Abstract
Background:
Primary percutaneous coronary intervention (PCI) is an emergent percutaneous catheter intervention in the setting of ST-segment elevations myocardial infarction (STEMI), without previous fibrinolytic treatment.
Aim:
To evaluate the feasibility and outcomes of primary percutaneous coronary interventions for STEMI in regional tertiary care cardiac centre in Bosnia and Herzegovina.
Methods:
Between January 2014 and December 2016, consecutive 549 STEMI patients who underwent primary PCI were prospectively enrolled in a primary PCI registry. The most of coronary angiography procedures were performed using the radial artery route. Patient demographics, risk factors, procedural characteristics, time variables and in-hospital events were assessed.
Results:
On admission, 297 (64.7%) of the patients were current smokers, 234 (42.6%) were hypertensive, 172 (31.3%) were diabetics, and 68 (12.3%) had cardiogenic shock. The mean duration of time from symptom onset to hospital arrival 193±118.2 minutes, and the mean door-to-balloon time was 37±11.3 minutes and median total ischemic time was 265(60-897) minutes. Infarct-related artery (IRA) was the left anterior descending artery in 47.1%, multivessel disease was present in 49.7%. Primary PCI involved balloon dilatation (2.7%) and stent implantation (97.3%). The incidence of postprocedural angiographic no-reflow was 6.7%. All-cause mortality occurred in 17 patients (3.1%).
Conclusion:
This study has shown feasibility and efficiency in performing of primary PCI with good outcomes in the first regional interventional center in Bosnia and Herzegovina. Experiences and results of our hospital can be very useful in creating primary PCI networks in our countries and developing countries as well.
Keywords: primary PCI, ST-segment elevations myocardial infarction, total ischemic time
1. INTRODUCTION
Acute ST-segment elevation myocardial infarction is the most dramatic manifestation of coronary artery disease (CAD) with high morbidity and mortality (1). Myocardial infarction was the first cause of death for males in Federation of Bosnia and Herzegovina in the last five years and fourth cause for both genders (2). Timely primary percutaneous coronary intervention has become the optimal strategy for the treatment of STEMI (3) and achieves rapid and more consistent reperfusion with low complication rate when compared to thrombolysis (4). Primary PCI is defined as intervention of the infarct related artery within 12 hour after the onset of symptoms, without prior thrombolytic therapy (3). Optimal treatment of STEMI in developed and most of developing countries is based on the establishing of networks between regional primary PCI capable hospitals connected by an efficient ambulance service. The goal of these networks is to provide optimal care while minimizing delays, in order to improve clinical outcomes. At these centers procedures ought to carry out systematically on a twenty-four hour, seven days a week (24/7) for all STEMI patients. There are no well-organized primary PCI networks in Bosnia and Herzegovina that cover all of regions 24/7. University Clinical Center Tuzla is the first primary PCI capable center organized 24/7 regime in Bosnia and Herzegovina. We aimed to evaluate the feasibility, results and outcomes of primary PCI. Experience and organization of this center could be a model for establishing other similar centers and creating primary PCI networks in whole country. This model might be effective in other developing countries.
2. MATERIAL AND METHODS
2.1. STUDY POPULATION
Total amount of 586 patients were admitted to our emergency department with STEMI between January 2014 and December 2016, and underwent emergent cardiac catheterization at Clinic for cardiovascular Disease, University Clinical Center Tuzla. Inclusion criteria were admission within 12 hours of onset of chest pain (18 hours for cardiogenic shock). Electrocardiogram (ECG) criteria were ST-segment elevation of at least 0.1 mV in minimum 2 consecutive leads (0.2mV for V1-V3) or new or presumably new left bundle branch block accompanying chest pain. After emergent angiography and baseline evaluation, 37 patients were excluded from the study with the following reasons: medical treatment due to very small diameter of infarct related artery (n=9), decision on bypass surgery (n=8), myocardial infarction with non-obstructive coronary arteries (MINOCA)(n=20). The remaining 549 patients (351males, 198 females; mean age 58.6±8.08) formed the study group who were underwent primary PCI.
