Abstract
The transforming growth factor β (TGF-β) family signaling pathway is conserved and ubiquitous in animals. In Drosophila, fewer representatives of each signaling component are present compared with vertebrates, simplifying mechanistic study of the pathway. Although there are fewer family members, the TGF-β family pathway still regulates multiple and diverse functions in Drosophila. In this review, we focus our attention on several of the classic and best-studied functions for TGF-β family signaling in regulating Drosophila developmental processes such as embryonic and imaginal disc patterning, but we also describe several recently discovered roles in regulating hormonal, physiological, neuronal, innate immunity, and tissue homeostatic processes.
Fewer representatives of TGF-β family signaling components are present in Drosophila compared to vertebrates. Despite this relative simplicity, the Drosophila TGF-β pathway still has numerous roles in development and homeostasis.
Core Drosophila TGF-β Family Signaling Components
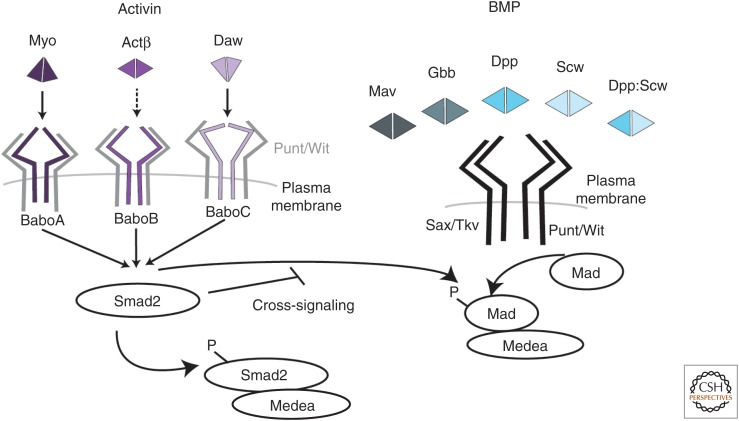
As in mammals, the Drosophila transforming growth factor β (TGF-β) family signaling pathway has two branches, initiated by different ligands, activins, and bone morphogenetic proteins (BMPs) that are defined by sequence conservation within families and the downstream signal transduction components that they use (Fig. 1). In both branches, canonical signaling begins upon extracellular ligand binding to a heteromeric receptor complex composed of two molecules each of type I and type II transmembrane serine/threonine kinases. Formation of this ligand–receptor complex results in phosphorylation of the GS domain within the type I receptor by the constitutively active type II receptor kinase. This phosphorylation event activates the type I receptor kinase and enables it to phosphorylate an appropriate receptor-activated Smad (R-Smad) substrate, either dSmad2 for the activin branch, or Mad for the BMP branch. The phosphorylated R-Smad then forms a complex with the Drosophila common-Smad (co-Smad), Medea, and this complex translocates to the nucleus where it can regulate transcriptional responses with a variety of cofactors. Some TGF-β signal transduction components, such as the type II receptors Punt and Wishful thinking (Wit) and the co-Smad Medea, are shared between the activin and BMP branches and often act redundantly, whereas others, such as the type I receptors and the R-Smads, are restricted to signaling within a single subfamily branch. Many accessory factors interact with core TGF-β family signaling components and are discussed throughout this review (Table 1).
Figure 1.
Core transforming growth factor β (TGF-β) family signaling components in Drosophila. Activin branch: Three ligands signal through the activin-specific type I receptor Babo. Each ligand is thought to have a dedicated receptor isoform, although evidence for Actβ-BaboB is lacking. Ligand binding induces formation of a receptor complex of both Babo and either of the type II receptors, Punt or Wishful thinking (Wit). Constitutively active type II receptors phosphorylate Babo, which activates dSmad2. Phosphorylated dSmad2 binds to Medea and translocates to the nucleus to regulate the transcriptional response. Bone morphogenetic protein (BMP) branch: Four ligands signal through shared the BMP-specific type I receptors Tkv and Sax and either Punt or Wit. The ligands are thought to primarily form homodimers, but there is one example of a heterodimer: Dpp–Scw. Mav is a divergent ligand based on sequence, but signals to activate Mad. Activation of type II receptors causes phosphorylation of type I receptors and subsequent phosphorylation of Mad, which then complexes with Medea to regulate transcription. In the absence of dSmad2, Babo can phosphorylate Mad and can also activate the Mad transcriptional response. Myo, Myoglianin; Actβ, Activin-β; Daw, Dawdle; Babo, Baboon; Mav, Maverick; Gbb, Glass-bottom boat; Dpp, Decapentaplegic; Scw, Screw; Sax, Saxophone; Tkv, Thickveins; Wit, Wishful thinking.
Table 1.
Transforming growth factor β (TGF-β) family accessory factors in Drosophila
| Gene name | Symbol | Function | Interaction |
|---|---|---|---|
| armadillo | arm | Transcription factor | Complexes with Mad |
| crossveinless | cv | Lipoprotein | Modulates Dpp movement |
| crossveinless 2 | cv-2 | BMP binding protein | Binds extracellular BMPs |
| dally-like protein | dlp | Heparan sulfate proteoglycan | Controls Dpp gradient by binding Dpp |
| division abnormally delayed | dally | Heparan sulfate proteoglycan | Controls Dpp gradient by binding Dpp |
| follistatin | fs | Secreted factor | Modulates ligand activity |
| pentagone | pent | Secreted factor | Interacts with heparan sulfate proteoglycans to control Dpp gradient |
| Plum | plum | Immunoglobulin protein | Genetic interaction with Myo |
| Schnurri | shn | Transcription factor | Complexes with Mad and Medea |
| Shrew | srw | Secreted factor | Role unknown |
| short gastrulation | sog | Secreted factor | Binds extracellular BMPs |
| Tolloid | tld | Protease | Binds extracellular BMPs, cleaves Sog |
| tolkin (also called tolloid-related) | tok, tlr | Protease | Cleaves extracellular BMP and activin ligands |
| twisted gastrulation | tsg | Secreted factor | Binds extracellular BMPs |
| Yorkie | yki | Transcription factor | Complexes with Mad |
Many additional proteins interact with core TGF-β pathway components and modulate pathway activation and output. Listed in the table are known accessory factors and their interactions with core TGF-β components.
BMP, Bone morphogenetic protein.
In the BMP signaling branch, three ligands—Decapentaplegic (Dpp), Glass-bottom boat (Gbb), and Screw (Scw)—signal through two type I receptors—Thickveins (Tkv) and Saxophone (Sax)—and either of two type II receptors—Wit or Punt. Although loss-of-function mutations in either tkv or sax are lethal, tkv overexpression can rescue sax mutants (Brummel et al. 1994), suggesting that it may be the primary BMP type I receptor, whereas Sax, in most cases, fine-tunes signaling levels. Downstream from the receptors, Mad is the primary R-Smad that appears to transduce all BMP signals in combination with the co-Smad Medea. One inhibitory Smad, Dad, appears to act specifically to modulate BMP signaling (Kamiya et al. 2008).
Within the activin subfamily, three ligands—activin-β (Actβ), Dawdle (Daw), and Myoglianin (Myo)—signal through the single type I receptor Baboon (Babo) and either Wit or Punt, and this branch uses dSmad2 as the R-Smad transcriptional transducer. In this pathway, each ligand appears to have unique functions, but double mutants have not yet been analyzed in detail to determine whether they also act redundantly. Consistent with the unique functional activities of the three ligands, three isoforms of Babo have been identified. Each differs in the ligand-binding domain and is produced through alternative splicing of a single exon (Jensen et al. 2009). Signaling assays in S2 cells show that Daw signals through BaboC (Jensen et al. 2009), whereas subsequent in vivo work indicates that Myo signals preferentially through BaboA (Zhu et al. 2008; Awasaki et al. 2011). It remains to be shown if Actβ signals through BaboB, and if there is any promiscuity in ligand binding to the different receptor isoforms. A handful of genes have been shown to be expressed downstream from dSmad2 in specific contexts (Zheng et al. 2003; Gibbens et al. 2011; Bai et al. 2013), but only one, Atg8a, is known to be a direct target, although a fully validated dSmad2 consensus binding sequence has not yet been determined. The seventh ligand, Maverick (Mav), is aptly named because it does not clean fit into either pathway by sequence similarity, although one report indicates that it signals through Tkv, which would be consistent with a BMP family member (Fuentes-Medel et al. 2012).
Unlike in vertebrates, only two non-Smad signaling pathways have been described in Drosophila. One involves the activin branch, where Rho-like GTPases and Lim kinase act downstream from Babo to limit mushroom body (MB) axon extension (Ng 2008), and the other is Lim kinase acting downstream from BMP signaling to regulate neuromuscular junction (NMJ) growth (Eaton and Davis 2005; Piccioli and Littleton 2014).
In addition to non-Smad-dependent signaling, one example of cross-pathway Smad activation has also been documented in Drosophila (Fig. 1). In this case, the activin receptor Babo can phosphorylate the BMP transducer Mad in cell culture and also in vivo when overexpressed together with Mad (Gesualdi and Haerry 2007; Peterson et al. 2012). However, whether this occurs under endogenous conditions is unknown. As discussed below, loss of dSmad2 protein does lead to enhanced Mad activity in vivo, but it is not clear whether this comes about as a result of enhanced carboxy-terminal phosphorylation of Mad or through some other modifications that happen only in the absence of dSmad2 (Peterson and O’Connor 2013).
As in vertebrates, many accessory factors regulate ligand production and availability and several are highlighted in Table 1. For example Drosophila follistatin (fs) can modulate ligand activity of both BMP and activin pathway ligands; inhibiting Dpp and Actβ while enhancing Daw activity (Bickel et al. 2008; Pentek et al. 2009). The BMP heterodimer Dpp–Scw binds to a complex of Short gastrulation and Twisted gastrulation (Sog–Tsg) and, as described below, is required for shuttling of the Dpp–Scw heterodimer during embryonic dorsal patterning (Shimmi et al. 2005b). Numerous potential coreceptors have also been identified.
Differential processing can also dramatically affect the signal range and activity of the ligand. Furin proteases facilitate maturation of the disulfide-linked ligand dimer by processing the inactive proprotein at any one of several potential “maturation sites.” For Dpp, three furin cleavage sites are processed in distinct ways, producing ligands with different amino-terminal sequences. These alternatively processed ligands have different activities, such as long-range or short-range diffusion, and variable stabilities (Kunnapuu et al. 2009; Sopory et al. 2010). They can also affect interactions with extracellular matrix (ECM) proteins, such as the Drosophila glypican Division abnormally delayed (Dally), through inclusion or exclusion of heparan sulfate proteoglycan (HSPG)-binding sites (Akiyama et al. 2008). In some cases, additional cleavage within the upstream prodomain is needed to fully release the prodomain from the mature ligand, and, in the two Drosophila examples, this processing is performed by a Tolloid/BMP-1 type protease (Serpe and O’Connor 2006; Kunnapuu et al. 2014). Lastly, there is an example in which cleavage of the BMP-type ligand Gbb does not take place at the normal maturation site, but instead at an upstream site leading to production of a very long mature ligand that, surprisingly, has bioactivity. The choice of maturation site seems to be tissue context-specific (Akiyama et al. 2012; Fritsch et al. 2012).
BMPs AND EARLY AXIAL PATTERNING IN Drosophila
The major body plan of most organisms is laid out during early embryonic development. BMPs play important roles in both eggshell patterning and axis formation. Dpp controls patterning of the follicle cells that produce the eggshell surrounding the oocytes (Dobens and Raftery 1998). In particular, Dpp signaling through the type I receptor Tkv and the type II receptor Wit specifies the location of the operculum and the respiratory dorsal appendage (Yakoby et al. 2008a,b; Marmion et al. 2013).
Once fertilized, maternal factors guide the initial subdivision of the embryo along the anterior/posterior (A/P) and dorsal/ventral (D/V) axes. At the time of cellularization, the products of the first zygotically expressed genes take over and further refine cell fates within each subdivision (Lynch et al. 2012). Two primary tissues are specified within the dorsal half of the embryo: the dorsal–lateral ectoderm that gives rise to portions of the epidermis, and the dorsal-most amnioserosa, a short-lived tissue that coordinates the movements of gastrulation. Classic genetic screens for embryonic lethal mutations first identified six zygotic genes—decapentaplegic (dpp), screw (scw), short gastrulation (sog), twisted gastrulation (tsg), tolloid (tld), and shrew (srw)—that guide the dorsal subdivision into these two tissues (Nusslein-Volhard et al. 1984; Wieschaus et al. 1984). Among these six genes, loss of dpp caused the strongest phenotype in which all dorsal cells adopted more lateral ectoderm fates (Arora and Nusslein-Volhard 1992; Ferguson and Anderson 1992; Wharton et al. 1993). The dpp gene encodes a factor with ∼75% identity to vertebrate BMP-2 and -4 (Padgett et al. 1987). As best described for vertebrates (Wu and Hill 2009; Wang et al. 2014; Brazil et al. 2015), BMPs control a wide range of developmental and physiological processes in higher eukaryotes. Remarkably, not only is the sequence of Dpp closely related to vertebrate BMPs, but it was also shown to be functionally equivalent to its vertebrate homologs. Thus, subcutaneous implantation of Dpp protein in rats leads to ectopic bone formation, while a BMP-4 transgene inserted into the Drosophila germline was able to partially rescue early patterning defects of dpp mutant embryos (Padgett et al. 1993; Sampath et al. 1993).
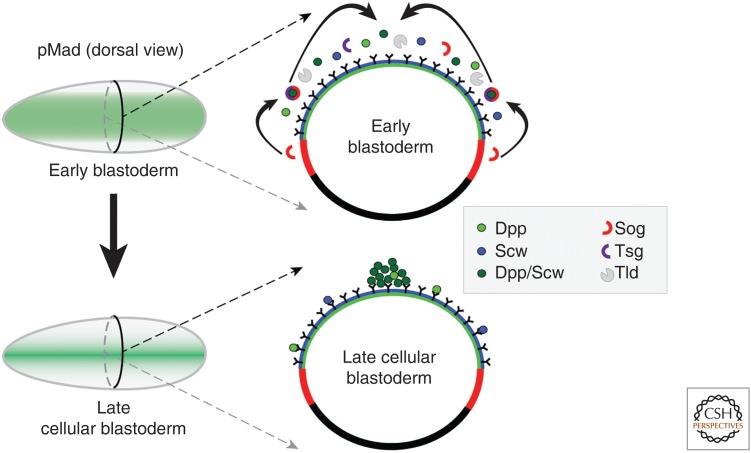
One of the more noteworthy properties of many TGF-β-type proteins is their ability to act as morphogens, that is, molecules that instruct cells to adopt specific fates in a concentration-dependent manner. Injection of dpp mRNA into early embryos, along with careful phenotypic analysis of a large number of embryonic lethal dpp mutant alleles, first suggested that Dpp likely had morphogenetic activity within the dorsal region, because low levels of Dpp activity specified dorsal ectoderm whereas high levels specified the formation of amnioserosa (Ferguson and Anderson 1992; Wharton et al. 1993). Subsequent comparison of the endogenous dpp mRNA pattern compared with protein revealed that the mRNA was uniformly transcribed throughout the entire dorsal half of the embryo, whereas the protein distribution changed with time (Shimmi et al. 2005a). When first detectable, the protein pattern mirrors that of the mRNA, but shortly thereafter its distribution is concentrated within a domain of 8 to 12 cells centered about the dorsal midline (Fig. 2). Using an antibody to detect phosphorylated Mad (pMad), the major BMP transcriptional transducer in Drosophila, revealed a similar temporal and spatial refinement in Dpp signal output, that is, the pMad signal is initially low and broad and is subsequently refined and intensified along the dorsal midline (Fig. 2) (Dorfman and Shilo 2001; Ray and Wharton 2001; Ross et al. 2001).
Figure 2.
Drosophila embryo patterning. Wider distribution of pMad (early blastoderm) is converted to a restricted pMad stripe (late blastoderm) through the action of Sog, Tsg, and Tld. The combined activities of these factors result in net flux of the Dpp–Scw heterodimer to the dorsal midline. Homodimers stay broadly distributed as a result of low affinity to Sog.
These observations trigger the obvious question of what controls the change in Dpp protein distribution and signaling output. Additional genetic, biochemical, and computational approaches provide a fairly complete picture of the molecular events that facilitate the formation of the embryonic Dpp activity gradient (Fig. 2) (O’Connor et al. 2006). Key to understanding this process was the elucidation of biochemical activities of Scw, Tld, Tsg, and Sog, four products encoded by the other previously mentioned zygotic genes that, when mutated, produce partially ventralized embryos. All four proteins are secreted and physically interact in various ways to form both stable and transient complexes with Dpp. The scw gene encodes a second BMP-type ligand that is uniformly expressed in the embryo (Arora et al. 1994), but can form a disulfide-linked heterodimer with Dpp in the dorsal domain (Shimmi et al. 2005b). Cell culture signaling assays showed that the Dpp–Scw heterodimer induces a much stronger phosphorylation of Mad on a per-mole basis than does either homodimer (Shimmi et al. 2005b). Because wit mutations cause no early embryonic phenotype (Marques et al. 2002), it is generally thought that Punt is the only functional type II receptor in the early embryo. Further genetic experiments revealed that, in addition to Punt, both BMP type I receptors Tkv and Sax are required for heterodimer-mediated synergistic signaling; however, the molecular mechanism responsible for synergy is not understood (Nguyen et al. 1998).
