Abstract
Introduction:
Varicella Zoster Virus (VZV) is associated with many disorders of the central and peripheral nervous systems including neuralgia, meningitis, meningoencephalitis, cerebellitis, vasculopathy, myelopathy, Ramsay-Hunt syndrome, and polyneuritis cranialis. Cranial nerves V, VI, VII, VIII, IX, X, XI, and/or XII may be affected. The neurological disorders caused by VZV usually present with rash, but may rarely present without rash.
Case report:
We herein present a case of polyneuritis cranialis without rash caused by VZV affecting cranial nerves VII, VIII, IX, and X. After excluding other causes of the condition, we diagnosed VZV infection based on VZV DNA in the CSF and an elevated anti-VZV IgG level in serum. The patient responded well to antiviral therapy.
Conclusion:
VZV infection should be kept in mind during the differential diagnosis of polyneuritis cranialis; it is important to note that VZV re-activation may occur without rash.
Keywords: Varicella Zoster Virus, Cranial Polyneuropathy
1. INTRODUCTION
Varicella Zoster Virus (VZV) is a neurotropic DNA virus as a member of the family Herpesviridae. It is the cause of varicella as primary infection and zoster as recurrence. (1). VZV produces a lot of disorders involving central and peripheral nervous system such as neuralgia, meningitis, meningoencephalitis, cerebellitis, vasculopathy, myelopathy, Ramsay Hunt syndrome and polyneuritis cranialis (2). Pathological mechanism is thought to be usually related to microinfarction of cranial nerves due to occlusion of vasa nervorum (3). These neurological disorders caused by VZV are usually seen with rash but rarely may present without rash. (4). In the literature some cases of polyneuritis cranialis caused by VZV have been reported. We herein present a case of polyneuritis cranialis without rash.
2. CASE REPORT
A 63 year-old man was admitted to our hospital with complaints of headake, dysphagia, hearing loss and left sided facial asymmetry continuing for 10 days. He was hospitalized to our clinic for further assesment. Detailed anamnesis revealed coronary artery disease with family history and smoking habit of one-pack-per day for 15 years. He had been using acetylsalicylic acid and propranolol. In the physical examination, his vital signs were normal, and there were no eruptions on his face, ears, mouth or pharynx. Neurological examination revealed hoarse voice, left sided peripheral facial asymmetry, hypoesthesia on the left side of the face and decreased gag reflex (Figure 1). The other neurological examinations were found normal. Blood cell counts, erythrocyte sedimentation rate, C-reactive protein, electrolytes, creatinine, hepatic enyzmes, thyroid tests, tumor markers, vitamins A, B1, B6 and E were in normal range. Magnetic resonance imaging (MRI) of brain demonstrated nonspecific ischemic gliotic focis. MRI venography, temporal MRI, neck computed tomography (CT), thorax CT were normal. Electromyography (EMG) demonstrated findings of axonal neuropathy on the left facial nerve (Figure 2). In the audiometry test pure tone averages of air and bone conduction were 44-36 dB in left and 41-35 dB in right, respectively (bilateral mild sensorineural hearing loss). Laryngeal fiberoscopy demonstrated left vocal cord paralysis (Abduction defect) (Figure 3). Lomber puncture revealed normal CSF pressure and levels of protein and chlorine while glucose level increased slightly. Cytological analysis of the CSF revealed lymphoid cell elevation (130/µl). Polymerase chain reaction (PCR) test for BK virus, Adenovirus, EBV, TBC, CMV, HSV1/2, JC virus, Brucella, atypical pneumonia agents in CSF were negative but only VZV-DNA was positive (+2/5 copy). Serum VZV IgG was elevated (749 mIU/ml) (Table 1).
Figure 1.
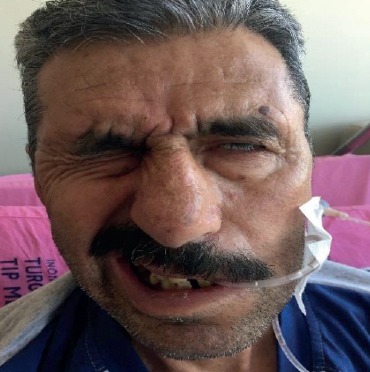
Left facial weakness
Figure 2.