2.2. INTERVENTIONAL PROCEDURE AND ADJUNCTIVE MEDICATIONS
All patients routinely received 300mg acetylsalicylic acid (ASA), clopidogrel (loading dose of 600mg) and with intravenous bolus of unfractionated heparin (100U/kg weight). We used glycoprotein IIb/IIIa inhibitor (tirofibran) as intracoronary bolus 10µg/kg followed by 0.15µg/kg/min intravenous infusion according to operator´s discretion. Coronary angiography procedures were performed by radial and femoral access. Infarct related artery was engaged with an appropriate sized guiding catheter and the culprit lesion was crossed with non-hydrophilic soft 0.014´´ guide wire. Balloon dilatation or stent implantation was performed by standard methods. According to our hospital protocol, bare metal stents (BMS) were used in most of the patients and drug-eluting stents (DES) were used when the patient or lesion characteristics were at high risk for restenosis. In case of multi-vessel disease, PCI is limited to IRA unless patient was in cardiogenic shock. Baseline and post-procedural TIMI flow grade were assessed. During procedures we used nonionic contrast media. Time from pain onset to hospital arrival, door-to-balloon time and total ischemic time were recorded.
Heparin therapy was stopped after the procedure of primary PCI in all cases except the cases of intra-aortic balloon counterpulsation (IABC) use where heparin was continued until its removal. Clopidogrel, ASA, beta-adrenergic blockers (β-blockers), angiotensin-converting-enzyme inhibitors (ACE-I) and statins were used as in-hospital standard therapy, if not contraindicated. In hospital adverse events (death, reinfarction, urgent CABG, bleeding and stroke) were noted.
2.3. STATISTICAL ANALYSIS
All the variables were entered into the Statistical Package for Social Sciences software, version 15 (SPSS Inc) for data analysis. Continuous variables like age were presented as means and standard deviations and timing variables like symptom onset to presentation at hospital, door-to-balloon time and total ischemic time were expressed as mean, median and range. Categorical variables like gender, risk factors, cardio-genic shock, left ventricular failure, multi-vessel disease, procedural success, mortality and outcomes were all reported as percentages.