Another important property of the heterodimer is that it is required for redistribution of Dpp during dorsal patterning since, in scw mutants, Dpp homodimers do not accumulate at the dorsal midline (Shimmi et al. 2005b; Wang and Ferguson 2005). The inability to concentrate the heterodimer at the midline is also observed in sog, tld, and tsg mutant embryos indicating that these gene products facilitate heterodimer movement. Computational modeling provided insight into the mechanism and relied on several key bits of biochemical data (Eldar et al. 2002; Mizutani et al. 2005; Shimmi et al. 2005b). The first was that the secreted factors Sog and Tsg could form a tripartite complex with BMP ligands (Ross et al. 2001). As with signaling, the Dpp–Scw heterodimer has the highest affinity for binding the Sog–Tsg complex (Shimmi et al. 2005b). The second pivotal observation was that tld encodes an astacin-like protease, related to vertebrate BMP-1 that cleaves Sog, but only when bound in a complex with a BMP (Shimell et al. 1991; Marques et al. 1997; Peluso et al. 2011). Once again, the heterodimer forms a higher affinity complex with Sog and Tsg than either homodimer, and this complex also provides the best platform to stimulate Sog cleavage by the Tld protease (Shimmi et al. 2005b). The key to understanding directed movement of the heterodimer toward the dorsal midline is the ventral lateral expression of Sog (Fig. 2). As Sog diffuses from its site of synthesis into the ventral lateral region, it first encounters Tsg, Dpp–Scw, and Tld at the boundary between the dorsal and ventral halves of the embryo. The complex of Sog, Tsg, and Dpp–Scw forms at the boundary and can either diffuse or is processed by Tld, releasing Dpp–Scw for further binding and diffusion. Although not intuitively obvious, the computational models show that, with the correct set of kinetic parameters, this process results in shuttling of the Dpp–Scw complex to the dorsal-most region (Eldar et al. 2002; Mizutani et al. 2005; Shimmi et al. 2005b).
Despite the apparent success of the first computational models, one problem was that, while they all predict shuttling, the shape of the output response was not what was actually observed. These models predict that pMad would first accumulate at the dorsal midline and then would intensify and “expand outward” away from the midline with time. However, experimentally, exactly the opposite was observed: the initial pMad signal is low and broad and intensifies and “contracts inward” toward the midline (Fig. 2). This has been referred to as the spatial bistability issue, and studies using mutations in Medea, the Drosophila co-Smad necessary for transducing all BMP signals, revealed that the contraction and intensification of the pMad output is due to positive feedback (Wang and Ferguson 2005). It appears that an early, broad, low-level BMP signal induces the transcription of unknown component(s) that then provide feedback and enhance binding of Dpp–Scw to its receptors. Subsequent models that incorporate such a feedback scheme were able to accurately capture the correct temporal and spatial refinement in the pMad pattern (Umulis et al. 2006; Umulis et al. 2010).
Although the exact molecular mechanism for enhancing BMP binding to its receptors remains unknown, the feedback mechanism appears to be surprisingly complex and involves induction of additional positive and negative regulators of Dpp signaling (Gavin-Smyth et al. 2013). The positive effector is eiger, which encodes a tumor necrosis factor α homolog that signals through c-Jun amino-terminal kinase (JNK) (Igaki et al. 2002; Moreno et al. 2002). The negative effector is crossveinless 2 (CV-2), an extracellular BMP-binding protein that can have positive or negative influences on BMP signals depending on context (Serpe et al. 2008). In the absence of both Eiger and CV-2, the robustness of the BMP signal is compromised and the number of fated amnioserosa cells is extremely variable. Although the negative effects of CV-2 may be attributed to its ability to bind and sequester BMP ligands, how Eiger signaling through Jnk promotes BMP signaling is unknown. It is still not clear if the positive effect of Eiger–Jnk signaling is at the level of enhancing BMP binding to its receptor, as the computational modeling requires, or if other BMP target genes yet to be discovered are involved in mediating positive feedback.
Although the computational models using feedback are likely correct in many aspects (Umulis et al. 2010), it is probable that some significant nuances will arise. To illustrate this point, consider that in every proposed computational model, the binding affinity of Sog for ligand is tightly constrained and needs to be in the picomolar range for effective ligand shuttling to take place (Eldar et al. 2002; Mizutani et al. 2005; Shimmi et al. 2005b). Thus, a potential problem arose when the affinity of Chordin, a vertebrate homolog of Sog, for BMP-2 was determined to be in the nanomolar range (Rentzsch et al. 2006). A likely solution to this problem was provided when it was determined that two type IV collagen-like molecules are also required for proper dorsal patterning (Wang et al. 2008; Sawala et al. 2012). Loss of these matrix proteins attenuates the formation of the strong dorsal-most Dpp–Scw signal. Furthermore, it was shown that Dpp–Scw and Sog could both bind to collagen and then be released in a complex through the action of Tsg binding. Tolloid, also interacts with collagen IV, via its amino-terminal CUB domains, and this association enhances cleavage of Sog (Winstanley et al. 2015). Modeling showed that the local concentrating effect of the collagen scaffold can greatly reduce the effective binding affinity of the ligand for Sog, thereby solving the problem presented by the high solution dissociation constant (Umulis et al. 2009).
Other refinements to the mechanism are also likely. For example, the Scw prodomain was found to require processing at a site distinct from the normal maturation site (Kunnapuu et al. 2014). A mutation that disrupts this processing results in a dominant-negative effect that might be brought about by interference of the altered heterodimer with the ECM interaction or the kinetics of processing and secretion of the heterodimer in vivo. The co-Smad Medea is also susceptible to covalent regulatory modifications that impact early dorsal patterning. Sumoylation, for example, promotes export of Medea from the nucleus, perhaps enabling nuclei to continuously monitor the presence of extracellular Dpp–Scw signal to activate target gene expression for an appropriate duration (Miles et al. 2008). Medea has also been shown to be ubiquitylated (Dupont et al. 2009), and the level of ubiquitylation affects the activity of Medea in regulating early dorsal patterning (Stinchfield et al. 2012). Lastly, of all the classic mutants identified that affect dorsal patterning, the role of shrew still remains unknown.
Later Embryonic Roles of Drosophila BMPs
Although the Screw ligand only appears to have a role in early dorsal patterning (Arora et al. 1994), dpp transcription and pMad output continue throughout embryogenesis with complex spatial dynamics (Dorfman and Shilo 2001). In addition, Gbb, a third Drosophila BMP-like factor that is closely related to the vertebrate BMP-5, -6 and -7, is broadly activated during gastrulation in mesodermal derivatives including muscle and fat body (Wharton et al. 1999). Despite some potential overlap in expression with Dpp, each of these two ligands plays largely independent roles during embryogenesis suggesting that heterodimers are not likely to be important at these stages. In addition, there is no evidence for any shuttling of ligands in embryos after the early blastoderm stage, and no obvious roles for sog, tsg, or tld have been described. Instead, most patterning and cell fate specification events regulated by Dpp and Gbb in later embryo development seem to involve short-range, unassisted ligand diffusion. For Gbb, embryonic activities include maintenance of the first midgut constriction through regulation of the homeotic gene antennapedia, and specification of the gastric ceca precursors at the anterior end of the midgut (Wharton et al. 1999). Dpp controls signaling from the dorsal ectoderm to the underlying mesoderm at two stages. In early gastrulating embryos, it induces expression of tinman, which regulates the formation of heart and visceral muscle (Staehling-Hampton and Hoffmann 1994; Frasch 1995). Later, during germband retraction, Dpp refines the cardiac field by limiting the number of specified pericardial cells (Johnson et al. 2003, 2007). Dpp also regulates foregut morphogenesis (Fuss and Hoch 1998; Nakagoshi et al. 1998), patterning of the second midgut constriction (Bienz 1994; Szuts and Bienz 2000), establishment of embryonic imaginal disc placodes (Goto and Hayashi 1997), posterior spiracle development (Takaesu et al. 2008), ectodermal cell movement during dorsal closure (Glise and Noselli 1997; Fernandez et al. 2007; Zahedi et al. 2008), and tracheal cell migration and branching (Vincent et al. 1997; Myat et al. 2005), by activating expression of two zinc finger transcription factors encoded by knirps and knirps-related (Chen et al. 1998). Interestingly, during Dpp-directed dorsal closure, the range of Dpp signaling is negatively regulated by mummy, which encodes a UDP-N-acetylglucosame pyrophosphorylase, a component necessary for N- and O-linked glycan modification of protein and lipids. One possibility is that a glycosylated receptor or ECM component might bind Dpp and limit its diffusion away from the ectoderm leading edge cells (Humphreys et al. 2013).
TGF-β FAMILY SIGNALING IN THE WING IMAGINAL DISC
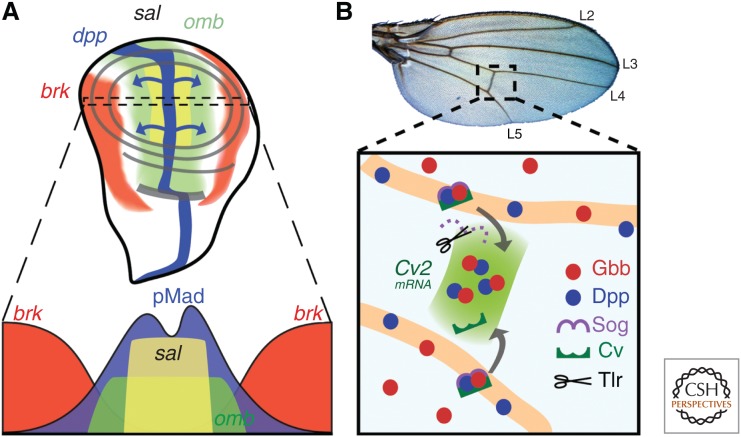
Drosophila larvae contain 15 distinct sac-like tissues called imaginal discs, which are mitotic and give rise to adult structures. Here, we will primarily focus on the wing imaginal disc because it is the best characterized system for understanding TGF-β signaling. Growth and patterning of imaginal discs is largely controlled by Dpp signaling (Hamaratoglu et al. 2014; Restrepo et al. 2014). In contrast, activin signals play a modest role in cell proliferation but not patterning (Brummel et al. 1999; Hevia and de Celis 2013; Peterson and O’Connor 2013). In the wing disc, as in the embryo, Dpp functions as a morphogen to specify different cell fates. In this tissue, Dpp is secreted from a narrow strip of cells abutting the A/P boundary, and spreads to both compartments, forming a gradient that regulates the expression of several target genes in a concentration-dependent manner (see Fig. 3A) (Lecuit et al. 1996; Nellen et al. 1996; Campbell and Tomlinson 1999; Jaźwińska et al. 1999; Minami et al. 1999). The spatially defined expression of Dpp target genes governs both growth and size, as well as patterning of the adult wing veins. Below, we will focus on how the Dpp gradient is formed, interpreted, and translated to produce a functional wing.
Figure 3.
Dpp signaling during Drosophila wing development. (A) During larval development, dpp (blue, top) is expressed along the A/P border and its gene product spreads to both compartments (blue arrows). High Dpp signaling in the middle of the disc results in activation of Mad (blue, below), which silences brk (red). The inverse gradients of pMad and Brk form the nested expression pattern of sal and omb. pMad is slightly lower in cells abutting the A/P axis due to local tkv down-regulation (Funakoshi et al. 2001). (B) Posterior crossvein (PCV) forms between the L4 and L5 veins (top), which begins during pupal development. Dpp–Gbb heterodimers bound by Sog (purple) and crossvein (dark green) allow for facilitated diffusion of ligands into the presumptive PCV space. Tolloid-related (Tlr) cleaves Sog and allows for signaling to occur. The initial broad signaling induces expression of CV-2, which further sharpens the signaling, resulting in PCV formation.
Models of Dpp Gradient Formation
Francis Crick initially proposed that morphogen gradients can form by simple diffusion (Crick 1970). To examine the role of Dpp diffusion in the wing disc, a Dpp–green fluorescent protein (GFP) fusion was created allowing the morphogen gradient to be directly visualized (Entchev et al. 2000; Teleman and Cohen 2000). Surprisingly, fluorescent recovery after photobleaching (FRAP) experiments using this Dpp–GFP suggested a diffusion coefficient that was too slow to account for production of the observed gradient in the wing disc (Kicheva and González-Gaitán 2008). This result led to the development of two alternative models, one involving multiple rounds of receptor-mediated ligand uptake and resecretion, termed transcytosis (Kicheva and González-Gaitán 2008), and a second based on directed transport of Dpp along extended actin-based filipodia, termed cytonemes (Ramirez-Weber and Kornberg 1999; Roy and Kornberg 2011).
Although the final resolution of the true mechanism may not be fully settled, single molecule imaging of tagged Dpp in the wing disc has allowed for measurements of the diffusion coefficient, revealing two distinct populations of Dpp that diffuse at different rates (Zhou et al. 2012). A significant portion of Dpp diffuses extremely slowly, as predicted for ligand bound to cell surface proteins such as receptors, and another pool of Dpp diffuses much more rapidly, consistent with free diffusion that is hindered only by the viscosity of the extracellular fluid and the tortuosity of intercellular paths (Zhou et al. 2012). Theoretical calculations suggest that this highly mobile pool of Dpp is sufficient to form the observed gradient in the disc. Furthermore, wild-type cells can sense Dpp even when completely surrounded by cells lacking Tkv. This observation argues against the need for receptor-mediated transcytosis (Schwank et al. 2011). To date, no strong support for cytoneme-mediated dispersal of Dpp throughout the wing disc has been reported, although it appears that cytonemes convey Dpp from the wing disc to other interacting tissues such as the trachea (Roy and Kornberg 2011; Roy et al. 2014).
Modifiers of the Dpp gradient
Because the FRAP studies and other microscopy techniques highlighted above suggest that most Dpp is bound to the cell surface, it is likely that any molecule regulating ligand–receptor interactions will dramatically affect either gradient formation or the signaling output. Indeed, genetic approaches identified the products of the genes division abnormally delayed (dally) and dally-like protein (dlp), which encode two members of the HSPG family, as proteins that potentially affect Dpp gradient formation and/or signal output (Fujise et al. 2003; Belenkaya et al. 2004; Akiyama et al. 2008). However, theoretical calculations now suggest that HSPGs do not restrict the fast free diffusion of Dpp (Zhou et al. 2012). Instead, they exert their primary effect on signaling output and not gradient formation, likely by facilitating Dpp–receptor interactions (Kuo et al. 2010).
Another less understood aspect of morphogen gradients is their ability to scale according to size of the tissue, (Wartlick et al. 2011a). A novel secreted factor, Pentagone (Pent), has been shown to be important for Dpp gradient scaling as the wing disc grows during the larval stages (Vuilleumier et al. 2010; Hamaratoglu et al. 2011). Pent contains follistatin-like, thyroglobulin type I and EF hand calcium-binding domains and interacts with HSPGs to alter the slope of the Dpp signaling output (Vuilleumier et al. 2010).
Because pent expression is negatively regulated by Dpp signaling, cells along the flanks sense less Dpp as the disc grows in size, and begin to produce Pent, which then through feedback appears to facilitate Dpp gradient expansion (Vuilleumier et al. 2010; Ben-Zvi et al. 2011). The ability of Pent to facilitate internalization of Dally and Dlp has been proposed to modify the ability of cells to trap and transduce BMP by fine-tuning the levels of the BMP reception system at the plasma membrane (Norman et al. 2016).
The Role of Gbb in Wing Growth and Patterning
Although the previous sections emphasize the critical role of Dpp as a morphogen that specifies wing growth and patterning, it is important to recognize that, just like in the embryo, a second BMP ligand (Gbb) is necessary for proper disc growth and wing patterning. Gbb is widely expressed in the wing disc, contrary to Dpp whose expression is restricted to cells along the A/P compartment boundary (Khalsa et al. 1998). Gbb loss results in similar wing growth and patterning defects, as does Dpp loss, although generally not as severe (Ray and Wharton 2001). As in the embryo, both heterodimers and homodimers of these BMP ligands likely exist, but their respective roles in activating the observed spatial and temporal BMP signal responses are not clear (Ray and Wharton 2001; Bangi and Wharton 2006). As in the embryo, genetic evidence suggests that BMP signal transduction in the wing disc uses heteromeric receptor complexes containing combinations of the type I receptors Tkv or Sax together with two molecules of the type II receptor Punt. Although Tkv appears to mediate signals from both Dpp and Gbb, Sax shows the novel ability to both promote and antagonize signaling, and this ability is proposed to be dependent on its receptor partner (Bangi and Wharton 2006). When partnered with Tkv, Sax is thought to facilitate BMP signaling, but, as a homodimer, it fails to signal effectively and instead sequesters Gbb. This model proposes a balance between antagonizing and promoting BMP signaling across the wing pouch that helps shape the BMP activity gradient.