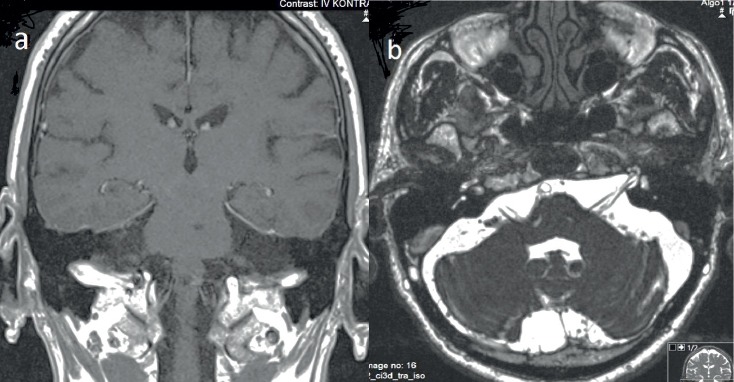
Brain MRI. A contrast-enhanced T1-weighted image (a) and T2-weighted non-contrast image (b) disclosed normal cranial nerves
Figure 3.

Laryngeal fiberoscopy; left vocal cord paralysis
We initiated intravenous acylovir in dose 3x750 mg./day. In the 6 th day of the teraphy the patient’s creatinin and urea levels were increased (respectively 2.31 mg/dl and 38 mg/dl.) After nephrological consultation, no primary kidney problem was detected, therefore we supposed that this situation was related to acylovir terapy. So we decreased the dose of acylovir to 2x750 mg/day and within 3 days the kidney functions turned back to normal. In follow-up evaluation after 10 days of antiviral therapy, his facial weakness declined but dysphagia remained same. The antiviral teraphy was maintained 21 days. He was fed by orogastric probe for 3 weeks. After 3 week his gag reflex came back poorly and he started oral feeding. In second month of the illness, his gag reflex was normal. His control laryngeal fiberoscopy was normal (Figure 4). Control audiometry was performed and pure tone averages of air and bone conduction showed a slight improvement on the left and remained nearly same on the right (36-34 dB and 41-39 dB, respectively). But clinically his hearing complaints declined. The neurological examination showed mild left peripheral facial neuropathy as sequela. In third month of the illness control CSF analyses revealed normal cell count. PCR test for all agents including VZV-DNA were negative. Serum VZV IgG showed significant decrease (9.9 mIU/ml).
Figure 4.
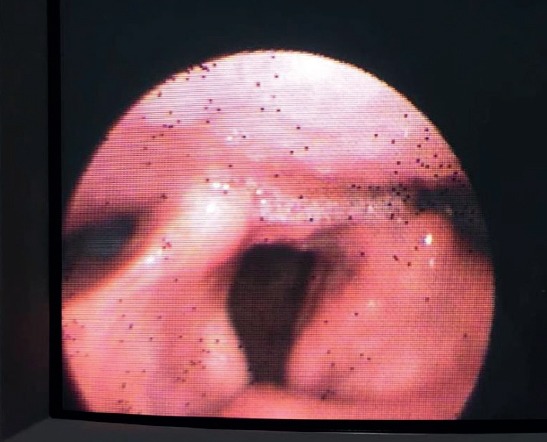
Laryngeal fiberoscopy control; normal vocal cord functions
The patient was fully informed and gave written consent prior to this report.
3. DISCUSSION
There have been various examples of polyneuritis cranialis caused by VZV (5). Cranial nerves V, VI, VII, VIII, IX, X, XI and XII may be affected. While especially, the involvement of cranial nerves V, VII and VIII with VZV is more prevalent, the other cranial nerves are affected lesser, relatively. In our case cranial nerves VII, VIII, IX and X were affected. Causes of polyneuritis cranialis include vascular diseases, infections, tumors, trauma, bone diseases, toxic causes, chemotherapy, surgery, vasculitis, inflammatory diseases and diabetes (2). Our patient did not have any story of malignancy, trauma, vasculitis, inflammatory diseases, diabetes and surgery. Some MRI findings have been reported in some cases related to involvement of multipl cranial nerves by VZV such as enlargement of cranial nerves with enhancement in the localization of auditory canal or swelling of nerves complex (6, 7). In a case report of VZV polyneuropathy which the IX and X. cranial nerves were involved, the MRI findings mimicked a small tumor (7). MRI findings of VZV polyneuritis cranialis have been suggested to be a temporary phenomenon in some case reports (8). Conversely, our patient did not display any similar MRI findings (Figure 2). Thus, it is important to take into consideration that polyneuropathy cranialis caused by VSV may present without MRI findings. Multiple cranial nerve involvement with rash by VZV is more prevalent and easily diagnosed. The main point of VZV reactivation is that it may present without rash. Infection of VZV without rash may cause various neurological disorders including chronic radicular pain, polyneuritis cranialis, cerebellitis, meningitis, vasculopathy (intracerebral and extracerebral arteries that causes ischemic or hemorrhagic stroke, dissection, arterial ectasia), myelopathy (myelitis and spinal cord infarction) (5, 9). Since the control CSF analysis revealed negative VZV-PCR and also no rash was detected in this period, we confirmed the diagnosis of VZV infection without rash. Antiviral and corticosteroid treatment has been recommended in some cases of polyneuritis cranialis by VZV (8, 10). Particularly, in a recent case report, urgent combined treatment with antiviral and agents was proposed (10). We initiated antiviral teraphy in our patient as soon as VZV infection was diagnosed. Since the disease was diagnosed on subacute period, the corticosteroid treatment was not applied. In the clinical follow up the pathological signs resolved by antiviral teraphy
4. CONLUSION
We herein presented a case of polyneuritis cranialis caused by VZV without rash of which the cranial nerves VII, VIII, IX and X were affected. Excluding the other causes of polyneuritis cranialis, we diagnosed VZV infection showing VZV-DNA in the CSF and elevated VZV IgG in the serum. This clinical situation responded well to antiviral teraphy. VZV infection should be kept in mind in the differential diagnosis of polyneuritis cranialis causes and the other important consideration is that VZV reactivation may ocur without rash.