3. RESULTS
Demographic and clinical characteristics of primary PCI patients are summarized in Table 1. The most often cardiac risk factor was smoking habits and it were observed in 297 (64.7%) patients. Hypertension and diabetes mellitus were present in 234 (42.6%) and 172 (31.3%) patients retrospectively. Cardiogenic shock was present on admission in 68 (12.3%) patients. Before admission in our center 11 patients (2.9%) had cardiac arrest. They were underwent measures of cardiopulmonary resuscitation successfully in emergency department or in ambulance.
Table 1.
Baseline clinical characteristics (n=549)
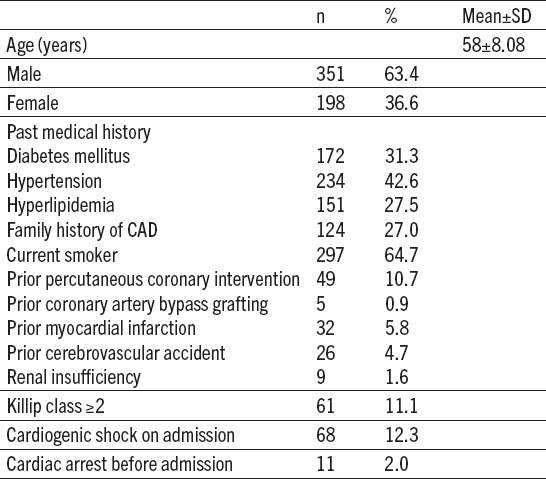
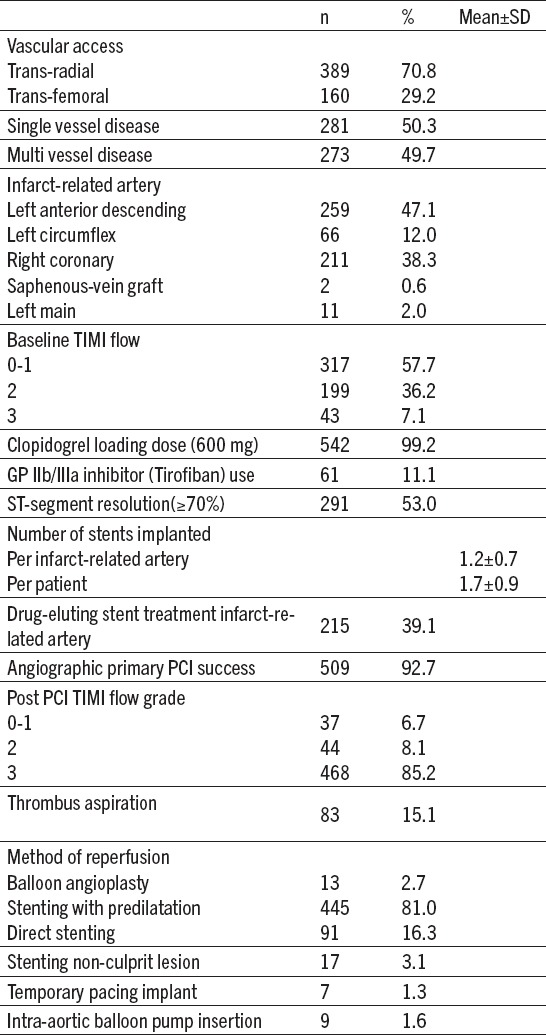
Mostly procedures were performed by trans-radial approach 389 (70.8%) patients. Left anterior descending coronary artery (LAD) was the most common IRA accounting for 259 (47.1%), followed by right coronary artery 211 (38.3%) patients. Multi-vessel disease was present in 273 (49.7%) patients. Baseline TIMI 0-1 flow was present in 317 patients (57.7%). Implantation of coronary stent of IRA was performed in 536 patients (97.3%); of these, 91 (16.3%) was direct stenting. Culprit vessels were treated in 215 patients (39.1%) with drug-eluting stents. After culprit vessel treatment we performed stenting of non-culprit lesion in 17 (3.1%) patients (Table 2).
Table 2.
Angiographic and procedural findings
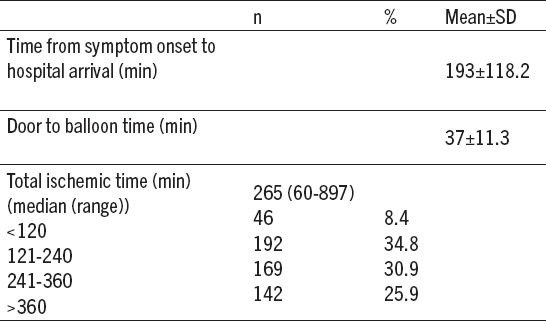
Timing intervals to primary PCI are summarized in Table 3. The median symptom onset to hospital arrival time was 193±118.2 minutes. The mean door-to-balloon (DTB) was 37 minutes in our clinic. The median total ischemic time was 265(90-897) min.
Table 3.
Timing variables
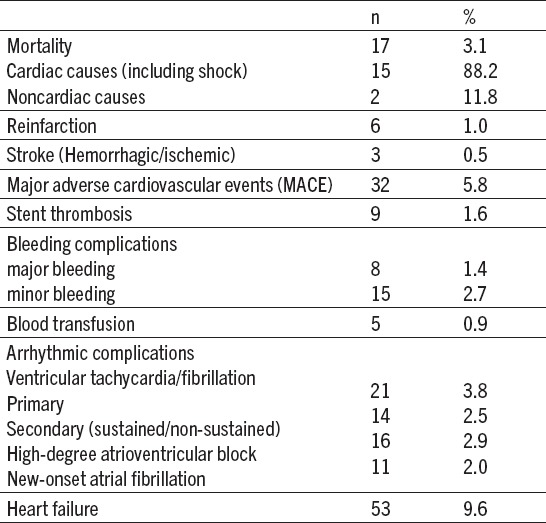
Death in the catheterization laboratory or within 24 hours of hospital admission occurred in 17 (3.1%) patients and (88.29%) of those cases died from cardiac cause. Cumulative major adverse cardiovascular events (MACE) were observed in 32 (5.8%) patients. Major bleeding complications were noted in 8 patients (1.4%). In-hospital stent thrombosis was present in 9 (1.6%) patients (Table 4).
Table 4.