Transcriptional Responses to BMP Signaling in the Wing Disc
The morphogen model predicts that target genes of BMP signaling should be differentially expressed within the morphogen gradient field. Indeed, spalt (sal), optomoter blind (omb), and brinker (brk) were identified early on as likely BMP-responsive genes (Fig. 3A) that are differentially expressed along the A/P axis (Lecuit et al. 1996; Nellen et al. 1996; Campbell and Tomlinson 1999; Jaźwińska et al. 1999; Minami et al. 1999). Both omb and sal encode transcription factors that are required for proper patterning and cell survival in the central region of the disc, whereas brk encodes a repressor that is expressed in the lateral regions of the disc. brk expression is negatively regulated in the center of the disc by silencer elements (SEs) that bind a heteromeric complex of transcription factors composed of pMad and Medea, together with another transcription factor, Schnurri (Kim et al. 1997; Affolter and Basler 2007). Surprisingly, the induction of omb and sal by Dpp actually occurs by repressing brk, which is itself a repressor of omb and sal. The result is that high levels of Dpp signaling directly silence brk in the center of the disc so that its expression pattern is an inverse complement to that of the Dpp gradient, low in the middle and high at the edges (Fig. 3A). The graded expression of brk in the more central regions of the disc in turn gives rise to the nested expression patterns of omb and sal (Fig. 3A).
Although expression of omb and sal are indirectly controlled by Dpp signaling, direct targets have also been identified, including dad, which codes for an inhibitory Smad (Tsuneizumi et al. 1997). Dad affects the robustness of the Dpp signaling gradient (Ogiso et al. 2011) by blocking Tkv-meditated phosphorylation of Mad and oligomerization of pMad (Inoue et al. 1998). Within the central region of the disc, dad expression is controlled by an activating element (AE) in its regulatory sequences that directly responds to Dpp signals. The AE DNA sequence is similar to that of the SE and recruits a trimeric pMad–Medea complex, but differs at certain nucleotide positions, such that the AE does not recruit Schnurri (Weiss et al. 2010). As a result, it is able to promote rather than repress transcription. The lateral boundary of dad activation is controlled by Brk repression. The combined activities of AE and SE elements, together with Brk-binding sites, appears to explain the bulk of BMP response gene expression patterns so far identified in the wing disc.
Role of Dpp in Regulating Tissue Growth
In addition to controlling patterning within the wing disc, Dpp also regulates tissue growth. Loss of dpp leads to very small discs (Spencer et al. 1982; Zecca et al. 1995) while gain of Dpp signaling throughout the disc produces overgrowth (Capdevila and Guerrero 1994; Burke and Basler 1996; Martin-Castellanos and Edgar 2002). Even expressing extra dpp within its normal source using dpp-Gal4 causes a mild enlargement of the larval wing disc with some extra vein defects (Entchev et al. 2000; Teleman and Cohen 2000). When expressed locally in clones, it can also lead to the formation of an entirely new secondary winglet attached to a normal wing (Zecca et al. 1995).
How BMP signaling can regulate both growth and patterning in the wing disc has been an enigma for quite some time. The dilemma arises because cell proliferation within the wing disc is uniform (Milan et al. 1996) and patterning is clearly coupled to gradient formation and interpretation. Explaining how uniform proliferation can be produced by a graded pattern of Dpp has proven difficult. Several hypotheses have been put forth including the idea that proliferation depends on the slope of the Dpp gradient (Day and Lawrence 2000), the rate of change in Dpp signal reception (Wartlick et al. 2011b), and the “growth equalization model” (Restrepo et al. 2014). At present, the mechanism for producing uniform growth is unclear because all the models have some conflicts with certain observations. The major conflict arises from analysis of brk mutants and expression. That Brk plays a central role in regulating wing disc growth was initially revealed by analysis of loss-of-function clones, which in the flanks of the disc also leads to winglet formation as does the gain of BMP signaling (Campbell and Tomlinson 1999). Subsequently, it was found that increased expression of brk in the entire disc leads to a severe reduction in disc size (Martin et al. 2004; Schwank et al. 2008) similar to loss of dpp. The real surprise, however, was that wing discs not only grow, but actually overgrow if they lose both Brk and Dpp (Campbell and Tomlinson 1999; Schwank et al. 2008, 2012). This observation suggests that neither the slope model nor the temporal dynamics model is correct. One alternative is that Dpp and Brk do not directly regulate proliferation of wing disc cells, but instead modulate an underlying nonuniform proliferation pattern that is the result of other growth promoting signals such as insulin–TOR and/or the Hippo–Fat, and other TGF-β signaling pathways (Restrepo et al. 2014).
Two additional studies using novel independent reagents, suggest that the Dpp gradient is not required for uniform growth (Hamaratoglu et al. 2011; Akiyama and Gibson 2015). In the first study, dpp is deleted from the cells in the wing disc that normally express Dpp, using a UAS>GAL4/FRT system (Akiyama and Gibson 2015). Surprisingly, the disc cells proliferate uniformly, resulting in a normal size tissue, even though brk expression is up-regulated in the wing pouch. This result argues against the Dpp-gradient and the growth equalization model and instead supports the temporal dynamics model perhaps due to other non-A/P boundary disc cells that still produce Dpp. In the second method, Dpp diffusion is restricted by a nanobody trapping method and once again there is uniform disc growth, supporting the growth equalization model (Harmansa et al. 2015). Going forward it will be important to reconcile these observations. Furthermore, if and how Gbb signaling contributes to disc growth in the absence of a Dpp gradient will be interesting to explore.
BMP Signaling in Pupal Wing Development
Dpp and Gbb also play important roles in vein specification and positioning, which begins in late larval stages and continues throughout pupal development (de Celis 2003; O’Connor et al. 2006). During the late larval stages, induction of the BMP targets, sal and omb, dictate where some of the longitudinal veins (LVs) will form (de Celis 1997, 2003). Soon after pupariation, dpp expression is lost at the A/P boundary and becomes localized to cells that form the LVs, and high levels of pMad are detected in these pre-vein cells (Conley et al. 2000). Intriguingly, the posterior crossvein (PCV), that forms slightly later at ∼20 h after puparium formation, also requires BMP signaling, but Dpp is not expressed in primordial PCV cells. Nevertheless, these cells receive high levels of BMP signal as evidenced by strong pMad staining (Ralston and Blair 2005). The mechanism appears to be similar to that used in the embryo where the Dpp or a Dpp–Gbb heterodimer is shuttled away from the primordial LVs and concentrated at the site of the future PCV (Matsuda and Shimmi 2012). Just as in the embryo, this shuttling process requires several extracellular BMP modulators, including Sog, CV-2, CV-1 (a Tsg homolog), and Tlr (a Tld homolog) (Fig. 3B) (O’Connor et al. 2006). One novel component not used for shuttling in the embryo is crossveinless-D, which encodes a vittelogenin-like lipoprotein that appears to regulate PCV formation by modulating Dpp movement as part of a lipid–Dpp–lipoprotein complex (Chen et al. 2012).
Mad Is a Focal Point For Integration of Cross-Pathway Signals
Dpp signals are integrated with numerous other growth and patterning signals during imaginal disc development. In many cases, this interaction occurs through Mad, and two important points of integration within the wing disc involve the Hippo and Wingless (Wg) signaling pathways (reviewed in Baena-Lopez et al. 2012). The Hippo pathway can promote disc growth via regulation of the nuclear localization of the transcription factor Yorkie (Yki; Yap in vertebrates), which binds to promoters of certain cyclins and the microRNA encoded by bantam (ban) (reviewed in Oh and Irvine 2010). The microRNA ban is particularly interesting because it also responds to Dpp signaling (Martin et al. 2004) and a direct link between the control of ban levels by Dpp and Hippo has been established (Oh and Irvine 2011). On its own, Yorkie is not able to bind DNA but can do so when in a complex with other transcription factors. Mad can form a complex with Yki (Oh and Irvine 2011), and this complex is mediated through a PPXY motif located in the linker region between the Mad homology 1 (MH1) DNA-binding domain of Mad and the carboxy-terminal MH2 domain, a more typical site of regulatory interaction between Mad and other proteins. The Mad–Yki complex was also shown to bind to a novel enhancer element in the ban promoter to augment ban transcription, thus providing an integration point for these two growth promoting signals (Oh and Irvine 2010).
Mad is also the focal point of interaction with the canonical Wg pathway (Eivers et al. 2009, 2011). Both in vivo and in vitro studies showed that unphosphorylated Mad forms a complex with Armadillo (homolog of vertebrate β-catenin) to regulate wg target genes in both the embryo and wing disc. Upon Dpp signaling, Mad is titrated away from this complex, resulting in Wg–reporters being significantly down-regulated (Eivers et al. 2011). Once again, the linker region between the MH1 and MH2 domains of Mad plays an important role in mediating regulatory interactions between the two pathways. The linker region possesses several serines and tyrosines, which are phosphorylated by various kinases to control Mad stability (Kretzschmar et al. 1997; Pera et al. 2003; Alarcon et al. 2009). In particular, glycogen synthase kinase-3 (GSK3)-mediated phosphorylation of the linker is important for initiating Mad degradation via Smurf1, and it was proposed that both the Wg and Dpp functions of Mad are terminated by GSK3-mediated phosphorylation of the linker (Eivers et al. 2011). An additional complexity in the interplay between these two pathways has also been uncovered where in one region of the wing blade, phosphorylation of the Mad linker by GSK3 does not lead to degradation. Instead, the linker-phosphorylated Mad controls certain cell divisions that lead to the formation of selected sensory bristles on the wing (Quijano et al. 2011).
A third signal intersection converging on Mad takes place between the activin and the BMP signaling branches in the wing disc. Although these two branches appear to have diverged from one another long ago, the activin-specific type I receptor Babo retains an ability to activate Mad by carboxy-terminal phosphorylation (Peterson et al. 2012). Whether this carboxy-terminal Mad phosphorylation activity is used during normal development is still not clear, but results from using a dSmad2 null mutant further supports the idea that cross-signaling by the activin pathway impinges on Mad activity (Sander et al. 2010; Peterson and O’Connor 2013). Interestingly, when dSmad2 protein is absent, Babo sends an ectopic signal that leads to pronounced overgrowth of the wing disc. The excessive growth is caused by down-regulation of brk, and this down-regulation requires binding of a Mad–Medea–Schnurri complex to the brk SEs. However, there was no detectable increase of carboxy-terminal Mad phosphorylation, leading to the intriguing unresolved issue of how Mad is hyperactivated by Babo in the absence of dSmad2. Taken together, these studies indicate that Mad is clearly at the nexus of integrating numerous signaling events that control wing growth and patterning.
BMP Signaling in Other Imaginal Discs
So far, our discussion has focused on the role of BMP signaling in wing disc growth and patterning because it is the best studied of the imaginal tissues. Not surprisingly, however, BMP signals also play important roles in the development of all imaginal discs. In fact, the name decapentaplegic indicates that all 15 (decapenta) discs are defective (plegic) (Spencer et al. 1982). Here, we briefly examine the role of BMP signaling in the leg, eye and genital imaginal discs. More detailed reviews cover the development of the eye (Curtiss and Mlodzik 2000), leg (Estella et al. 2012), and genital discs (Keisman and Baker 2001; Sánchez et al. 2001; Estrada et al. 2003).
In the leg disc, Dpp synergizes with Wg to give proximal/distal (PD) positional information to the tissue with distal being the center (Lecuit and Cohen 1997; Estella and Mann 2008). Along the A/P boundary of the leg disc dpp is expressed in the dorsal compartment and wg in the ventral. This creates a zone in the center of the disc where both ligands are present at high concentrations, which is required to induce the expression of the distal marker distal less (dll). Cells surrounding the dll expression domain sense moderate levels of Dpp and Wg and turn on dachshund (dac), a medial leg marker. Cells in the periphery give rise to proximal domains of the leg and sense low levels of the ligands leading to expression of homothorax and teashirt. Patterning of dll and dac are thought to result from brk repression, a mechanism that poses striking similarity with the wing disc (Estella and Mann 2008). Thus, although the details of patterning in the leg disc are different from those in the wing, the basic function of Dpp as a morphogen that functions, in part, through the repression of brk, is nevertheless the same in both tissues.
In the eye imaginal disc, the responses to Dpp are notably different from those in other discs. In this tissue, dpp is expressed only in the morphogenetic furrow, which migrates posterior to anterior during late larval stages (Baker 2001). The Dpp signaling response varies depending on the position of the cells relative to the furrow. Cells anterior of the furrow are stimulated to proliferate, whereas cells in and abutting the furrow undergo cell-cycle arrest (Baker 2001). Cell-cycle arrest is necessary for proper eye differentiation because mutant clones defective in Dpp signaling are delayed in entering G1 arrest and do not fully differentiate (Horsfield et al. 1998; Firth et al. 2010). Although the mechanism for the dual nature of Dpp signaling to induce both cell proliferation and G1 arrest is unresolved, it is likely that multiple signaling pathways, including those activated by Wg, Hedgehog (Hh), Notch, and EGF, finely tune this process (Amore and Casares 2010).
Dpp signaling also plays an important role in genital disc development, which gives rise to the adult internal and external genitalia and analia. Several distinguishing characteristics of this disc set it apart from the others. It is the only disc that is located medially, formed by the fusion of cells originating from three embryonic segments, it is an unpaired disc (i.e., not one for each side of the larva) and it is sexually dimorphic (Sánchez and Guerrero 2001; Estrada et al. 2003). Nevertheless, the genital disc has some similarity to other discs, such as the leg, in the sense that hh expression in the posterior compartment induces expression of dpp and wg in the anterior compartment, which in turn induce dll expression that is required for development of the disc in both males and females (Gorfinkiel et al. 1999; Sánchez and Guerrero 2001; Sánchez et al. 2001).
TGF-β FAMILY SIGNALING IN THE NERVOUS SYSTEM
Activins and BMPs at the Neuromuscular Junction
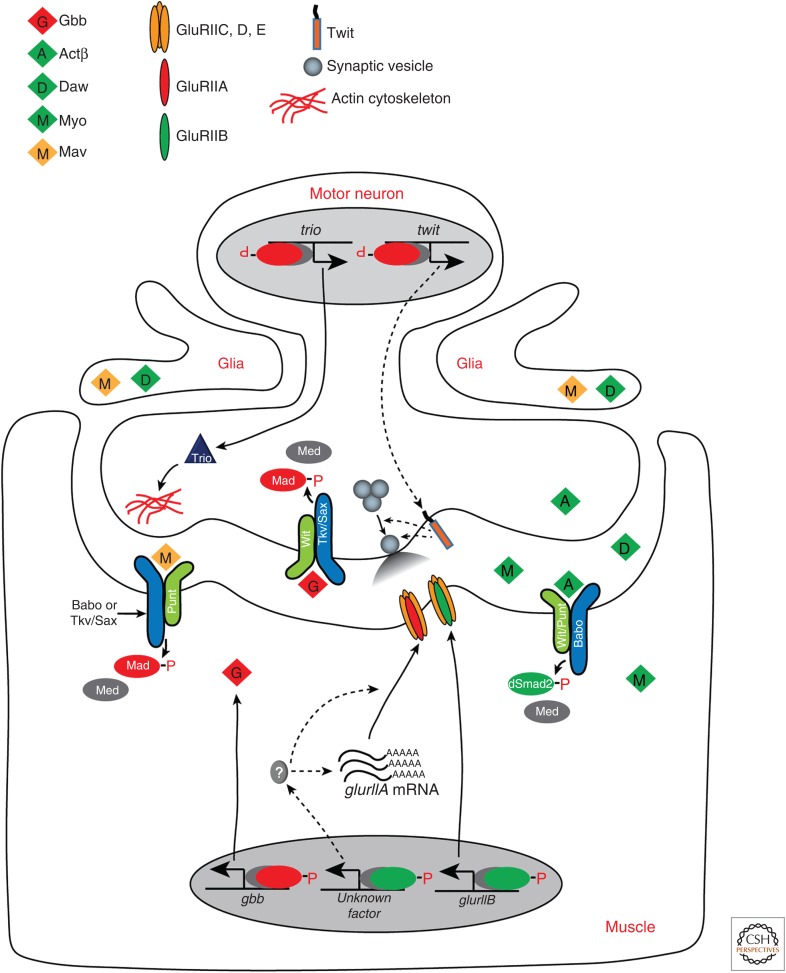
The Drosophila NMJ, a collection of synapses made on body wall muscles by motor neurons, has long served as an excellent model for studying synapse formation and function. The NMJ grows substantially during larval life to accommodate the rapidly growing body wall muscles, and several trans-synaptic signaling pathways have been shown to play important roles in coordinating the pre- and postsynaptic development at the larval NMJ (Dani and Broadie 2012). Both branches of TGF-β family signaling have important roles at the NMJ (Fig. 4), and BMP signaling has been particularly well studied. The primary ligand involved is Gbb, which is expressed in the muscles and the central nervous system (CNS) beginning at the embryonic stage (McCabe et al. 2003). Mutations in gbb cause a loss of pMad accumulation in motor neuron nuclei as well as a reduced NMJ size, aberrant presynaptic ultrastructure, and defective neurotransmission (McCabe et al. 2003). The defective synaptic growth of gbb null mutants is only fully rescued when gbb expression is restored in muscles and not in the CNS. These observations suggest that the Gbb signal acts in a retrograde fashion to control presynaptic growth in the motor neurons (McCabe et al. 2003).
