydroxyapatite acid and 40% beta-tricalcium phosphate (β-TCP). Osteoconductivity of Maxresorb® is achieved through a matrix of interrelated pores and very high porosity. On one hand, high macroporosity of Maxresorb® ensures the effective growth of osteogenic cells and promotes bone regeneration. On the other hand, microporosity and roughness of the surface of Maxresorb® facilitate the diffusion of biological fluids and binding of cells, making this material an excelent basis for the migration of cells that produce bone layers and bind signaling molecules, which can accelerate the integration and regeneration of tissues. It is a 100% synthetic bone replacement with controlled resorptive properties and excellent handling characteristics (14).
The results of our study showed a significant reduction in probing depth of for both groups of patients, one and six months after the performed surgical therapy. By comparing the results between the groups, we did not get significantly better results in group 2, where we used Maxresorb® (Bottis dental) bone replacement.
The results of our research disagree with the findings of a study by Gokhale, S.T., whose results showed a significant improvement in all clinical parameters at sites treated with Bio-Oss™ compared to control sites. In addition, they believe that further long-term clinical studies with histological evaluation of results are needed to better understand the regeneration of the periodontium (15). This difference in the obtained results can be explained by different properties of the materials used in regenerative therapy during the research.
In his research, Chhina, S. compared treatment results using standard flap surgery and flap surgery with the addition of free gingival graft. The results of treatment in the group with free gingival graft were better (16), while the research by Khash et al. implementing the combined use of the bioresorptive Atrisorb membrane and bone graft did not show clinically better treatment results. The authors believe that long-term studies with a larger sample are needed, which would be aimed at comparing bone grafts and combination of GTR with bone graft, followed by histological evaluation of periodontal healing (16, 17). We agree that further research in this area is needed. Even though in our study we applied bone replacement during the open flap surgery in group 2, and open flap surgery without bone replacement in group 1, we obtained a significant reduction in the probing depth, which was retained at the second control after six months. We believe that a technically well conducted flap operation, during which the root area conditioning is performed with minimal soft tissue damage, creates good preconditions for periodontal healing without the use of bone replacement materials. We agree with other authors (15, 17, 18) that studies with histological evaluation of periodontal healing would be useful.
Perumal et al. have concluded that, within the limits of the conducted study, the microsurgical approach can significantly improve the initial treatment outcome and postoperative pain, but it does not yield better results long-term (19). We believe that minimally invasive periodontal therapy can achieve long-lasting good results, clinically monitored through the reduction of pocket probing depth, even without the use periodontal microsurgery, as we have demonstrated in our research.
5. CONCLUSION
Based on a detailed analysis of clinical parameters and statistical data processing, it can be concluded that surgical periodontal therapy, with and without bone replacement, resulted in a statistically significant reduction in the depth of the periodontal pocket probing in both groups. The results showed that there was no statistically significant difference between group 1 and group 2. We believe that future research should be focused on testing the effectiveness of new regenerative methods and materials (bone replacements with various properties, membranes, and surgical methods) that will result in better treatment results with predictable outcomes.
Footnotes
• Authors’ contributions: Conception and design: MGV and SH; Acquisition, analysis and interpretation of data: MGV, SH and EP; Drafting the article: MGV and EP; Revising it critically for important intellectual content: MGV and SH.
REFERENCES
- 1.Savage A, Eaton KA, Moles DR, Needleman I. A systematic review of definitions of periodontitis and methods that have been used to identify this disease. J Clin Periodontol. 2009;36:458–67. doi: 10.1111/j.1600-051X.2009.01408.x. [DOI] [PubMed] [Google Scholar]
- 2.Haffajee AD, Cugini MA, Dibart S, Smith C, Kent RL, Jr, Socransky SS. The effect of SRP on the clinical and microbiological parameters of periodontal diseases. J Clin Periodontol. 1997;24:324–34. doi: 10.1111/j.1600-051x.1997.tb00765.x. [DOI] [PubMed] [Google Scholar]
- 3.Reynolds MA, Kao RT, Camargo PM, Caton JG, Clem DS, Fiorellini JP, Geisinger ML, Mills MP, Nares S, Nevins ML. Periodontal regeneration–intrabony defects: a consensus report from the AAP Regeneration Workshop. J Periodontol. 2015 Feb;86(2 Suppl):S105–7. doi: 10.1902/jop.2015.140378. [DOI] [PubMed] [Google Scholar]