In-hospital events
4. DISCUSSION
In this review, we aimed to show our outcomes and results of primary PCI during three-year period of time in our tertiary hospital. We evaluated demographic, angiographic, and procedural characteristics of the patients and in-hospital adverse cardiovascular events. Primary PCI is now considered the gold standard of treatment for STEMI. Randomized clinical trials shown superior efficacy and safety of primary PCI over hospital fibrinolysis (3, 5). Both European and American guidelines state that primary PCI is the preferred therapeutic option in patients with STEMI admitted within 90 min after diagnosis (3, 6). Hence, primary PCI has become routine practice in our clinic. Our tertiary cardiology center started 24hours/7days primary PCI in 2013. Before that, we performed primary PCI from 08.00 to 16.00 except weekend.
For the patient’s perspective, total ischemic time or time to treatment (either starting fibrinolysis or passing a wire through the culprit vessel) is possible the most important determinant of not only myocardial salvage but also mortality (7). It should be reduced as much as possible. It starts from the onset of symptoms of myocardial infarction to the initiation of reperfusion. The importance of time to treatment has been shown in studies by Tarantini et al.(8). Mean pain to balloon time was 90±40 min, 110±107 min, and 137±97 min in patients without myocardial transmural necrosis (TN) and severe microvascular obstruction (SMO), with TN but without SMO, or with both TN and SMO, respectively (p=0.001). Multivariate analysis showed that time delay was significantly associated both with TN (odds ratio per 30 min, 1.37, p=0.032), and SMO (odds ratio per 30 min, 1.21; p=0.021). In AMI patients with impaired coronary perfusion undergoing PCI, the risk of TN and SMO.
Increases with the duration of the ischemic time (8). Thus, the amount of salvageable myocardium is time dependent and gave birth to the concept of “time is muscle”. In this study median total ischemic time was 265(60-897) minutes. Various reasons are responsible for the prolonged of the ischemic time as a lack of awareness, paucity of transport facilities, financial difficulties, and inaccurate diagnosis (9).
The mean door-to-balloon time of our center was 37±11.3 minutes, which is shorter than many reference centers (10, 11) and in none of our patients, DTB time exceeded 60 minutes as suggested in the guidelines. This delay reflects the organization and performance of the PCI-capable hospital. McNamara et al.(12) showed a close relationship between in-hospital mortality and DTB time. Every 15-minute decrease in the treatment time between 150 and 90 minutes was associated with a decrease of 6.3 mortalities in 1000 deaths.
The mortality of STEMI is influenced by many factors, among them: age, Killip class, time delay to treatment, mode of treatment, history of prior myocardial infarction, diabetes mellitus, renal failure, number of diseased coronary arteries, ejection fraction and treatment.
In-hospital mortality in our hospital was 3.1% (88.2%-cardiac causes including cardiogenic shock and 11.8%-noncardiac causes).
The in-hospital mortality of unselected STEMI patients in the national registries of the 32 European countries (threated PPCI and fibrinolysis) varies between 6% and 14%. (3,12) In-hospital mortality after primary PCI varies from center to center; from 3.2% (13), 4.2 % (14), 4.4% (15). There are a few reasons for low level of in-hospital mortality; decreased DTB times, performing procedures by experienced interventional cardiologists, efficacy STEMI treatment protocol in our center, advances in anticoagulant and antiaggregant treatments. On the other hand, some patients underestimated symptoms, and they came in interventional center too late for primary PCI or they stayed at home. Thereby, some patients died at home or in other hospitals and they were not included in this study.
Incidence of major bleeding was 8(1.4%) and minor bleeding was 15(2.7%). Main reason for low level of bleeding was trans radial approach (TRA) as a dominant vascular access for primary PCI. We used TRA in 389 patients (70.8%) according to recommendations (16). Also, a significant decrease was observed in the amount of bleeding with controlled anticoagulation regimens, early sheath removal, use of smaller-diameter cannulation, and increased experience of interventional cardiologists (17).
Stent thrombosis (ST) is the most feared complication of coronary stent treatment because of its morbidity and mortality. The reported incidence of early stent thrombosis is 1.5% and the rate of this complication is higher in STEMI patients compared to elective procedures, as stent implantation during acute myocardial infarction is a risk factor for stent thrombosis (18, 19). In our study, in-hospital stent thrombosis was observed in 19 patients (1.6%). The incidence of stent thrombosis was similar to the reported rates in the literature.