Figure 4.
Bone morphogenetic protein (BMP) and activin signaling at the Drosophila neuromuscular junction (NMJ). Motor neuron-released Actβ induces Babo-mediated activation of dSmad2, facilitating association with Medea in the muscle. The pdSmad2-Medea complex then activates the transcription of glurIIB and an unknown factor(s) that controls posttranscriptional processing or mRNA stability of glurIIA. On the other hand, muscle-released Gbb binds Wit and the Tkv-Sax complex in the motor neuron, and stimulates Mad phosphorylation. The resultant pMad–Medea complex activates the transcription of trio and twit whose products promote synaptic growth and control spontaneous vesicle release, respectively. The expression of Gbb in the muscle is fine-tuned by glia-released Mav. The dotted lines in this model depict speculations that should be verified in future studies.
Similar phenotypes in pMad level, NMJ size, neurotransmitter release, and synaptic ultrastructure were also observed in mutations of the type II receptor wit (Aberle et al. 2002; Marques et al. 2002). The expression of wit is restricted to motor neurons in the neuromuscular system and restoration of wit in the motor neurons rescues synaptic size, supporting the idea that Wit acts as the primary type II receptor for Gbb in motor neurons (Aberle et al. 2002; Marques et al. 2002). Likewise, null mutations in all other BMP signal transduction components, including tkv, sax, mad, and medea, phenocopy the gbb and wit null mutations indicating that canonical BMP signaling is the main regulator of synaptic growth at the NMJ (Rawson et al. 2003; McCabe et al. 2004).
The identities of target genes in motor neurons that regulate synaptic growth are largely unknown. However, recent studies using molecular genetic tools that allow for temporal regulation of Gbb signaling at the NMJ identified some molecular mechanisms through which Gbb affects synapse structure and function. These studies showed that synaptic structure and function use genetically separate pathways with an early transient embryonic signal needed for synaptic growth, whereas continuous Gbb signaling is required for maturation of neurotransmitter release properties (Berke et al. 2013). One BMP target gene required for regulating synaptic growth is trio, which encodes a guanine nucleotide exchange factor (GEF) that acts on Rac GTPases. (Ball et al. 2010). Because previous studies show that GTPases control axon growth and guidance through alterations in the actin cytoskeleton (Dickson 2001; Fan et al. 2003), it was postulated that Trio could promote synaptic growth also by modulation of the actin cytoskeleton via Rac (Ball et al. 2010). However, a direct relationship between Rac activation and changes in actin filaments is yet to be shown. In parallel to stimulating Trio expression, Wit signaling also leads to stimulation of Lim domain kinase 1 (Limk1) activity through a non-canonical pathway. Because Lim kinase is implicated in rapid bouton budding in response to elevated synaptic activity and synaptic stabilization (Eaton and Davis 2005; Piccioli and Littleton 2014), this may be yet another means by which the Gbb signal influences synaptic growth.
In addition to promoting NMJ growth and presynaptic structural maturation, BMP signaling also plays a pivotal role in regulating synaptic transmission at the NMJ. Strong BMP pathway mutants display reductions in the amplitude of evoked junctional potential (EJP) and the frequency of miniature EJPs (mEJP) (Aberle et al. 2002; Marques et al. 2002; McCabe et al. 2003; Rawson et al. 2003). Unlike synaptic growth that is predominantly regulated by retrograde BMP signaling, the defective synaptic transmission phenotypes of gbb mutants are only completely rescued when gbb expression is restored by a pan-neuronal driver (Aberle et al. 2002; McCabe et al. 2003), indicating that presynaptic Gbb is required for proper neurotransmission. The smaller magnitude of EJPs has also implied that homeostatic regulation of synaptic efficacy is compromised in the BMP pathway mutants. It was later shown that BMP signaling is continuously required in the motor neurons to confer competence for the rapid induction of synaptic homeostasis (Goold and Davis 2007). In terms of the effects on neurotransmission, the only BMP-responsive target gene identified to date is twit, which encodes a Ly6 neurotoxin-like molecule. Restoring twit expression in wit mutant motor neurons partially rescues neurotransmitter release defects, although the molecular mechanism remains obscure (Kim and Marques 2012).
Because BMP signaling has profound effects on NMJ structure and function, it is not surprising that its activity is tightly controlled to prevent uncoordinated growth of the synapses. Multiple molecular mechanisms have been implicated in regulating BMP signaling at the NMJ. In muscles, Gbb release is inhibited by Drosophila Cdc42-interacting protein 4 (dCIP4) acting downstream of Cdc42 and activating the Wsp (Drosophila orthologue of mammalian Wiskott–Aldrich syndrome protein, Wasp) pathway (Nahm et al. 2010a). The Cdc42-Wsp pathway is, in turn, inhibited by Drosophila Rich (dRich), a Cdc42-selective GAP (Nahm et al. 2010b). Therefore, a complex regulatory loop appears to be used to control the release of Gbb. In the presynaptic motor neurons, hyperactivation of BMP signaling is prohibited by Highwire, a ubiquitin ligase that presumably targets Medea for protein degradation (McCabe et al. 2004). The activated receptors are also subject to negative regulation by internalization. Mutations in nwk, spin, and spict whose products act in, or interact with, endocytic machinery result in overgrowth of the synapses (Sweeney and Davis 2002; Wang et al. 2007; O’Connor-Giles et al. 2008). Furthermore, genetic interactions of these genes with BMP receptors have been documented (Sweeney and Davis 2002; Wang et al. 2007; O’Connor-Giles et al. 2008). Finally, retrograde signaling of Gbb also occurs in the central motor circuit, which is necessary to strengthen the synaptic activity. Therefore, it appears that the retrograde signaling is a general theme by which Gbb regulates synaptic development and function (Baines 2004). On the other hand, release of Gbb from the presynaptic side is promoted by Crimpy, a homolog of vertebrate Cysteine-rich transmembrane BMP regulator 1 (CRIM1), which binds Gbb and delivers it to a dense core vesicle (DCV) (James and Broihier 2011). Interestingly, the ectodomain of Crimpy is also released together with Gbb from the DCV on exocytosis. This has been proposed to help discriminate the presynaptic and postsynaptic pools of Gbb, thus setting the directionality of BMP signaling at the NMJ.
The retrograde Gbb signaling at the NMJ results in accumulation of pMad in the nuclei of motor neurons. In addition to the nuclear pMad, another pool of pMad has been identified. This pool of pMad is localized to the presynaptic side of the synapses at the NMJ and is regulated differently from the nuclear pMad (Sulkowski et al. 2014, 2016). Specifically, the level of synaptic pMad is independent of Gbb, but is positively regulated by the activity of postsynaptic iGluRs containing glutamate receptor IIA (type A receptor). The synaptic pMad in turn stabilize the A-type glutamate receptors suggesting a positive feedback loop between the presynaptic and postsynaptic molecules. This has been proposed to be a mechanism to coordinate the maturation of synapses to the activity.
In contrast to BMP signaling, whose role in synaptic formation and function is well documented, relatively little is known about the role of the activin pathway in the regulation of NMJ development and activity, even though all three activin ligands are expressed in at least one cell type of the neuromuscular system (Brummel et al. 1999; Lo and Frasch 1999; Zheng et al. 2003; Serpe and O’Connor 2006; Gesualdi and Haerry 2007). Studies of babo, dSmad2, and actβ mutants indicate that Actβ is a motor neuron-derived anterograde signal that is necessary for achieving normal levels of GluRIIA and GluRIIB densities in muscle at the postsynaptic side of the NMJ (Fig. 4), as well as proper resting membrane potential of the muscle (Kim and O’Connor 2014). Actβ appears to control the synaptic GluRIIA density through a posttranscriptional mechanism while it regulates the GluRIIB density via dSmad2-dependent transcriptional activation.
Lastly, glia-secreted Mav, a more distantly related TGF-β family ligand whose signaling branch is unclear, has been implicated in regulating Gbb release from the muscle (Fuentes-Medel et al. 2012). This may provide a way to finely tune presynaptic axon growth and budding with muscle growth. Together, the findings suggest that the two branches of TGF-β family signaling play distinct roles in regulating synapse growth, maturation and function at the NMJ (Fig. 4).
Activin Signaling Stimulates Neuroblast Proliferation
In addition to its role at the NMJ, activin signaling also has other roles in the nervous system including regulation of neuroblast proliferation, control of neurite outgrowth and target selection, as well as neuronal remodeling during metamorphosis. These activities are accomplished by deployment of the activin-type ligands, that is, Actβ, Daw, and Myo, from different source cells in the nervous system acting on different target cells. In most cases, this signaling is canonical through Babo and dSmad2, but there is one example in which noncanonical signaling appears to be used.
One major function of activin signaling in the nervous system is to stimulate production of neurons, which are generated from neuroblasts in the brain. Loss-of-function mutations in babo and dSmad2 produce small brains due to fewer neuroblasts and reduced proliferation of neuroblasts and their daughter cells, whereas reexpression of Babo specifically in neuroblasts rescues brain size (Zhu et al. 2008). The ligands Actβ and Daw have been implicated in this process. actβ is expressed in numerous neurons within the central brain and ventral ganglia, whereas daw is expressed in subsets of glial cells. Myo may also be involved, since it, too, is expressed in many glial cells (Lo and Frasch 1999). Mechanistically, activin signaling appears to control proliferation at the S to M phase transition of the cell cycle (Zhu et al. 2008).
Activin Signaling and Axon Guidance and Target Selection
In addition to generating neurons, activin signaling also regulates several aspects of axon targeting in different contexts. In the embryonic nervous system, activin signaling plays a role in guiding motor neuron axons to their target muscles. In daw mutant embryos, motor neurons leaving the CNS have pathfinding defects in both intersegmental nerve and segmental nerve roots (Parker et al. 2006; Serpe and O’Connor 2006). These defects are also seen in babo and dSmad2 mutant embryos (Parker et al. 2006; Serpe and O’Connor 2006) indicating that canonical signaling is responsible. Because expressing dominant-negative forms of either Babo or Punt in the motor neurons also cause pathfinding defects, the target of Daw signaling appears to be the motor neuron itself and not the muscle (Parker et al. 2006). In addition, because Daw expression in either the muscle or glia rescues pathfinding defects and excess Daw expression is not detrimental, Daw appears to provide a permissive cue for motor neuron axon guidance (Parker et al. 2006). Interestingly, mutants in tlr also phenocopy the pathfinding defects of daw mutants (Serpe and O’Connor 2006). Tlr was found to cleave the prodomain of Daw, as well as Actβ and Myo, and, in the case of Daw, cleavage enhances signaling in cell culture assays. These observations suggest that, as described above for Scw, prodomain processing of activin-like ligands at sites distinct from the normal furin maturation sites may be important for full signaling activity, perhaps by stimulating complete dissociation of the prodomain from the carboxy-terminal ligand domain (Serpe and O’Connor 2006). Similar activating cleavages in prodomains of several vertebrate ligands have also been reported (Wolfman et al. 2003).
During the larval stage, photoreceptor axons must be targeted properly for light and color information to be processed correctly, and Actβ plays a role in regulating proper photoreceptor axon termination in the medulla. Clonal loss of babo or dSmad2 in R7 photoreceptors causes their axons to target to the correct layer, but move laterally and enter the wrong column (Ting et al. 2007). Actβ is expressed in R7 photoreceptors, and Actβ RNAi in the photoreceptors also causes axon mis-targeting (Ting et al. 2007). These studies indicate that Actβ expression in R7 photoreceptors restricts their targeting to a single column in the medulla through an autocrine signaling mechanism (Ting et al. 2007).
Activin also signals in a juxtacrine manner to negatively regulate dendritic branching and termination of medulla interneurons, which receive input from photoreceptors. In this case, Actβ derived from R7 or R8 photoreceptors signals to Dm8 or Tm20 medulla neurons, respectively, to regulate their dendritic field size (Ting et al. 2014). Loss-of-function babo and dSmad2 mutant clones in medulla interneurons produce expanded dendrites, whereas neurons with increased reception of activin signals have reduced dendrites. In loss-of-function mutants the expanded dendrites form ectopic membrane connections with presynaptic sites on multiple photoreceptors, potentially disrupting visual processing (Ting et al. 2014).
Activin signals also function to regulate MB neuron β-lobe axon growth. MB neurons are responsible for olfactory learning and memory. Expression of activated Babo causes axon truncation, whereas loss of babo results in overgrowth (Ng 2008). This is independent of dSmad2 and requires the actin cytoskeleton regulators Limk1, Rho, and Rac. The identity of the responsible ligand has not been determined. This example is one of only two cases in Drosophila in which non-Smad signaling is responsible for mediating a response.
Activin Signaling and Neuronal Remodeling
Apart from roles in axon guidance and target selection, activin signaling is also required during metamorphosis for MB remodeling. The MB undergoes stereotypic remodeling during the pupal phase to eliminate larval-specific connections and to form new connections required for proper adult behaviors. The larval MB has two major branches of γ neurons: medial and dorsal. Both branches are pruned during metamorphosis, and only the medial lobe remains in the adult MB. Mutations in babo and dSmad2 prevent pruning of dorsal and medial γ neurons of the MB, and these defects are rescued by expressing the proper wild-type gene in the MB neurons (Zheng et al. 2003). One major target of activin signaling in the remodeling MB neurons is the gene encoding the ecdysone receptor EcR-B1. Ectopic expression of EcR-B1 in the MB neurons partially rescues the pruning defect (Zheng et al. 2003). Although it was originally suggested that Actβ was the responsible ligand based on RNAi phenotypes, further studies using loss-of-function mutants show that glial-derived Myo provides the signal (Awasaki et al. 2011). In this example, Myo appears to signal primarily through the BaboA isoform (Awasaki et al. 2011) and can use either Punt or Wit as a type II receptor because only when both are eliminated is a MB remodeling defect observed (Zheng et al. 2003).
This signaling event is also noteworthy because efficient signaling requires a novel coreceptor in addition to the normal type I and type II receptors. Genetic screens for additional factors that regulate MB remodeling identified plum, a gene that codes for an immunoglobulin superfamily protein. Mutations in plum lead to loss of EcR-B1 expression and defective MB remodeling. Because these phenotypes can be rescued by expressing activated Babo in MB neurons, but not by overexpressing Plum in babo mutant MB clones, Plum appears to act upstream of receptor activation (Yu et al. 2013). Limited structure-function studies show that the extracellular domain of Plum is required for rescue, but the cytoplasmic domain is dispensable (Yu et al. 2013). At present, the best guess is that Plum acts to either stabilize receptor complexes and/or to facilitate Myo binding to the receptor complex, although no biochemical interactions between any of these components have been reported.
ROLE OF TGF-β FAMILY SIGNALING IN PHYSIOLOGICAL AND METABOLIC HOMEOSTASIS
Roles of TGF-β family signaling in developmental processes are well established and extensively studied. Nevertheless, TGF-β family signaling components continue to be expressed throughout the life span of an animal and in various adult tissues, signifying potential roles of the TGF-β family signaling pathways in key physiological or homeostatic processes. Investigating the role of TGF-β family signaling in physiology is an active field of research, and the involvement of the TGF-β family in the regulation of physiological homeostasis in Drosophila larvae and adults has been shown in a number of studies. In this section, we highlight our understanding of how TGF-β family signaling in Drosophila regulates the following four key homeostatic and physiological processes: (1) metabolic homeostasis, (2) hormonal signaling, (3) innate immunity, and (4) tissue homeostasis.
TGF-β Family Signaling in Metabolic Homeostasis
TGF-β family signaling components are expressed in metabolically active tissues in both vertebrates and invertebrates, and their roles in regulating metabolism are gaining attention (Andersson et al. 2008; Bertolino et al. 2008; Zamani and Brown 2011; Ghosh and O’Connor 2014). In Drosophila, both the TGF-β family ligand Dawdle (Daw) and the BMP ligand Glass-bottom boat (Gbb) have been implicated in metabolic regulation.
The activin-like ligand Daw regulates insulin/insulin-like growth factor signaling (IIS) in feeding third instar larvae (Ghosh and O’Connor 2014). Consistent with loss of IIS, daw mutant larvae show a significant increase in circulating sugar (glucose + trehalose) concentration, total triacylglycerol and glycogen content, and a significant reduction in Akt activation in the peripheral tissues (Ghosh and O’Connor 2014). Although these mutant animals did not show any change in expression of dilps in the insulin producing cells (IPCs), they were defective in the release of Dilps from the IPCs (Ghosh and O’Connor 2014).
Independent of its role in regulating IIS, canonical Smad signaling downstream from Daw also regulates the internal pH balance, most likely by altering the output of acidic TCA cycle intermediates primarily in the fat body (FB) (Ghosh and O’Connor 2014). FB-specific RNA sequencing of daw mutants and control flies revealed increased expression of multiple nuclear-encoded mitochondrial genes, including enzymes of the TCA cycle, electron transport chain, β-oxidation and ketogenesis in the daw mutants (Ghosh and O’Connor 2014). Additionally, metabolomics analysis revealed increased accumulation of multiple TCA cycle intermediates in these mutants. These results point toward a transcriptional role of Daw in mitochondrial biogenesis, energy metabolism, TCA cycle activity and pH balance. The involvement of Daw in expression of nuclear encoded mitochondrial genes may be reminiscent of the proposed role of activin A in mitochondrial biogenesis in vertebrates (Li et al. 2009; Zamani and Brown 2011).