- 4.Needleman IG, Worthington HV, Giedrys-Leeper E, Tucker RJ. Guided tissue regeneration for periodontal infra-bony defects. Cochrane Database Syst Rev. 2006 Apr 19;2:CD001724. doi: 10.1002/14651858.CD001724.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Thakkar DJ, Deshpande NC, Dave DH, Narayankar SD. A comparative evaluation of extraction socket preservation with demineralized freeze driedbone allograft alone and along with platelet-rich fibrin: A clinical and radiographic study. Contemp Clin Dent. 2016 Jul-Sep;7(3):371–6. doi: 10.4103/0976-237X.188567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yukna RA, Krauser JT, Callan DP, Evans GH, Cruz R, Martin M. Multi-center clinical comparison of combination anorganic bovine-derived hydroxyapatite matrix (ABM)/cell binding peptide (P-15) and ABM in human periodontal osseous defects 6-month results. J Periodontol. 2000;71:1671–9. doi: 10.1902/jop.2000.71.11.1671. [DOI] [PubMed] [Google Scholar]
- 7.Nevins ML, Camelo M, Lynch SE, Schenk RK, Nevins M. Evaluation of periodontal regeneration following grafting intrabony defects with Bio-Oss collagen: a human histologic report. Int J Periodontics Restorative Dent. 2003 Feb;23(1):9–17. [PubMed] [Google Scholar]
- 8.Fatima G, Shivamurthy R, Thakur S, Baseer MA. Evaluation of anorganic bovine-derived hydroxyapatite matrix/cell binding peptide as a bone graft material in the treatment of human periodontal infrabony defects: A clinico-radiographic study. J Indian Soc Periodontol. 2015 Nov-Dec;19(6):651–8. doi: 10.4103/0972-124X.164766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graziani L, Laurell L, Tonetti M, Gottlow J, Berglundh T. Periodontal wound healing following GTR therapy of dehiscence-type defects in the monkey: Short-, medium-and long-term healing. J Clin Periodontol. 2005;32:905–14. doi: 10.1111/j.1600-051X.2005.00789.x. [DOI] [PubMed] [Google Scholar]
- 10.Bembi NN, Bembi S, Mago J, Baweja GK, Baweja PS. Comparative Evaluation of Bioactive Synthetic NovaBone Putty and Calcified Algae-derived Porous Hydroxyapatite Bone Grafts for the Treatment of Intrabony Defects. Int J Clin Pediatr Dent. 2016;9(4):285–290. doi: 10.5005/jp-journals-10005-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gothi R, Bansal M, Kaushik M, Khattak BP, Sood N, Taneja V. A comparative evaluation of freeze dried bone allograft and decalcified freeze dried bone allograft in the treatment of intrabony defects: A clinical and radiographic study. J Indian Soc Periodontol. 2015 Jul-Aug;19(4):411–5. doi: 10.4103/0972-124X.154169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chacko NL, Abraham S, Rao HN, Sridhar N, Moon N, Barde DH A. Clinical and Radiographic Evaluation of Periodontal Regenerative Potential of PerioGlas® A Synthetic Resorbable Material in Treating Periodontal Infrabony Defects. J Int Oral Health. 2014 Jun;6(3):20–6. [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan A, Kedige S. Periodontal bone regeneration in intrabony defects using osteoconductive bone graft versus combination of osteoconductive and osteostimulative bone graft: A comparative study. Dent Res J (Isfahan) 2015 Jan-Feb;12(1):25–30. doi: 10.4103/1735-3327.150307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eriberto Bressan, et al. Donor Age-Related Biological Properties of Human Dental Pulp Stem Cells Change in Nanostructured Scaffolds. PLOS One. 2012 Nov;17(11):e49146. doi: 10.1371/journal.pone.0049146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gokhale ST, Dwarakanath CD. The use of a natural osteoconductive porous bone mineral (Bio-Oss™) in infrabony periodontal defects. J Indian Soc Periodontol. 2012;16:247–52. doi: 10.4103/0972-124X.99270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chhina S. A 12 Months Clinical and Radiographic Study to Assess the Efficacy of Open Flap Debridement and Subepithelial Connective Tissue Graft in Management of Supracrestal Defects. J Int Oral Health. 2015 Aug;7(8):108–13. [PMC free article] [PubMed] [Google Scholar]
- 17.Khashu H, Vandana KL. Clinical and radiographic evaluation of human periodontal osseous defect (mandibular grade II furcation) treated with PepGen P-15 and a bioresorbable membrane (Atrisorb) J Indian Soc Periodontol. 2012 Oct;16(4):569–76. doi: 10.4103/0972-124X.106917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reddy MS, Aichelmann-Reidy ME, Avila-Ortiz G, Klokkevold PR, Murphy KG, Rosen PS, Schallhorn RG, Sculean A, Wang HL. Periodontal regeneration - furcation defects: a consensus report from the AAP RegenerationWorkshop. J Periodontol. 2015 Feb;86(2 Suppl):S131–3. doi: 10.1902/jop.2015.140379. [DOI] [PubMed] [Google Scholar]
- 19.Perumal MP, Ramegowda AD, Lingaraju AJ, Raja JJ. Comparison of microsurgical and conventional open flap debridement: A randomized controlledtrial. J Indian Soc Periodontol. 2015 Jul-Aug;19(4):406–10. doi: 10.4103/0972-124X.156884. [DOI] [PMC free article] [PubMed] [Google Scholar]