Compliance with Ethical Standards:
Footnotes
• This article does not contain any studies with human participants performed by any of the authors and no funding for this study.
• All authors have no conflict of interest about this papers submission and to declare.
• All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
• Informed consent was obtained from all individual participants included in the case report.
• Statement on consent for publication of this case report was obtained from the patient.
• Approval of the case presentation was taken from the patient.
REFERENCES
- 1.Sauerbrei A. Diagnosis, antiviral therapy, and prophylaxis of varicella-zoster virus infections. [cited February 12, 2016];Eur J Clin Microbiol Infect Dis. 2016 35(5):723–34. doi: 10.1007/s10096-016-2605-0. doi:10.1007/s10096-016-2605-0. [DOI] [PubMed] [Google Scholar]
- 2.Carroll CG, Campbell WW. Multiple cranial neuropathies. [cited February 12, 2009];Semin Neurol. 2009 29(1):53–65. doi:10.1055/s-0028-1124023. [Google Scholar]
- 3.Rhonda GK, Straus SE. Postherpetic neuralgia: pathogenesis, treatment and prevention. [cited July 4, 1996];N Engl J Med. 1996 355(1):32–42. doi: 10.1056/NEJM199607043350107. doi:10.1056/NEJM199607043350107. [DOI] [PubMed] [Google Scholar]
- 4.Gilden DH, Dueland AN, Devlin ME, Mahalingam R, Cohrs R. Varicella-zoster virus reactivation without rash. [cited August 1, 1992];J Infect Dis. 1992 166(1):S30–S34. doi: 10.1093/infdis/166.supplement_1.s30. doi:10.1093/infdis/166.Supplement 1.S30. [DOI] [PubMed] [Google Scholar]
- 5.Gilden D, Cohrs RJ, Mahalingam R, Nagel MA. Neurological Disease Produced by Varicella Zoster Virus Reactivation Without Rash. [cited April 14, 2011];Curr Top Microbiol Immunol. 2010 342:243–53. doi: 10.1007/82_2009_3. doi:10.1007/82_2009_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yoshida T, Fujisaki N, Nakachi R, Sueyoshi T, Suwazono S, Suehara M. Persistent Hiccups and Vomiting with Multiple Cranial Nerve Palsy in a Case of Zoster Sine Herpete. [cited October 15, 2014];Intern Med. 2014 53(20):2373–6. doi: 10.2169/internalmedicine.53.1348. doi:10.2169/internalmedicine.53.1348. [DOI] [PubMed] [Google Scholar]
- 7.Adachi M. A case of Varicella zoster virus polyneuropathy: involvement of the glossopharyngeal and vagus nerves mimicking a tumor. [cited June 19, 2008];AJNR Am J Neuroradiol. 2008 29(9):1743–5. doi: 10.3174/ajnr.A1141. doi:10.3174/ajnr.A1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nisa L, Landis BN, Giger R, Leuchter I. Pharyngolaryngeal Involvement by Varicella-Zoster Virus. [cited June 12, 2013];Journal of Voice. 2013 27(5):636–41. doi: 10.1016/j.jvoice.2013.02.011. doi:10.1016/j.jvoice.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 9.Gilden D. Varicella-Zoster Virus Infections. [cited December 21, 2015];Continuum (Minneap Minn) 2015 21(6):1692–1703. doi: 10.1212/CON.0000000000000246. doi:10.1212/CON.0000000000000246. [DOI] [PubMed] [Google Scholar]
- 10.Taniguchi D, Nakahara PhDT, Nakajima S, Nakazato T, Mikasa M, Furukawa PhDY. Successful treatment with acyclovir and a corticosteroid for lower cranial polyneuropathy in zoster sine herpete: a case report. [cited October 28, 2015];Rinsho Shinkeigaku. 2015 55(12):932–5. doi: 10.5692/clinicalneurol.cn-000781. doi:10.5692/clinicalneurol.cn-000781. [DOI] [PubMed] [Google Scholar]