Daw may regulate metabolic processes in a systemic dose-dependent manner. Expressing daw in any larval tissue could completely rescue larval lethality associated with mutations in daw, and had a graded effect in rescuing the metabolic phenotypes of daw mutants depending on the size of the tissue. Analyses of phospho-dSmad2 (pdSmad2) levels in the FB showed that Smad2 activation in the larval FB is primarily mediated by Daw and that the FB is poised to respond to a wide range of Daw signaling. Consistent with the endocrine nature of Daw signaling, expressing daw in a variety of larval tissues could rescue pdSmad2 levels in a daw mutant FB (Ghosh and O’Connor 2014). These results indicate that Daw acts like a metabolic hormone capable of dose-dependently regulating cellular metabolism. Intriguingly, unlike most hormones, daw is expressed in multiple larval tissues, and it will be interesting to learn how each of these tissues uses this metabolic ligand for systemic communication. One possibility is that daw expression in each tissue is regulated by different environmental stimuli and that Daw then acts on other tissues to bring about systemic changes to cope with the stimuli.
The involvement of the Drosophila TGF-β family ligand Daw in sugar homeostasis is further shown by work showing that nutrient-sensitive expression of Daw is required for inducing glucose repression (GR) in the Drosophila intestine (Chng et al. 2014). GR is a phenomenon whereby feeding of excessive dietary glucose leads to repression of amylase in the gut, presumably in an effort to protect the animal against deleterious effects of a high sugar diet (Hickey and Benkel 1982; Benkel and Hickey 1986; Musselman et al. 2011; Na et al. 2013). Smad signaling activated by Babo and dSmad2 mediates GR by negatively regulating the expression of digestive enzymes (amylases, hydrolases, and lipases) in the gut in response to high sugar diet (Chng et al. 2014). This effect is mediated by the Babo isoform C in the gut, which is specific to Daw signaling (Jensen et al. 2009; Chng et al. 2014). Importantly, in response to high sugar diet, Daw is released from the adult fat body and signals to the gut to repress expression of digestive enzymes including amylase, further confirming the endocrine nature of Daw signaling. It is interesting to note that expression of oxido-reductase genes is also significantly suppressed in the gut when flies are kept on a high sugar diet. Because a high sugar diet increases Daw signaling in the gut, this finding is consistent with a role for Daw signaling in repressing the expression of mitochondrial genes, including oxido-reductases, similar to what is seen in whole animal daw mutants (Ghosh and O’Connor 2014).
Additional evidence suggests that Daw is one of the primary effectors of a conserved sugar-sensing pathway mediated by the ChREBP/MondoA-Mlx complex (Mattila et al. 2015). The MondoA-Mlx transcription factors are activated by sugars and are essential for mediating multiple downstream processes essential for conversion of sugars to lipids and usage of sugars. The daw promoter contains a carbohydrate response element that is occupied by Mlx in both S2 cells and in Drosophila larvae indicating that daw expression is directly regulated by Mondo-A/Mlx. Additionally, Daw is required for and regulates the expression of a second key target of Mondo-A/Mlx called sugarbabe (sug) (Mattila et al. 2015). These findings put Daw firmly in a major sugar sensing and sugar metabolism pathway in Drosophila. Whether activin signaling in vertebrates is also a downstream target and effector of a Mondo-A/Mlx complex remains to be determined.
Daw has also been implicated in life span extension in Drosophila. A screen for transcriptional targets of dFOXO identified daw as a potential target, and muscle-specific knockdown of daw leads to increased life span (Bai et al. 2013). Loss of daw in the muscle affects life span possibly by increasing macro-autophagy that helps remove age-related accumulation of protein aggregates in the muscle, thereby improving muscle function (Bai et al. 2013). Interestingly, daw-RNAi in the muscle can reduce Dilp2 levels in circulation, whereas daw-RNAi in the FB leads to an increase in Dilp2 in the hemolymph (Bai et al. 2013). Although these observations support a role of Daw in regulating IIS in the adult fly as well, it remains to be explained how tissue-specific knockdown of a ligand, which clearly works as an endocrinal systemic signal in the larva, can lead to opposing phenotypes in the adult. The relative contributions of the muscle and FB to circulating Daw levels and potential compensatory changes in daw expression when it is knocked down in any single tissue could explain some of these findings.
The BMP ligand Gbb has also been proposed to regulate metabolic processes, initially based on a striking morphological defect exhibited by gbb mutants in which the FB takes on a glassy appearance instead of its normal opaqueness (hence, the name Glass bottom boat). Characterization of these mutant larvae revealed that the FB shows nutrient storage and morphological characteristics resembling a state of starvation (Ballard et al. 2010). Additionally, gbb mutants show alterations in the expression of multiple starvation response genes. Surprisingly, monitoring lipid uptake using a fluorescently labeled lipid derivative (Bo-C12) reveals that the gbb mutants show an increased rate of nutrient uptake, indicating defects in the ability to store nutrients. Although Gbb produced by the FB regulates FB morphology and nutrient storage in a cell-autonomous manner, Gbb signaling in the FB may only be partially responsible for the increased nutrient uptake phenotype observed in gbb mutants (Ballard et al. 2010). The mechanistic details of how Gbb affects usage of absorbed nutrients remains unexplained.
TGF-β Family Signaling Regulates Hormonal Control of Drosophila Development
Like other insects, Drosophila uses two primary hormonal signals, juvenile hormone (JH) and ecdysone (E), to coordinately regulate transitions in larval development. TGF-β family signaling appears to work upstream of these hormonal signals by controlling the production of both these hormones, presumably in response to developmental or environmental cues (Gibbens et al. 2011; Huang et al. 2011). Both gain and loss-of-function studies show that activin signaling is required in the prothoracic gland (PG), the site of E production and release, to generate the large pulse of E necessary for entering metamorphosis (Gibbens et al. 2011). In the absence of canonical activin signaling in the PG, the larvae arrest at the third instar stage and do not undergo metamorphosis. This phenotype results from a requirement for activin signaling in the PG to make it competent to respond to two other hormonal signals, prothoracicotropic hormone (PTTH) and insulin. In the absence of activin signaling, expression of Torso, the PTTH receptor, and InR, the insulin receptor and several other downstream components in the insulin signaling pathway, are all down-regulated in the PG. Because each of these two signaling pathways is required for high-level expression of several key E biosynthetic enzymes, their simultaneous loss eliminates production of the E pulse that is required for initiating metamorphosis. A remaining question is the identity of the TGF-β ligand that signals in the PG because loss of any one ligand (actβ, daw, or myo) does not show larval developmental arrest phenotypes. This observation suggests redundancy among the ligands for providing competence to the PG for reception of PTTH and insulin signals.
In contrast to the involvement of activin signaling in the control of E production, JH synthesis is under the control of the BMP ligand Dpp (Huang et al. 2011). JH is critical for determining the nature of developmental transitions in insects. In the presence of JH, E induces a larval–larval molt, whereas, in the absence of JH, E induces a larval–pupal or pupal–adult molt. Using an elegant genetic screen, Tkv and Mad were identified as positive regulators of JH signaling in the larva (Huang et al. 2011). JH is synthesized in the corpus allata (CA), and glutamatergic signals emanating from the brain induce dpp expression in the CA. Dpp, then cell-autonomously induces production of the JH-synthesizing enzyme JH acid methytransferase to stimulate JH production in the developing larvae.
TGF-β Family Signaling in Drosophila Innate Immunity
In vertebrates, TGF-β signaling plays diverse roles in the regulation of immune responses (Li et al. 2006). This is also true in Drosophila in which both the BMP and activin pathways regulate different aspects of the innate immune response (Clark et al. 2011). Expression of both daw and dpp responds to immune challenge, although the nature and context of the response differ between the two ligands. Expression of daw goes up in response to an infection. In this case, Daw appears to play an important role in suppressing infection-induced activation of the immune response, in an effort to protect the animal against immune-induced pathology. In contrast, dpp expression increases and persists in response to sterile wounding, and Dpp has been proposed to suppress unnecessary expression of antimicrobial peptides (AMP). The presence of a nearby Mad-Medea-Schnurri SE in many AMP genes suggests that Dpp may directly regulate AMP gene expression (Clark et al. 2011).
Role of TGF-β Family Signaling in Tissue Homeostasis
Adult tissues showing a rapid turnover of living cells require a constant supply of differentiated cells to replenish old ones to maintain tissue homeostasis. Adult stem cells accomplish this crucial task of tissue homeostasis. Each adult stem cell undergoes a stereotypical asymmetric cell division giving rise to one progenitor cell that commits to differentiation and one stem cell that ensures maintenance of a healthy population of precursor cells throughout the life of an animal. This behavior of stem cells and stem cell identity itself are maintained by specialized microenvironments or “niches” generated by support cells. Studies using Drosophila have shown crucial involvement of TGF-β family signaling components in stem cell niche maintenance and stem cell self-renewal.
In the Drosophila female germline, two or three germline stem cells (GSCs) are located in close contact with the niche cells (terminal filament cells and cap cells) at the tip of each ovariole (Fig. 5A) (Lin 2002; Yamashita et al. 2005). TGF-β family signaling plays a crucial role during both the development of this niche at late larval stages and during maintenance of the adult GSCs. In the late larval stages, the activin receptor Babo is required for proliferation of somatic precursor cells that give rise to the terminal filament (TF) cells. Additionally, during the final differentiation of these precursor cells activin signaling interacts with ecdysone signaling to regulate expression of the target gene Br-Z1, and thereby regulates differentiation of the TF cells (Lengil et al. 2015). In the adult female germline, GSCs undergo asymmetric cell division that give rise to one GSC and a cystoblast (CB). Each CB undergoes four rounds of synchronous divisions to produce 16 interconnected cystocytes (CC) that ultimately differentiate into ovarian follicles (Lin 2002). The Drosophila BMP ligand Dpp plays a prominent role in maintenance of GSCs in this tissue. Dpp is produced and released by the TF and cap cells, resulting in a shallow gradient that elicits a strong BMP response in the GSCs but not in the distally located CBs (Xie and Spradling 1998, 2000; Kai and Spradling 2003). BMP signaling in the GSC functions cell-autonomously through Mad and Medea to maintain GSC identity and inhibit differentiation (Xie and Spradling 1998). Excessive BMP signaling induced by ectopic overexpression of dpp produces enlarged germaria filled with cells that resemble GSCs, while null mutations in sax encode the BMP type I receptor and shorten the half-life of GSCs (Xie and Spradling 1998; Kai and Spradling 2003). The effect of BMP signaling on GSC maintenance is primarily mediated by the BMP target gene bag-of-marbles (bam) (Chen and McKearin 2003; Song et al. 2004). Expression of bam is directly regulated by binding of the Mad–Medea complex at repressor elements in the promoter region of bam (Chen and McKearin 2003; Song et al. 2004). Consequently, bam expression is high in differentiating, early germ cells and has been shown to be necessary and sufficient for differentiation of female GSCs.
Figure 5.
Bone morphogenetic protein (BMP) signaling in Drosophila female and male germline. (A) Schematic of the role of BMP signaling in the Drosophila female germline. BMP signaling mediated by Dpp released from the TF and cap cells regulates germinal stem cell (GSC) identity by inhibiting bam expression. (B) Schematic of the role of BMP signaling in Drosophila male germline. BMP ligands Dpp and Gbb released from the hub cells form a morphogen gradient that determines the number of transient amplifications the spermatogonia undergoes before differentiating into sperm. TF, Terminal filament cell; CB, cystoblast.
The Drosophila BMP ligands, Dpp and Gbb have also been shown to repress bam levels in the male germline. The function of this regulation differs between the male germline and the female germline. In the female germline, bam expression initiates differentiation of the stem cell progeny. However, in the male germline, expression of bam determines the number of transient-amplifying divisions that spermatogonia undergo before differentiating into spermatocytes (Fig. 5B) (Shivdasani and Ingham 2003; Schulz 2004). BMP signaling in the male germline may also play a permissive role in the survival and proliferation of the GSCs but does not determine GSC fate (Shivdasani and Ingham 2003).
Apart from the germline tissue, BMP signaling also plays a role in tissue homeostasis in the adult midgut. Based on morphological and functional characteristics, the Drosophila adult midgut can be broadly divided into three regions, the anterior midgut (AM), the middle midgut (MM) and the posterior midgut (PM) (Fig. 6). Each of these compartments houses intestinal stem cells (ISCs), which are responsible for the renewal of the various cell types in the midgut (Micchelli and Perrimon 2006; Ohlstein and Spradling 2006). The ISCs in the Drosophila midgut divide to produce a daughter cell called the enteroblast (EB), which, depending on the level of Notch signaling, differentiates into either an enterocyte (EC) or an enteroendocrine cell.
Figure 6.
Dpp signaling in Drosophila gut. Schematic of the Drosophila midgut. dpp-Gal4-driven expression of GFP reveals two clusters of dpp expressing cells in the midgut, at the junction of the middle midgut (MM) and near the posterior end of the posterior midgut (PM). The expression of dpp from the first cluster of cells was shown to be necessary for specification of the copper cells and establishment of the copper cell region (CCR). A–C show the three proposed models of where dpp is expressed and released, and how Dpp regulates intestinal stem cell (ISC) proliferation and renewal. The validity of any of these models remains to be supported by additional studies. (A) Dpp released from the visceral muscle (orange) contains enterocyte (EC) cell injury-induced proliferation of ISCs. (B) Dpp released from trachea indirectly inhibits ISC proliferation by protecting the loss of EC cells. (C) Dpp released from the ECs acts as an autocrine signal to inhibit EC signals that induce ISC proliferation.
In the adult midgut, Dpp is expressed and secreted by a group of ECs that are located at the boundary between the PM and MM (Fig. 6). Anterior to this junction, and contained within the MM, is an acidic region called the copper cell region (CCR). The CCR houses specialized “copper” cells, which secrete acids that maintain the lumen of the CCR at low pH. Dpp signaling plays an essential role in the specification of the copper cells from EBs (Guo et al. 2013; Li et al. 2013a). Disrupting the Dpp gradient in the MM by overexpressing dpp in all ECs causes an increase in the presence of copper cells in the CCR and a progressive expansion of the CCR in the AM (Li et al. 2013a). Conversely, silencing mad or tkv in the CCR leads to a complete loss of the low luminal pH associated with CCR, and a loss of Labial-expressing cells in the CCR, indicating a loss of functional copper cells (Guo et al. 2013).
BMP signaling may also play an important role in regulating the choice between ISC and EB cell fate in the intestine. Loss of BMP signaling in precursor cells has been shown to cause rapid loss of ISCs accompanied by an increase in the number of EB (Tian and Jiang 2014). Additionally, the strength of BMP signaling is asymmetric in ISC and EB cell pairs, with the basally located ISCs showing much stronger BMP signaling than apically located EBs, indicating a role for BMP signaling in maintaining ISC identity. Consistent with these observations, loss of BMP in precursor cells leads to formation of more EB–EB pairs, and gain of BMP in the precursor cells results in formation of large ISC-like cell clusters and favors symmetric ISC–ISC division of ISCs (Tian and Jiang 2014).
Apart from the physiological role in specifying copper cells and maintaining ISC identity, BMP signaling has also been proposed to regulate ISC proliferation in the AM and PM regions (Guo et al. 2013; Li et al. 2013b; Tian and Jiang 2014). The source of Dpp or Gbb that mediates this role and the nature of this regulation remain controversial based on seemingly contradictory results. Work from two groups suggests that Dpp signaling inhibits ISC proliferation in the midgut (Guo et al. 2013; Li et al. 2013a). One shows that Dpp secreted from the trachea indirectly affects ISC proliferation by protecting the loss of ECs (Fig. 6B). The second group suggested that Dpp released from visceral muscle in response to intestinal injury works directly on the ISCs to inhibit ISC proliferation and thereby helps contain injury-induced proliferation of ISCs (Fig. 6A) (Guo et al. 2013). In contrast, a third study proposed that both Gbb and Dpp released from the ECs indirectly regulate ISC proliferation by acting on the ECs and inhibiting ISC proliferation signals (Fig. 6C) (Tian and Jiang 2014). Another showed that Dpp can have a dual role in ISC proliferation, and that in response to gut damage, Dpp released from the hemocytes activates ISC proliferation by signaling through Sax and Smox. Subsequently, to prevent overproliferation, the ISCs switch on tkv expression and Dpp signaling through Tkv and Mad inhibits ISC proliferation (Ayyaz et al. 2015). Although more work will be necessary to fully understand the source of BMP ligands and the mechanism by which they regulate ISC proliferation and maintenance, these results clearly highlight a significant role of BMP signaling in maintaining Drosophila midgut homeostasis.