The no-reflow phenomenon remains a significant challenge for STEMI patients. It is associated with poor prognosis that is independent of and beyond that provided by other relevant clinical factors including the infarct size (20). It is an angiographic phenomenon characterized by evidence of slow-flow in the affected vessel (TIMI flow 0-1) and lack of contrast uptake “blush” by the subtended myocardium, leading to a potential dissociation between coronary revascularization and myocardial perfusion in STEMI. The rate of this complication varies from 5% to 40% in different series, depending on the method to evaluate successful reperfusion (21, 22). No-reflow is associated with high short- and long-term mortality and morbidity rates. In the GUSTO-IIb trial, 30-day mortality was 1.6% in patients with TIMI 3 flow, 19.9% in patients with TIMI 2 flow, and 20% in patients with TIMI 0-1 flow (23). In our study, angiographic no-reflow rate was 6.7%. Our results of no-flow phenomenon were similar to the reported rates in the other studies.
The main limitation of our study is its retrospective design. In retrospective studies, the reliability of data depends on regular and accurate recording of patients data by responsible physicians and hospital staff. In addition, some data could not be obtained from the archive. Another limitation is the lack of long-term cardiovascular events, which is planned to be included in a future study.
This study has shown feasibility and efficiency in performing of primary PCI with good outcomes in the first regional interventional center in Bosnia and Herzegovina. In the future our goals should be: decreasing of reperfusion time, which should be achieved by increasing public awareness, reducing patient delay and improving the service of first medical contact (emergency medical system). Experiences and results of our hospital can be very useful in creating primary PCI networks in our countries and developing countries as well.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM. Executive summary: heart disease and stroke statistics - 2012 update: a report from the American Heart Association. Circulation. 2012;125:188–97. doi: 10.1161/CIR.0b013e3182456d46. [DOI] [PubMed] [Google Scholar]
- 2.Zavod za javno zdravstvo FBIH. Izvještaj o zdravstvenom stanju stanovništva Federacije BIH u 2014 godini. Zavod za javno zdravstvo Federacije BIH. 2015 [Google Scholar]
- 3.Steg G, James S, Atar D, Badano L, Bldmstrom-Lundqvist C, Di Mario C, et al. Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2012;33:2569–2619. [Google Scholar]
- 4.Keeley EC, Boura JA, Grines CL. Primary angioplasty vs. intravenous thrombolytic therapy for acute myocardial infarction: a quantitative review of 23 randomised trials. Lancet. 2003;361:13–20. doi: 10.1016/S0140-6736(03)12113-7. [DOI] [PubMed] [Google Scholar]
- 5.Andersen HR, Nielsen TT, Rasmussen K, Thuesen L, Kelbaek H, et al. A comparison of coronary angioplasty with fibrinolytic therapy in acute myocardial infarction. N Engl J Med. 2003;349:733–42. doi: 10.1056/NEJMoa025142. [DOI] [PubMed] [Google Scholar]
- 6.O’Gara P, Kushner F, Ascheim D, Casey D, et al. 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction;A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:00–00. doi: 10.1161/CIR.0b013e3182742c84. [DOI] [PubMed] [Google Scholar]
- 7.De Luca G, Suryapranata H, Ottervanger JP, Antman EM. Time delay to treatment and mortality in primary angioplasty for acute myocardial infarction: every minute of delay counts. Circulation. 2004;109:1223–e1225. doi: 10.1161/01.CIR.0000121424.76486.20. [DOI] [PubMed] [Google Scholar]
- 8.Tarantini G, Cacciavillani L, Corbetti F, et al. Duration of ischemia is a major determinant of transmurality and severe microvascular obstruction after primary angioplasty: a study performed with contrast enhanced magnetic resonance. J Am Coll Cardiol. 2005;46:1229–e1235. doi: 10.1016/j.jacc.2005.06.054. [DOI] [PubMed] [Google Scholar]