SUMMARY AND OUTLOOK
Studies of TGF-β family signaling in Drosophila have uncovered a wide variety of roles for both activins and BMPs in the regulation of diverse processes, including developmental patterning, neural development and function, innate immunity, as well as metabolic and tissue homeostasis. However, numerous questions remain. Several noncanonical signal transducers were found to interact with TGF-β family signaling, but most of the factors have not been identified. Accessory factors to core TGF-β family signaling components, such as the putative Myo coreceptor Plum, need to be further investigated to determine the nature of their interactions with the canonical signaling machinery. Other interesting areas of study include unraveling how many ligands can signal in different directions at structures like the synapse, and, importantly, how their downstream effects can be compartmentalized. Determining to what degree different ligands act systemically through the circulatory system also requires attention, as does the issue of differential processing within ligand prodomains and how that might affect ligand activity. The mechanistic basis of how TGF-β family signaling regulates metabolic homeostasis still remains to be explained, and additional roles of the pathway in regulating other aspects of physiology need to be explored. Moreover, identification of nutritional and environmental cues that work upstream to TGF-β family signals to regulate their roles in metabolism and homeostasis is also an important unresolved question. Undoubtedly, addressing these issues will keep Drosophila researchers busy for some time, and the results should also help inform studies of TGF-β family signaling in other systems.
ACKNOWLEDGMENTS
The authors thank MaryJane O’Connor and Aidan Peterson for critical reading of the manuscript and for helpful suggestions. This work is supported by Grant GM R01 GM95746 and R35 GM118029 to M.B.O.
Footnotes
Editors: Rik Derynck and Kohei Miyazono
Additional Perspectives on The Biology of the TGF-β Family available at www.cshperspectives.org
REFERENCES
- Aberle H, Haghighi AP, Fetter RD, McCabe BD, Magalhães TR, Goodman CS. 2002. wishful thinking encodes a BMP type II Receptor that regulates synaptic growth in Drosophila. Neuron 33: 545–558. [DOI] [PubMed] [Google Scholar]
- Affolter M, Basler K. 2007. The decapentaplegic morphogen gradient: From pattern formation to growth regulation. Nat Rev Genet 8: 663–674. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Gibson MC. 2015. Decapentaplegic and growth control in the developing Drosophila wing. Nature 527: 375–378. [DOI] [PubMed] [Google Scholar]
- Akiyama T, Kamimura K, Firkus C, Takeo S, Shimmi O, Nakato H. 2008. Dally regulates Dpp morphogen gradient formation by stabilizing Dpp on the cell surface. Dev Biol 313: 408–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama T, Marques G, Wharton KA. 2012. A large bioactive BMP ligand with distinct signaling properties is produced by alternative proconvertase processing. Sci Signal 5: ra28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alarcon C, Zaromytidou AI, Xi Q, Gao S, Yu J, Fujisawa S, Barlas A, Miller AN, Manova-Todorova K, Macias MJ, et al. 2009. Nuclear CDKs drive Smad transcriptional activation and turnover in BMP and TGF-β pathways. Cell 139: 757–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amore G, Casares F. 2010. Size matters: The contribution of cell proliferation to the progression of the specification Drosophila eye gene regulatory network. Dev Biol 344: 569–577. [DOI] [PubMed] [Google Scholar]
- Andersson O, Korach-Andre M, Reissmann E, Ibáñez CF, Bertolino P. 2008. Growth/differentiation factor 3 signals through ALK7 and regulates accumulation of adipose tissue and diet-induced obesity. Proc Natl Acad Sci 105: 7252–7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arora K, Nusslein-Volhard C. 1992. Altered mitotic domains reveal fate map changes in Drosophila embryos mutant for zygotic dorsoventral patterning genes. Development 114: 1003–1024. [DOI] [PubMed] [Google Scholar]
- Arora K, Levine MS, O’Connor MB. 1994. The screw gene encodes a ubiquitously expressed member of the TGF-β family required for specification of dorsal cell fates in the Drosophila embryo. Genes Dev 8: 2588–2601. [DOI] [PubMed] [Google Scholar]
- Awasaki T, Huang Y, O’Connor MB, Lee T. 2011. Glia instruct developmental neuronal remodeling through TGF-β signaling. Nat Neurosci 14: 821–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyaz A, Li H, Jasper H. 2015. Haemocytes control stem cell activity in the Drosophila intestine. Nat Cell Biol 17: 736–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baena-Lopez LA, Nojima H, Vincent JP. 2012. Integration of morphogen signalling within the growth regulatory network. Curr Opin Cell Biol 24: 166–172. [DOI] [PubMed] [Google Scholar]
- Bai H, Kang P, Hernandez AM, Tatar M. 2013. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet 9: e1003941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines RA. 2004. Synaptic strengthening mediated by bone morphogenetic protein-dependent retrograde signaling in the Drosophila CNS. J Neurosci 24: 6904–6911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker NE. 2001. Cell proliferation, survival, and death in the Drosophila eye. Semin Cell Dev Biol 12: 499–507. [DOI] [PubMed] [Google Scholar]
- Ball RW, Warren-Paquin M, Tsurudome K, Liao EH, Elazzouzi F, Cavanagh C, An BS, Wang TT, White JH, Haghighi AP. 2010. Retrograde BMP signaling controls synaptic growth at the NMJ by regulating trio expression in motor neurons. Neuron 66: 536–549. [DOI] [PubMed] [Google Scholar]
- Ballard SL, Jarolimova J, Wharton KA. 2010. Gbb/BMP signaling is required to maintain energy homeostasis in Drosophila. Dev Biol 337: 375–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangi E, Wharton K. 2006. Dual function of the Drosophila Alk1/Alk2 ortholog Saxophone shapes the Bmp activity gradient in the wing imaginal disc. Development 133: 3295–3303. [DOI] [PubMed] [Google Scholar]
- Belenkaya TY, Han C, Yan D, Opoka RJ, Khodoun M, Liu H, Lin X. 2004. Drosophila Dpp morphogen movement is independent of dynamin-mediated endocytosis but regulated by the glypican members of heparan sulfate proteoglycans. Cell 119: 231–244. [DOI] [PubMed] [Google Scholar]
- Benkel BF, Hickey DA. 1986. Glucose repression of amylase gene expression in Drosophila melanogaster. Genetics 114: 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Zvi D, Pyrowolakis G, Barkai N, Shilo BZ. 2011. Expansion-repression mechanism for scaling the Dpp activation gradient in Drosophila wing imaginal discs. Curr Biol 21: 1391–1396. [DOI] [PubMed] [Google Scholar]
- Berke B, Wittnam J, McNeill E, Van Vactor DL, Keshishian H. 2013. Retrograde BMP signaling at the synapse: A permissive signal for synapse maturation and activity-dependent plasticity. J Neurosci 33: 17937–17950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolino P, Holmberg R, Reissmann E, Andersson O, Berggren P-O, Ibáñez CF. 2008. Activin B receptor ALK7 is a negative regulator of pancreatic β-cell function. Proc Natl Acad Sci 105: 7246–7251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel D, Shah R, Gesualdi SC, Haerry TE. 2008. Drosophila follistatin exhibits unique structural modifications and interacts with several TGF-β family members. Mech Dev 125: 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M. 1994. Homeotic genes and positional signalling in the Drosophila viscera. Trends Genet 10: 22–26. [DOI] [PubMed] [Google Scholar]
- Brazil DP, Church RH, Surae S, Godson C, Martin F. 2015. BMP signalling: Agony and antagony in the family. Trends Cell Biol 25: 249–264. [DOI] [PubMed] [Google Scholar]
- Brummel TJ, Twombly V, Marques G, Wrana JL, Newfeld SJ, Attisano L, Massague J, O’Connor MB, Gelbart WM. 1994. Characterization and relationship of Dpp receptors encoded by the saxophone and thick veins genes in Drosophila. Cell 78: 251–261. [DOI] [PubMed] [Google Scholar]
- Brummel T, Abdollah S, Haerry TE, Shimell MJ, Merriam J, Raftery L, Wrana JL, O’Connor MB. 1999. The Drosophila Activin receptor Baboon signals through dSmad2 and controls cell proliferation but not patterning during larval development. Genes Dev 13: 98–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke R, Basler K. 1996. Dpp receptors are autonomously required for cell proliferation in the entire developing Drosophila wing. Development 122: 2261–2269. [DOI] [PubMed] [Google Scholar]
- Campbell G, Tomlinson A. 1999. Transducing the Dpp morphogen gradient in the wing of Drosophila: Regulation of Dpp targets by brinker. Cell 96: 553–562. [DOI] [PubMed] [Google Scholar]
- Capdevila J, Guerrero I. 1994. Targeted expression of the signaling molecule decapentaplegic induces pattern duplications and growth alterations in Drosophila wings. EMBO J 13: 4459–4468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, McKearin D. 2003. Dpp signaling silences bam transcription directly to establish asymmetric divisions of germline stem cells. Curr Biol 13: 1786–1791. [DOI] [PubMed] [Google Scholar]
- Chen CK, Kuhnlein RP, Eulenberg KG, Vincent S, Affolter M, Schuh R. 1998. The transcription factors Knirps and Knirps related control cell migration and branch morphogenesis during Drosophila tracheal. Development 125: 4959–4968. [DOI] [PubMed] [Google Scholar]
- Chen J, Honeyager SM, Schleede J, Avanesov A, Laughon A, Blair SS. 2012. Crossveinless d is a vitellogenin-like lipoprotein that binds BMPs and HSPGs, and is required for normal BMP signaling in the Drosophila wing. Development 139: 2170–2176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chng W-BA, Sleiman MSB, Schüpfer F, Lemaitre B. 2014. Transforming growth factor β/activin signaling functions as a sugar-sensing feedback loop to regulate digestive enzyme expression. Cell Rep 9: 336–348. [DOI] [PubMed] [Google Scholar]
- Clark RI, Woodcock KJ, Geissmann F, Trouillet C, Dionne MS. 2011. Multiple TGF-β superfamily signals modulate the adult Drosophila immune response. Curr Biol 21: 1672–1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley Ca, Silburn R, Singer MA, Ralston A, Rohwer-Nutter D, Olson DJ, Gelbart W, Blair SS. 2000. Crossveinless 2 contains cysteine-rich domains and is required for high levels of BMP-like activity during the formation of the cross veins in Drosophila. Development 127: 3947–3959. [DOI] [PubMed] [Google Scholar]
- Crick F. 1970. Diffusion in embryogenesis. Nature 225: 420–422. [DOI] [PubMed] [Google Scholar]
- Curtiss J, Mlodzik M. 2000. Morphogenetic furrow initiation and progression during eye development in Drosophila: The roles of decapentaplegic, hedgehog and eyes absent. Development 127: 1325–1336. [DOI] [PubMed] [Google Scholar]
- Dani N, Broadie K. 2012. Glycosylated synaptomatrix regulation of trans-synaptic signaling. Dev Neurobiol 72: 2–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day SJ, Lawrence PA. 2000. Measuring dimensions: The regulation of size and shape. Development 127: 2977–2987. [DOI] [PubMed] [Google Scholar]
- de Celis JF. 1997. Expression and function of decapentaplegic and thickveins during the differentiation of the veins in the Drosophila wing. Development 124: 1007–1018. [DOI] [PubMed] [Google Scholar]
- de Celis JF. 2003. Pattern formation in the Drosophila wing: The development of the veins. BioEssays 25: 443–451. [DOI] [PubMed] [Google Scholar]
- Dickson BJ. 2001. Rho GTPases in growth cone guidance. Curr Opin Neurobiol 11: 103–110. [DOI] [PubMed] [Google Scholar]
- Dobens LL, Raftery LA. 1998. Drosophila oogenesis: A model system to understand TGF-β/Dpp directed cell morphogenesis. Ann NY Acad Sci 857: 245–247. [DOI] [PubMed] [Google Scholar]
- Dorfman R, Shilo BZ. 2001. Biphasic activation of the BMP pathway patterns the Drosophila embryonic dorsal region. Development 128: 965–972. [DOI] [PubMed] [Google Scholar]
- Dupont S, Mamidi A, Cordenonsi M, Montagner M, Zacchigna L, Adorno M, Martello G, Stinchfield MJ, Soligo S, Morsut L, et al. 2009. FAM/USP9x, a deubiquitinating enzyme essential for TGF-β signaling, controls Smad4 monoubiquitination. Cell 136: 123–135. [DOI] [PubMed] [Google Scholar]
- Eaton BA, Davis GW. 2005. LIM kinase1 controls synaptic stability downstream of the type II BMP receptor. Neuron 47: 695–708. [DOI] [PubMed] [Google Scholar]
- Eivers E, Fuentealba LC, Sander V, Clemens JC, Hartnett L, De Robertis EM. 2009. Mad is required for wingless signaling in wing development and segment patterning in Drosophila. PLoS ONE 4: e6543–e6543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eivers E, Demagny H, Choi RH, De Robertis EM. 2011. Phosphorylation of Mad controls competition between wingless and BMP signaling. Sci Signal 4: ra68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar A, Dorfman R, Weiss D, Ashe H, Shilo BZ, Barkai N. 2002. Robustness of the BMP morphogen gradient in Drosophila embryonic patterning. Nature 419: 304–308. [DOI] [PubMed] [Google Scholar]
- Entchev EV, Schwabedissen A, González-Gaitán M. 2000. Gradient formation of the TGF-β homolog Dpp. Cell 103: 981–991. [DOI] [PubMed] [Google Scholar]
- Estella C, Mann RS. 2008. Logic of Wg and Dpp induction of distal and medial fates in the Drosophila leg. Development 135: 627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estella C, Voutev R, Mann RS. 2012. A dynamic network of morphogens and transcription factors patterns the fly leg. Curr Top Dev Biol 98: 173–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estrada B, Casares F, Sánchez-Herrero E. 2003. Development of the genitalia in Drosophila melanogaster. Differentiation 71: 299–310. [DOI] [PubMed] [Google Scholar]
- Fan X, Labrador JP, Hing H, Bashaw GJ. 2003. Slit stimulation recruits Dock and Pak to the roundabout receptor and increases Rac activity to regulate axon repulsion at the CNS midline. Neuron 40: 113–127. [DOI] [PubMed] [Google Scholar]
- Ferguson EL, Anderson KV. 1992. Decapentaplegic acts as a morphogen to organize dorsal–ventral pattern in the Drosophila embryo. Cell 71: 451–461. [DOI] [PubMed] [Google Scholar]
- Fernandez BG, Arias AM, Jacinto A. 2007. Dpp signalling orchestrates dorsal closure by regulating cell shape changes both in the amnioserosa and in the epidermis. Mech Dev 124: 884–897. [DOI] [PubMed] [Google Scholar]
- Firth LC, Bhattacharya A, Baker NE. 2010. Cell cycle arrest by a gradient of Dpp signaling during Drosophila eye development. BMC Dev Biol 10: 28–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasch M. 1995. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature 374: 464–467. [DOI] [PubMed] [Google Scholar]
- Fritsch C, Sawala A, Harris R, Maartens A, Sutcliffe C, Ashe HL, Ray RP. 2012. Different requirements for proteolytic processing of bone morphogenetic protein 5/6/7/8 ligands in Drosophila melanogaster. J Biol Chem 287: 5942–5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuentes-Medel Y, Ashley J, Barria R, Maloney R, Freeman M, Budnik V. 2012. Integration of a retrograde signal during synapse formation by glia-secreted TGF-β ligand. Curr Biol 22: 1831–1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujise M, Takeo S, Kamimura K, Matsuo T, Aigaki T, Izumi S, Nakato H. 2003. Dally regulates Dpp morphogen gradient formation in the Drosophila wing. Development 130: 1515–1522. [DOI] [PubMed] [Google Scholar]
- Funakoshi Y, Minami M, Tabata T. 2001. mtv shapes the activity gradient of the Dpp morphogen through regulation of thickveins. Development 128: 67–74. [DOI] [PubMed] [Google Scholar]
- Fuss B, Hoch M. 1998. Drosophila endoderm development requires a novel homeobox gene which is a target of Wingless and Dpp signalling. Mech Dev 79: 83–97. [DOI] [PubMed] [Google Scholar]
- Gavin-Smyth J, Wang YC, Butler I, Ferguson EL. 2013. A genetic network conferring canalization to a bistable patterning system in Drosophila. Curr Biol 23: 2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesualdi SC, Haerry TE. 2007. Distinct signaling of Drosophila Activin/TGF-β family members. Fly (Austin) 1: 212–221. [DOI] [PubMed] [Google Scholar]
- Ghosh AC, O’Connor MB. 2014. Systemic activin signaling independently regulates sugar homeostasis, cellular metabolism, and pH balance in Drosophila melanogaster. Proc Natl Acad Sci 111: 5729–5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbens YY, Warren JT, Gilbert LI, O’Connor MB. 2011. Neuroendocrine regulation of Drosophila metamorphosis requires TGFβ/Activin signaling. Development 138: 2693–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glise B, Noselli S. 1997. Coupling of Jun amino-terminal kinase and Decapentaplegic signaling pathways in Drosophila morphogenesis. Genes Dev 11: 1738–1747. [DOI] [PubMed] [Google Scholar]
- Goold CP, Davis GW. 2007. The BMP ligand Gbb gates the expression of synaptic homeostasis independent of synaptic growth control. Neuron 56: 109–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorfinkiel N, Sánchez L, Guerrero I. 1999. Drosophila terminalia as an appendage-like structure. Mech Dev 86: 113–123. [DOI] [PubMed] [Google Scholar]
- Goto S, Hayashi S. 1997. Specification of the embryonic limb primordium by graded activity of Decapentaplegic. Development 124: 125–132. [DOI] [PubMed] [Google Scholar]
- Guo Z, Driver I, Ohlstein B. 2013. Injury-induced BMP signaling negatively regulates Drosophila midgut homeostasis. J Cell Biol 201: 945–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, de Lachapelle AM, Pyrowolakis G, Bergmann S, Affolter M. 2011. Dpp signaling activity requires Pentagone to scale with tissue size in the growing Drosophila wing imaginal disc. PLoS Biol 9: e1001182–e1001182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamaratoglu F, Affolter M, Pyrowolakis G. 2014. Dpp/BMP signaling in flies: From molecules to biology. Semin Cell Dev Biol 32: 128–136. [DOI] [PubMed] [Google Scholar]
- Harmansa S, Hamaratoglu F, Affolter M, Caussinus E. 2015. Dpp spreading is required for medial but not for lateral wing disc growth. Nature 527: 317–322. [DOI] [PubMed] [Google Scholar]
- Hevia CF, de Celis JF. 2013. Activation and function of TGFβ signalling during Drosophila wing development and its interactions with the BMP pathway. Dev Biol 377: 138–153. [DOI] [PubMed] [Google Scholar]
- Hickey DA, Benkel B. 1982. Regulation of amylase activity in Drosophila melanogaster: Effects of dietary carbohydrate. Biochem Genet 20: 1117–1129. [DOI] [PubMed] [Google Scholar]
- Horsfield J, Penton A, Secombe J, Hoffman FM, Richardson H. 1998. Decapentaplegic is required for arrest in G1 phase during Drosophila eye development. Development 125: 5069–5078. [DOI] [PubMed] [Google Scholar]
- Huang J, Tian L, Peng C, Abdou M, Wen D, Wang Y, Li S, Wang J. 2011. DPP-mediated TGF signaling regulates juvenile hormone biosynthesis by activating the expression of juvenile hormone acid methyltransferase. Development 138: 2283–2291. [DOI] [PubMed] [Google Scholar]
- Humphreys GB, Jud MC, Monroe KM, Kimball SS, Higley M, Shipley D, Vrablik MC, Bates KL, Letsou A. 2013. Mummy, a UDP-N-acetylglucosamine pyrophosphorylase, modulates DPP signaling in the embryonic epidermis of Drosophila. Dev Biol 381: 434–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igaki T, Kanda H, Yamamoto-Goto Y, Kanuka H, Kuranaga E, Aigaki T, Miura M. 2002. Eiger, a TNF superfamily ligand that triggers the Drosophila JNK pathway. EMBO J 21: 3009–3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H, Imamura T, Ishidou Y, Takase M, Udagawa Y, Oka Y, Tsuneizumi K, Tabata T, Miyazono K, Kawabata M. 1998. Interplay of signal mediators of decapentaplegic (Dpp): Molecular characterization of mothers against dpp, Medea, and daughters against dpp. Mol Biol Cell 9: 2145–2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James RE, Broihier HT. 2011. Crimpy inhibits the BMP homolog Gbb in motoneurons to enable proper growth control at the Drosophila neuromuscular junction. Development 138: 3273–3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaźwińska a, Kirov N, Wieschaus E, Roth S, Rushlow C. 1999. The Drosophila gene brinker reveals a novel mechanism of Dpp target gene regulation. Cell 96: 563–573. [DOI] [PubMed] [Google Scholar]
- Jensen PA, Zheng X, Lee T, O’Connor MB. 2009. The Drosophila Activin-like ligand Dawdle signals preferentially through one isoform of the Type-I receptor Baboon. Mech Dev 126: 950–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AN, Bergman CM, Kreitman M, Newfeld SJ. 2003. Embryonic enhancers in the dpp disk region regulate a second round of Dpp signaling from the dorsal ectoderm to the mesoderm that represses Zfh-1 expression in a subset of pericardial cells. Dev Biol 262: 137–151. [DOI] [PubMed] [Google Scholar]
- Johnson AN, Burnett LA, Sellin J, Paululat A, Newfeld SJ. 2007. Defective decapentaplegic signaling results in heart overgrowth and reduced cardiac output in Drosophila. Genetics 176: 1609–1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kai T, Spradling A. 2003. An empty Drosophila stem cell niche reactivates the proliferation of ectopic cells. Proc Natl Acad Sci 100: 4633–4638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya Y, Miyazono K, Miyazawa K. 2008. Specificity of the inhibitory effects of Dad on TGF-β family type I receptors, Thickveins, Saxophone, and Baboon in Drosophila. FEBS Lett 582: 2496–2500. [DOI] [PubMed] [Google Scholar]
- Keisman EL, Baker BS. 2001. The Drosophila sex determination hierarchy modulates wingless and decapentaplegic signaling to deploy dachshund sex-specifically in the genital imaginal disc. Development 128: 1643–1656. [DOI] [PubMed] [Google Scholar]
- Khalsa O, Yoon JW, Torres-Schumann S, Wharton KA. 1998. TGF-β/BMP superfamily members, Gbb-60A and Dpp, cooperate to provide pattern information and establish cell identity in the Drosophila wing. Development 125: 2723–2734. [DOI] [PubMed] [Google Scholar]
- Kicheva A, González-Gaitán M. 2008. The Decapentaplegic morphogen gradient: A precise definition. Curr Opin Cell Biol 20: 137–143. [DOI] [PubMed] [Google Scholar]
- Kim NC, Marques G. 2012. The Ly6 neurotoxin-like molecule target of wit regulates spontaneous neurotransmitter release at the developing neuromuscular junction in Drosophila. Dev Neurobiol 72: 1541–1558. [DOI] [PubMed] [Google Scholar]
- Kim MJ, O’Connor MB. 2014. Anterograde activin signaling regulates postsynaptic membrane potential and GluRIIA/B abundance at the Drosophila neuromuscular junction. PLoS ONE 9: e107443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Johnson K, Chen HJ, Carroll S, Laughon A. 1997. Drosophila Mad binds to DNA and directly mediates activation of vestigial by Decapentaplegic. Nature 388: 304–308. [DOI] [PubMed] [Google Scholar]
- Kretzschmar M, Doody J, Massagué J. 1997. Opposing BMP and EGF signalling pathways converge on the TGF-β family mediator Smad1. Nature 389: 618–622. [DOI] [PubMed] [Google Scholar]
- Kunnapuu J, Bjorkgren I, Shimmi O. 2009. The Drosophila DPP signal is produced by cleavage of its proprotein at evolutionary diversified furin-recognition sites. Proc Natl Acad Sci 106: 8501–8506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunnapuu J, Tauscher PM, Tiusanen N, Nguyen M, Loytynoja A, Arora K, Shimmi O. 2014. Cleavage of the Drosophila screw prodomain is critical for a dynamic BMP morphogen gradient in embryogenesis. Dev Biol 389: 149–159. [DOI] [PubMed] [Google Scholar]
- Kuo WJ, Digman MA, Lander AD. 2010. Heparan sulfate acts as a bone morphogenetic protein coreceptor by facilitating ligand-induced receptor hetero-oligomerization. Mol Biol Cell 21: 4028–4041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. 1997. Proximal-distal axis formation in the Drosophila leg. Nature 388: 139–145. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Brook W, Ng M, Calleja M, Sun H, Cohen S. 1996. Two distinct mechanisms for long-range patterning by Decapentaplegic in the Drosophila wing. Nature 381: 387–393. [DOI] [PubMed] [Google Scholar]
- Lengil T, Gancz D, Gilboa L. 2015. Activin signaling balances proliferation and differentiation of ovarian niche precursors and enables adjustment of niche numbers. Development 142: 883–892. [DOI] [PubMed] [Google Scholar]
- Li MO, Wan YY, Sanjabi S, Robertson AKL, Flavell RA. 2006. Transforming growth factor-β regulation of immune responses. Annu Rev Immunol 24: 99–146. [DOI] [PubMed] [Google Scholar]
- Li L, Shen JJ, Bournat JC, Huang L, Chattopadhyay A, Li Z, Shaw C, Graham BH, Brown CW. 2009. Activin signaling: Effects on body composition and mitochondrial energy metabolism. Endocrinology 150: 3521–3529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Qi Y, Jasper H. 2013a. Dpp signaling determines regional stem cell identity in the regenerating adult Drosophila gastrointestinal tract. Cell Rep 4: 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Zhang Y, Han L, Shi L, Lin X. 2013b. Trachea-derived dpp controls adult midgut homeostasis in Drosophila. Dev Cell 24: 133–143. [DOI] [PubMed] [Google Scholar]
- Lin H. 2002. The stem-cell niche theory: Lessons from flies. Nat Rev Genet 3: 931–940. [DOI] [PubMed] [Google Scholar]
- Lo PC, Frasch M. 1999. Sequence and expression of myoglianin, a novel Drosophila gene of the TGF-β superfamily. Mech Dev 86: 171–175. [DOI] [PubMed] [Google Scholar]
- Lynch JA, El-Sherif E, Brown SJ. 2012. Comparisons of the embryonic development of Drosophila, Nasonia, and Tribolium. Wiley Interdiscip Rev Dev Biol 1: 16–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marmion RA, Jevtic M, Springhorn A, Pyrowolakis G, Yakoby N. 2013. The Drosophila BMPRII, wishful thinking, is required for eggshell patterning. Dev Biol 375: 45–53. [DOI] [PubMed] [Google Scholar]
- Marques G, Musacchio M, Shimell MJ, Wunnenberg-Stapleton K, Cho KW, O’Connor MB. 1997. Production of a Dpp activity gradient in the early Drosophila embryo through the opposing actions of the SOG and TLD proteins. Cell 91: 417–426. [DOI] [PubMed] [Google Scholar]
- Marques G, Bao H, Haerry TE, Shimell MJ, Duchek P, Zhang B, O’Connor MB. 2002. The Drosophila BMP type II receptor Wishful Thinking regulates neuromuscular synapse morphology and function. Neuron 33: 529–543. [DOI] [PubMed] [Google Scholar]
- Martin FA, Perez-Garijo A, Moreno E, Morata G. 2004. The brinker gradient controls wing growth in Drosophila. Development 131: 4921–4930. [DOI] [PubMed] [Google Scholar]
- Martin-Castellanos C, Edgar BA. 2002. A characterization of the effects of Dpp signaling on cell growth and proliferation in the Drosophila wing. Development 129: 1003–1013. [DOI] [PubMed] [Google Scholar]
- Matsuda S, Shimmi O. 2012. Directional transport and active retention of Dpp/BMP create wing vein patterns in Drosophila. Dev Biol 366: 153–162. [DOI] [PubMed] [Google Scholar]
- Mattila J, Havula E, Suominen E, Teesalu M, Surakka I, Hynynen R, Kilpinen H, Vaananen J, Hovatta I, Kakela R, et al. 2015. Mondo-Mlx mediates organismal sugar sensing through the Gli-similar transcription factor Sugarbabe. Cell Rep 13: 350–364. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Marques G, Haghighi AP, Fetter RD, Crotty ML, Haerry TE, Goodman CS, O’Connor MB. 2003. The BMP homolog Gbb provides a retrograde signal that regulates synaptic growth at the Drosophila neuromuscular junction. Neuron 39: 241–254. [DOI] [PubMed] [Google Scholar]
- McCabe BD, Hom S, Aberle H, Fetter RD, Marques G, Haerry TE, Wan H, O’Connor MB, Goodman CS, Haghighi AP. 2004. Highwire regulates presynaptic BMP signaling essential for synaptic growth. Neuron 41: 891–905. [DOI] [PubMed] [Google Scholar]
- Micchelli CA, Perrimon N. 2006. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature 439: 475–479. [DOI] [PubMed] [Google Scholar]
- Milan M, Campuzano S, Garcia-Bellido A. 1996. Cell cycling and patterned cell proliferation in the wing primordium of Drosophila. Proc Natl Acad Sci 93: 640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles WO, Jaffray E, Campbell SG, Takeda S, Bayston LJ, Basu SP, Li M, Raftery LA, Ashe MP, Hay RT, et al. 2008. Medea SUMOylation restricts the signaling range of the Dpp morphogen in the Drosophila embryo. Genes Dev 22: 2578–2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami M, Kinoshita N, Kamoshida Y, Tanimoto H, Tabata T. 1999. brinker is a target of Dpp in Drosophila that negatively regulates Dpp-dependent genes. Nature 398: 242–246. [DOI] [PubMed] [Google Scholar]
- Mizutani CM, Nie Q, Wan FY, Zhang YT, Vilmos P, Sousa-Neves R, Bier E, Marsh JL, Lander AD. 2005. Formation of the BMP activity gradient in the Drosophila embryo. Dev Cell 8: 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno E, Yan M, Basler K. 2002. Evolution of TNF signaling mechanisms: JNK-dependent apoptosis triggered by Eiger, the Drosophila homolog of the TNF superfamily. Curr Biol 12: 1263–1268. [DOI] [PubMed] [Google Scholar]
- Musselman LP, Fink JL, Narzinski K, Ramachandran PV, Hathiramani SS, Cagan RL, Baranski TJ. 2011. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis Model Mech 4: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myat MM, Lightfoot H, Wang P, Andrew DJ. 2005. A molecular link between FGF and Dpp signaling in branch-specific migration of the Drosophila trachea. Dev Biol 281: 38–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na J, Musselman LP, Pendse J, Baranski TJ, Bodmer R, Ocorr K, Cagan R. 2013. A Drosophila model of high sugar diet-induced cardiomyopathy. PLoS Genet 9: e1003175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M, Kim S, Paik SK, Lee M, Lee S, Lee ZH, Kim J, Lee D, Bae YC. 2010a. dCIP4 (Drosophila Cdc42-interacting protein 4) restrains synaptic growth by inhibiting the secretion of the retrograde Glass bottom boat signal. J Neurosci 30: 8138–8150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nahm M, Long AA, Paik SK, Kim S, Bae YC, Broadie K, Lee S. 2010b. The Cdc42-selective GAP rich regulates postsynaptic development and retrograde BMP transsynaptic signaling. J Cell Biol 191: 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagoshi H, Hoshi M, Nabeshima Y, Matsuzaki F. 1998. A novel homeobox gene mediates the Dpp signal to establish functional specificity within target cells. Genes Dev 12: 2724–2734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellen D, Burke R, Struhl G, Basler K. 1996. Direct and long-range action of a Dpp morphogen gradient. Cell 85: 357–368. [DOI] [PubMed] [Google Scholar]
- Ng J. 2008. TGFβ signals regulate axonal development through distinct Smad-independent mechanisms. Development 135: 4025–4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M, Park S, Marques G, Arora K. 1998. Interpretation of a BMP activity gradient in Drosophila embryos depends on synergistic signaling by two type I receptors, SAX and TKV. Cell 95: 495–506. [DOI] [PubMed] [Google Scholar]
- Norman M, Vuilleumier R, Springhorn A, Gawlik J, Pyrowolakis G. 2016. Pentagone internalises glypicans to fine-tune multiple signalling pathways. eLife 5: e13301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslein-Volhard C, Wieschaus E, Kluding H. 1984. Mutations affecting the pattern of the larval cuticle in Drosophila melanogaster. I: Zygotic loci on the second chromosome. Wilhelm Roux’s Arch Dev Biol 193: 267–282. [DOI] [PubMed] [Google Scholar]
- O’Connor MB, Umulis D, Othmer HG, Blair SS. 2006. Shaping BMP morphogen gradients in the Drosophila embryo and pupal wing. Development 133: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor-Giles KM, Ho LL, Ganetzky B. 2008. Nervous wreck interacts with thickveins and the endocytic machinery to attenuate retrograde BMP signaling during synaptic growth. Neuron 58: 507–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogiso Y, Tsuneizumi K, Masuda N, Sato M, Tabata T. 2011. Robustness of the Dpp morphogen activity gradient depends on negative feedback regulation by the inhibitory Smad, Dad. Dev Growth Differ 53: 668–678. [DOI] [PubMed] [Google Scholar]
- Oh H, Irvine KD. 2010. Yorkie: The final destination of Hippo signaling. Trends Cell Biol 20: 410–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H, Irvine KD. 2011. Cooperative regulation of growth by Yorkie and Mad through bantam. Dev Cell 20: 109–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlstein B, Spradling A. 2006. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature 439: 470–474. [DOI] [PubMed] [Google Scholar]
- Padgett RW, St Johnston RD, Gelbart WM. 1987. A transcript from a Drosophila pattern gene predicts a protein homologous to the transforming growth factor-β family. Nature 325: 81–84. [DOI] [PubMed] [Google Scholar]
- Padgett RW, Wozney JM, Gelbart WM. 1993. Human BMP sequences can confer normal dorsal-ventral patterning in the Drosophila embryo. Proc Natl Acad Sci 90: 2905–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker L, Ellis JE, Nguyen MQ, Arora K. 2006. The divergent TGF-β ligand Dawdle utilizes an activin pathway to influence axon guidance in Drosophila. Development 133: 4981–4991. [DOI] [PubMed] [Google Scholar]