- 9.Xavier D, Pais P, Devereaux PJ, et al. CREATE Registry Investigators. Treatment and outcomes of acute coronary syndromes in India (CREATE): a prospective analysis of registry data. Lancet. 2008;371:1435–e1442. doi: 10.1016/S0140-6736(08)60623-6. [DOI] [PubMed] [Google Scholar]
- 10.Cannon CP, Gibson CM, Lambrew CT, Shoultz DA, Levy D, French WJ, et al. Relationship of symptom-onset-to-balloon time and door-to-balloon time with mortality in patients undergoing angioplasty for acute myocardial infarction. JAMA. 2000;283:2941–7. doi: 10.1001/jama.283.22.2941. [DOI] [PubMed] [Google Scholar]
- 11.McNamara RL, Wang Y, Herrin J, Curtis JP, Bradley EH, Magid DJ, et al. Effect of door- to -balloon time on mortality in patients with ST- segment elevation myocardial infarction. J Am Coll Cardiol. 2006;47:2180–6. doi: 10.1016/j.jacc.2005.12.072. [DOI] [PubMed] [Google Scholar]
- 12.Mandelzweig L, Battler A, Boyko V, Bueno H, Danchin N, Filippatos G, Gitt A, Hasdai D, Hasin Y, Marrugat J, Van de Werf F, Wallentin L, Behar S. The second Euro Heart Survey on acute coronary syndromes: characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur Heart J. 2006;27:2285–93. doi: 10.1093/eurheartj/ehl196. [DOI] [PubMed] [Google Scholar]
- 13.Kumbhani DJ, Cannon CP, Fonarow GC, Liang L, Askari AT, Peacock WF. Association of hospital primary angioplasty volume in ST-segment elevation myocardial infarction with quality and outcomes. JAMA. 2009;302(20):2207–13. doi: 10.1001/jama.2009.1715. [DOI] [PubMed] [Google Scholar]
- 14.Subbana V, Lakshmananb A, Victora SM, Pakshirajana B, et al. Outcome of primary PCI e An Indian tertiary care center experience. Indian heart journal. 2014;66:25–30. doi: 10.1016/j.ihj.2013.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kırma C, Oduncu V, Tanalp AC, Erkol A, et al. Primary angioplasty in a high-volume tertiary center in Turkey: in-hospital clinical outcomes of 1625 patients. Arch Turk Soc Cardiol. 2011;39(4):300–7. doi: 10.5543/tkda.2011.01231. doi:10.5543/tkda.2011.01231. [DOI] [PubMed] [Google Scholar]
- 16.Diletti1 R, Yetginl T, Manintveld1 O, et al. Percutaneous coronary interventions during ST-segment elevation myocardial infarction: current status and future perspectives. EuroIntervention. 2014;10:T13–T22. doi: 10.4244/EIJV10STA4. [DOI] [PubMed] [Google Scholar]
- 17.Keeley EC, Hillis LD. Primary PCI for myocardial infarction with ST-segment elevation. N Engl J Med. 2007;356:47–54. doi: 10.1056/NEJMct063503. [DOI] [PubMed] [Google Scholar]
- 18.Laarman GJ, Suttorp MJ, Dirksen MT, van Heerebeek L, Kiemeneij F, Slagboom T, et al. Paclitaxel- eluting versus uncoated stents in primary percutaneous coronary intervention. N Engl J Med. 2006;355:1105–13. doi: 10.1056/NEJMoa062598. [DOI] [PubMed] [Google Scholar]
- 19.Iakovou I, Schmidt T, Bonizzoni E, Ge L, Sangiorgi GM, Stankovic G, et al. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–30. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- 20.Hiroshi I. No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nature Clinical Practice of Cardiovascular Medicine. 2006;3:499–506. doi: 10.1038/ncpcardio0632. [DOI] [PubMed] [Google Scholar]
- 21.Rezkalla SH, Kloner RA. Coronary no-reflow phenomenon: from the experimental laboratory to the cardiac catheterization laboratory. Catheter Cardiovasc Interv. 2008;72:950–7. doi: 10.1002/ccd.21715. [DOI] [PubMed] [Google Scholar]
- 22.Eeckhout E, Kern MJ. The coronary no-reflow phenomenon: a review of mechanisms and therapies. Eur Heart J. 2001;22:729–39. doi: 10.1053/euhj.2000.2172. [DOI] [PubMed] [Google Scholar]
- 23.Berger PB, Ellis SG, Holmes DR, Jr, Granger CB, Criger DA, Betriu A, et al. Relationship between delay in performing direct coronary angioplasty and early clinical outcome in patients with acute myocardial infarction: results from the global use of strategies to open occluded arteries in Acute Coronary Syndromes (GUSTO -IIb) trial. Circulation. 1999;100:14–20. doi: 10.1161/01.cir.100.1.14. [DOI] [PubMed] [Google Scholar]