- Peluso CE, Umulis D, Kim YJ, O’Connor MB, Serpe M. 2011. Shaping BMP morphogen gradients through enzyme–substrate interactions. Dev Cell 21: 375–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pentek J, Parker L, Wu A, Arora K. 2009. Follistatin preferentially antagonizes activin rather than BMP signaling in Drosophila. Genesis 47: 261–273. [DOI] [PubMed] [Google Scholar]
- Pera EM, Ikeda A, Eivers E, De Robertis EM. 2003. Integration of IGF, FGF, and anti-BMP signals via Smad1 phosphorylation in neural induction. Genes Dev 17: 3023–3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AJ, O’Connor MB. 2013. Activin receptor inhibition by Smad2 regulates Drosophila wing disc patterning through BMP-response elements. Development 140: 649–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson AJ, Jensen PA, Shimell M, Stefancsik R, Wijayatonge R, Herder R, Raftery LA, O’Connor MB. 2012. R-Smad competition controls activin receptor output in Drosophila. PLoS ONE 7: e36548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccioli ZD, Littleton JT. 2014. Retrograde BMP signaling modulates rapid activity-dependent synaptic growth via presynaptic LIM kinase regulation of cofilin. J Neurosci 34: 4371–4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quijano JC, Stinchfield MJ, Newfeld SJ. 2011. Wg signaling via Zw3 and mad restricts self-renewal of sensory organ precursor cells in Drosophila. Genetics 189: 809–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston A, Blair SS. 2005. Long-range Dpp signaling is regulated to restrict BMP signaling to a crossvein competent zone. Dev Biol 280: 187–200. [DOI] [PubMed] [Google Scholar]
- Ramirez-Weber FA, Kornberg TB. 1999. Cytonemes: Cellular processes that project to the principal signaling center in Drosophila imaginal discs. Cell 97: 599–607. [DOI] [PubMed] [Google Scholar]
- Rawson JM, Lee M, Kennedy EL, Selleck SB. 2003. Drosophila neuromuscular synapse assembly and function require the TGF-β type I receptor saxophone and the transcription factor Mad. J Neurobiol 55: 134–150. [DOI] [PubMed] [Google Scholar]
- Ray RP, Wharton KA. 2001. Context-dependent relationships between the BMPs gbb and dpp during development of the Drosophila wing imaginal disk. Development 128: 3913–3925. [DOI] [PubMed] [Google Scholar]
- Rentzsch F, Zhang J, Kramer C, Sebald W, Hammerschmidt M. 2006. Crossveinless 2 is an essential positive feedback regulator of Bmp signaling during zebrafish gastrulation. Development 133: 801–811. [DOI] [PubMed] [Google Scholar]
- Restrepo S, Zartman JJ, Basler K. 2014. Coordination of patterning and growth by the morphogen DPP. Curr Biol 24: R245–255. [DOI] [PubMed] [Google Scholar]
- Ross JJ, Shimmi O, Vilmos P, Petryk A, Kim H, Gaudenz K, Hermanson S, Ekker SC, O’Connor MB, Marsh JL. 2001. Twisted gastrulation is a conserved extracellular BMP antagonist. Nature 410: 479–483. [DOI] [PubMed] [Google Scholar]
- Roy S, Kornberg TB. 2011. Direct delivery mechanisms of morphogen dispersion. Sci Signal 4: pt8. [DOI] [PubMed] [Google Scholar]
- Roy S, Huang H, Liu S, Kornberg TB. 2014. Cytoneme-mediated contact-dependent transport of the Drosophila decapentaplegic signaling protein. Science 343: 1244624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, Rashka KE, Doctor JS, Tucker RF, Hoffmann FM. 1993. Drosophila transforming growth factor β superfamily proteins induce endochondral bone formation in mammals. Proc Natl Acad Sci 90: 6004–6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez L, Guerrero I. 2001. The development of the Drosophila genital disc. BioEssays 23: 698–707. [DOI] [PubMed] [Google Scholar]
- Sánchez L, Gorfinkiel N, Guerrero I. 2001. Sex determination genes control the development of the Drosophila genital disc, modulating the response to Hedgehog, Wingless and Decapentaplegic signals. Development 128: 1033–1043. [DOI] [PubMed] [Google Scholar]
- Sander V, Eivers E, Choi RH, De Robertis EM. 2010. Drosophila Smad2 opposes Mad signaling during wing vein development. PLoS ONE 5: e10383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawala A, Sutcliffe C, Ashe HL. 2012. Multistep molecular mechanism for bone morphogenetic protein extracellular transport in the Drosophila embryo. Proc Natl Acad Sci 109: 11222–11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz C. 2004. A misexpression screen reveals effects of bag-of-marbles and TGF class signaling on the Drosophila male germ-line stem cell lineage. Genetics 167: 707–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Restrepo S, Basler K. 2008. Growth regulation by Dpp: An essential role for Brinker and a non-essential role for graded signaling levels. Development 135: 4003–4013. [DOI] [PubMed] [Google Scholar]
- Schwank G, Dalessi S, Yang SF, Yagi R, de Lachapelle AM, Affolter M, Bergmann S, Basler K. 2011. Formation of the long range Dpp morphogen gradient. PLoS Biol 9: e1001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwank G, Yang SF, Restrepo S, Basler K. 2012. Comment on “Dynamics of dpp signaling and proliferation control.” Science 335: 401. [DOI] [PubMed] [Google Scholar]
- Serpe M, O’Connor MB. 2006. The metalloprotease Tolloid-related and its TGF-β-like substrate Dawdle regulate Drosophila motoneuron axon guidance. Development 133: 4969–4979. [DOI] [PubMed] [Google Scholar]
- Serpe M, Umulis D, Ralston A, Chen J, Olson DJ, Avanesov A, Othmer H, O’Connor MB, Blair SS. 2008. The BMP-binding protein Crossveinless 2 is a short-range, concentration-dependent, biphasic modulator of BMP signaling in Drosophila. Dev Cell 14: 940–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimell MJ, Ferguson EL, Childs SR, O’Connor MB. 1991. The Drosophila dorsal-ventral patterning gene tolloid is related to human bone morphogenetic protein 1. Cell 67: 469–481. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Ralston A, Blair SS, O’Connor MB. 2005a. The crossveinless gene encodes a new member of the Twisted gastrulation family of BMP-binding proteins which, with Short gastrulation, promotes BMP signaling in the crossveins of the Drosophila wing. Dev Biol 282: 70–83. [DOI] [PubMed] [Google Scholar]
- Shimmi O, Umulis D, Othmer H, O’Connor MB. 2005b. Facilitated transport of a Dpp/Scw heterodimer by Sog/Tsg leads to robust patterning of the Drosophila blastoderm embryo. Cell 120: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shivdasani AA, Ingham PW. 2003. Regulation of stem cell maintenance and transit amplifying cell proliferation by TGF-β signaling in Drosophila spermatogenesis. Curr Biol 13: 2065–2072. [DOI] [PubMed] [Google Scholar]
- Song X, Wong MD, Kawase E, Xi R, Ding BC, McCarthy JJ, Xie T. 2004. Bmp signals from niche cells directly repress transcription of a differentiation-promoting gene, bag of marbles, in germline stem cells in the Drosophila ovary. Development 131: 1353–1364. [DOI] [PubMed] [Google Scholar]
- Sopory S, Kwon S, Wehrli M, Christian JL. 2010. Regulation of Dpp activity by tissue-specific cleavage of an upstream site within the prodomain. Dev Biol 346: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer FA, Hoffmann FM, Gelbart WM. 1982. Decapentaplegic: A gene complex affecting morphogenesis in Drosophila melanogaster. Cell 28: 451–461. [DOI] [PubMed] [Google Scholar]
- Staehling-Hampton K, Hoffmann FM. 1994. Ectopic decapentaplegic in the Drosophila midgut alters the expression of five homeotic genes, dpp, and wingless, causing specific morphological defects. Dev Biol 164: 502–512. [DOI] [PubMed] [Google Scholar]
- Stinchfield MJ, Takaesu NT, Quijano JC, Castillo AM, Tiusanen N, Shimmi O, Enzo E, Dupont S, Piccolo S, Newfeld SJ. 2012. Fat facets deubiquitylation of Medea/Smad4 modulates interpretation of a Dpp morphogen gradient. Development 139: 2721–2729. [DOI] [PubMed] [Google Scholar]
- Sulkowski M, Kim YJ, Serpe M. 2014. Postsynaptic glutamate receptors regulate local BMP signaling at the Drosophila neuromuscular junction. Development 141: 436–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MJ, Han TH, Ott C, Wang Q, Verheyen EM, Lippincott-Schwartz J, Serpe M. 2016. A novel, noncanonical BMP pathway modulates synapse maturation at the Drosophila neuromuscular junction. PLoS Genet 12: e1005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney ST, Davis GW. 2002. Unrestricted synaptic growth in spinster—A late endosomal protein implicated in TGF-β-mediated synaptic growth regulation. Neuron 36: 403–416. [DOI] [PubMed] [Google Scholar]
- Szuts D, Bienz M. 2000. An autoregulatory function of Dfos during Drosophila endoderm induction. Mech Dev 98: 71–76. [DOI] [PubMed] [Google Scholar]
- Takaesu NT, Bulanin DS, Johnson AN, Orenic TV, Newfeld SJ. 2008. A combinatorial enhancer recognized by Mad, TCF and Brinker first activates then represses dpp expression in the posterior spiracles of Drosophila. Dev Biol 313: 829–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teleman AA, Cohen SM. 2000. Dpp gradient formation in the Drosophila wing imaginal disc. Cell 103: 971–980. [DOI] [PubMed] [Google Scholar]
- Tian A, Jiang J. 2014. Intestinal epithelium-derived BMP controls stem cell self-renewal in Drosophila adult midgut. eLife 3: e01857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, Herman T, Yonekura S, Gao S, Wang J, Serpe M, O’Connor MB, Zipursky SL, Lee CH. 2007. Tiling of r7 axons in the Drosophila visual system is mediated both by transduction of an activin signal to the nucleus and by mutual repulsion. Neuron 56: 793–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting CY, McQueen Philip G, Pandya N, Lin TY, Yang M, Reddy OV, O’Connor Michael B, McAuliffe M, Lee CH. 2014. Photoreceptor-derived activin promotes dendritic termination and restricts the receptive fields of first-order Interneurons in Drosophila. Neuron 81: 830–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuneizumi K, Nakayama T, Kamoshida Y, Kornberg TB, Christian JL, Tabata T. 1997. Daughters against dpp modulates dpp organizing activity in Drosophila wing development. Nature 389: 627–631. [DOI] [PubMed] [Google Scholar]
- Umulis DM, Serpe M, O’Connor MB, Othmer HG. 2006. Robust, bistable patterning of the dorsal surface of the Drosophila embryo. Proc Natl Acad Sci 103: 11613–11618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umulis D, O’Connor MB, Blair SS. 2009. The extracellular regulation of bone morphogenetic protein signaling. Development 136: 3715–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umulis DM, Shimmi O, O’Connor MB, Othmer HG. 2010. Organism-scale modeling of early Drosophila patterning via bone morphogenetic proteins. Dev Cell 18: 260–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent S, Ruberte E, Grieder NC, Chen CK, Haerry T, Schuh R, Affolter M. 1997. DPP controls tracheal cell migration along the dorsoventral body axis of the Drosophila embryo. Development 124: 2741–2750. [DOI] [PubMed] [Google Scholar]
- Vuilleumier R, Springhorn A, Patterson L, Koidl S, Hammerschmidt M, Affolter M, Pyrowolakis G. 2010. Control of Dpp morphogen signalling by a secreted feedback regulator. Nature 12: 611–617. [DOI] [PubMed] [Google Scholar]
- Wang YC, Ferguson EL. 2005. Spatial bistability of Dpp-receptor interactions during Drosophila dorsal-ventral patterning. Nature 434: 229–234. [DOI] [PubMed] [Google Scholar]
- Wang X, Shaw WR, Tsang HT, Reid E, O’Kane CJ. 2007. Drosophila spichthyin inhibits BMP signaling and regulates synaptic growth and axonal microtubules. Nat Neurosci 10: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Harris RE, Bayston LJ, Ashe HL. 2008. Type IV collagens regulate BMP signalling in Drosophila. Nature 455: 72–77. [DOI] [PubMed] [Google Scholar]
- Wang RN, Green J, Wang Z, Deng Y, Qiao M, Peabody M, Zhang Q, Ye J, Yan Z, Denduluri S, et al. 2014. Bone morphogenetic protein (BMP) signaling in development and human diseases. Genes Dis 1: 87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Julicher F, Gonzalez-Gaitan M. 2011a. Understanding morphogenetic growth control—Lessons from flies. Nat Rev Mol Cell Biol 12: 594–604. [DOI] [PubMed] [Google Scholar]
- Wartlick O, Mumcu P, Kicheva A, Bittig T, Seum C, Julicher F, Gonzalez-Gaitan M. 2011b. Dynamics of Dpp signaling and proliferation control. Science 331: 1154–1159. [DOI] [PubMed] [Google Scholar]
- Weiss A, Charbonnier E, Ellertsdóttir E, Tsirigos A, Wolf C, Schuh R, Pyrowolakis G, Affolter M. 2010. A conserved activation element in BMP signaling during Drosophila development. Nat Struct Mol Biol 17: 69–76. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Ray RP, Gelbart WM. 1993. An activity gradient of decapentaplegic is necessary for the specification of dorsal pattern elements in the Drosophila embryo. Development 117: 807–822. [DOI] [PubMed] [Google Scholar]
- Wharton KA, Cook JM, Torres-Schumann S, de Castro K, Borod E, Phillips DA. 1999. Genetic analysis of the bone morphogenetic protein-related gene, gbb, identifies multiple requirements during Drosophila development. Genetics 152: 629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Nusslein-Volhard C, Kluding H. 1984. Krüppel, a gene whose activity is required early in the zygotic genome for normal embryonic segmentation. Dev Biol 104: 172–186. [DOI] [PubMed] [Google Scholar]
- Winstanley J, Sawala A, Baldock C, Ashe HL. 2015. Synthetic enzyme-substrate tethering obviates the Tolloid-ECM interaction during Drosophila BMP gradient formation. eLife 4: e05508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman NM, McPherron AC, Pappano WN, Davies MV, Song K, Tomkinson KN, Wright JF, Zhao L, Sebald SM, Greenspan DS, et al. 2003. Activation of latent myostatin by the BMP-1/tolloid family of metalloproteinases. Proc Natl Acad Sci 100: 15842–15846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu MY, Hill CS. 2009. Tgf-β superfamily signaling in embryonic development and homeostasis. Dev Cell 16: 329–343. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. 1998. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell 94: 251–260. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling A. 2000. A niche maintaining germ line stem cells in the Drosophila ovary. Science 290: 328–330. [DOI] [PubMed] [Google Scholar]
- Yakoby N, Bristow CA, Gong D, Schafer X, Lembong J, Zartman JJ, Halfon MS, Schüpbach T, Shvartsman SY. 2008a. A combinatorial code for pattern formation in Drosophila oogenesis. Dev Cell 15: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakoby N, Lembong J, Schüpbach T, Shvartsman SY. 2008b. Drosophila eggshell is patterned by sequential action of feedforward and feedback loops. Development 135: 343–351. [DOI] [PubMed] [Google Scholar]
- Yamashita YM, Fuller MT, Jones DL. 2005. Signaling in stem cell niches: Lessons from the Drosophila germline. J Cell Sci 118: 665–672. [DOI] [PubMed] [Google Scholar]
- Yu XM, Gutman I, Mosca TJ, Iram T, Özkan E, Garcia KC, Luo L, Schuldiner O. 2013. Plum, an immunoglobulin superfamily protein, regulates axon pruning by facilitating TGF-β signaling. Neuron 78: 456–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi B, Shen W, Xu X, Chen X, Mahey M, Harden N. 2008. Leading edge-secreted Dpp cooperates with ACK-dependent signaling from the amnioserosa to regulate myosin levels during dorsal closure. Dev Dyn 237: 2936–2946. [DOI] [PubMed] [Google Scholar]
- Zamani N, Brown CW. 2011. Emerging roles for the transforming growth factor-β superfamily in regulating adiposity and energy expenditure. Endocr Rev 32: 387–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zecca M, Basler K, Struhl G. 1995. Sequential organizing activities of engrailed, hedgehog and decapentaplegic in the Drosophila wing. Development 121: 2265–2278. [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang J, Haerry TE, Wu AYH, Martin J, O’Connor MB, Lee C-HJ, Lee T. 2003. TGF-β signaling activates steroid hormone receptor expression during neuronal remodeling in the Drosophila brain. Cell 112: 303–315. [DOI] [PubMed] [Google Scholar]
- Zhou S, Lo WC, Suhalim JL, Digman Ma, Gratton E, Nie Q, Lander AD. 2012. Free extracellular diffusion creates the Dpp morphogen gradient of the Drosophila wing disc. Curr Biol 22: 668–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu CC, Boone JQ, Jensen PA, Hanna S, Podemski L, Locke J, Doe CQ, O’Connor MB. 2008. Drosophila Activin-β and the activin-like product Dawdle function redundantly to regulate proliferation in the larval brain. Development 135: 513–521. [DOI] [PubMed] [Google Scholar]