Abstract
In the current study, we aim to measure T1rho (T1ρ) in the hippocampus in the brain of control, Alzheimer’s disease (AD), Parkinson’s disease (PD), and PD patients with dementia (PDD), and to determine efficacy of T1ρ in differentiating these cohorts. With informed consent, 53 AD patients, 62 PD patients, 11 PDD patients, and 46 age-matched controls underwent a standardized clinical assessment including mini-mental state examination (MMSE) and brain T1ρ MRI on a 1.5-T clinical-scanner. T1ρ maps were generated by fitting each pixel’s intensity as a function of the spin-lock pulse duration. In control, AD, PD and PDD, mean ± SE T1ρ values in the right hippocampus (RH) were 92.15 ± 2.00, 99.65 ± 1.98, 85.68 ± 1.87, 102.47 ± 4.66 ms while in the left hippocampus (LH) these values were 90.16 ± 1.82, 99.53 ± 1.91, 84.33 ± 2.03, 95.33 ± 4.64 ms. Significant difference for both RH and LH T1ρ across the groups (p < 0.001) was observed. Both RH and LH T1ρ were significantly increased in AD compared to control (p = 0.034, p = 0.001) and PD (p < 0.001, p < 0.001). In control, both RH and LH T1ρ values were significantly increased compared to PD (p = 0.031, p = 0.027) while compared to PDD only the RH T1ρ value was significantly decreased (p = 0.043). Both RH and LH T1ρ values in PD were significantly lower than PDD (p = 0.004, p = 0.032). No significant correlation between the T1ρ and age as well as between T1ρ and MMSE scores was observed. The serial measurement of T1ρ in both AD and PD may provide the nature of disease progression and may contribute to their early diagnosis.
Keywords: T1rho MRI, Hippocampus, Alzheimer’s disease, Parkinson disease, Parkinson disease with dementia
Introduction
Alzheimer’s disease (AD) accounts for 50–60% of cases of dementia in the elderly [21]. Parkinson’s disease (PD) is associated with the loss of dopaminergic neurons in the substantia nigra. Dementia is common and affects approximately 40% of PD patients during the course of the disease, the risk for the development of dementia in PD being six times higher than in non-PD age-matched controls [23].
Early and accurate diagnosis of both AD and PD are crucial for the patient’s treatment. However, the diagnosis of both diseases is difficult in elderly patients because some of the key symptoms also may be manifestations of normal aging in both cases. Structural neuroimaging with MRI has the potential in the early diagnosis of both AD and PD [22, 28]. It has been shown that both AD and PD individuals showed high medial temporal lobe (MTL) atrophy compared to controls [29]. However, the contribution from normal age-related atrophy cannot easily be ruled out. Different neuroimaging studies have been proposed in the last few years to better characterize different structural or functional patterns that distinguish PD and PD patients with dementia (PDD) [6, 13]. A non-significant difference for MTL atrophy (MTA) between PD and PDD has been shown earlier [29].
Voxel based morphometry (VBM) has been used to measure the changes in brain volume in different neurodegenerative diseases [6, 13, 26]. However, VBM is not without its limitations. Spatial normalization is used to map each image to a template image in standard space, which has the potential to introduce errors since changes in brain volume are only on the order of a few percent. Although VBM detects atrophy of the hippocampus, these results may be insensitive to the detection of subtle changes/atrophy in areas of high variance, especially in small structures such as the hippocampus, resulting in a high variability among the elderly and diseased groups.
Functional imaging studies, such as single photon emission computed tomography (SPECT), positron emission tomography (PET), magnetic resonance spectroscopy, and diffusion tensor imaging have been proposed in studying AD, PD and PDD [8, 11, 12, 15–17, 27]. It has been demonstrated that SPECT may not be a useful tool in the clinical differential diagnosis among PD, PDD and lewy body dementia and poor signal-to-noise ratios of the MRI techniques ultimately limits their sensitivity and reproducibility.
We have previously demonstrated that an alternate MRI contrast mechanism, T1ρ (T1rho), which is the spin lattice relaxation time constant in the rotating frame, can distinguish between AD and age-matched controls [5, 10]. In biological tissues, T1ρ relaxation may have contributions from several interactions. The interactions that are studied using this methodology can be broadly categorized into (1) dipole-dipole, (2) chemical exchange and (3) scalar-coupling processes [4]. Depending upon the tissue type, more than one mechanism may be operative simultaneously but with different relative contributions. In biological tissues, frequency dependence of relaxation rates or relaxation dispersion may arise from (1) rotational motion of a fraction of water bound to proteins, (2) exchange of protons on macromolecules with bulk water and (3) the non-averaged residual dipolar interaction of spin associated with oriented macromolecules in the tissue. T1ρ MRI has the capability to probe protein content in various tissues [14] and has been utilized to delineate brain tumors, characterize breast cancer tissue, and monitor the level of cartilage degeneration [1, 24, 25].
The aim of the current study was to measure T1ρ in the hippocampus in the brain of control, AD, PD, and PDD patients, and to determine whether T1ρ values can be used in differentiating these cohorts.
Materials and methods
Patient selection
The Institutional Review Board of University of Pennsylvania approved the study protocols. In the current study, we included 53 AD patients (mean age ± SD = 77.5 ± 8.1 years), 62 PD patients (mean age ± SD = 70.5 ± 6.1 years) without dementia, 11 PDD patients (mean age ± SD = 76.2 ± 3.7 years), and 46 age-matched controls (mean age ± SD = 71.2 ± 9.8 years).
All patients underwent a standardized clinical assessment including medical history, physical and neurological examination, psychometric evaluation, and brain MRI. The mini-mental state examination (MMSE) was used as a measure of general cognitive function. Diagnoses were made in conference by a team made up of a neurologist, neuropsychologists, a neurophysiologist and a psychiatrist. Diagnoses was made according the National Institute of Neurological and Communicative Diseases and Stroke/Alzheimer’s Disease and Related Disorders Association criteria (NINCDS–ADRDA) for probable AD [19], the consensus criteria for PDD [18] and the UK Parkinson’s Disease Society Brain Bank criteria for Parkinson’s disease [9]. All Alzheimer’s disease subjects met the criteria for probable Alzheimer’s disease, while all Parkinson’s disease and PDD subjects met the clinical diagnostic criteria for Parkinson’s disease. Patients were excluded if they had a history of significant electrocardiographic (ECG) abnormalities; hematologic disorders; active malignancy within 5 years; or clinically important depressive, neuropsychiatric, cerebrovascular, or respiratory disease. Patients with hemorrhagic lesions as detected on MRI were also excluded from the study. The control group consisted of patients who presented to our memory clinic with subjective complaints, and underwent exactly the same diagnostic work-up as the AD and PD patients.
MRI protocol
Written informed consent was obtained from each patient before they underwent MRI. All volunteers underwent MRI on a 1.5-T Siemens Sonata clinical scanner using the vendor-supplied 8-channel head coil. For T1ρ MRI, a fluid-attenuated T1ρ pre-encoded Turbo Spin-Echo pulse sequence was used [2]. The imaging parameters were: TR/TE = 2,000/12 ms, TSL (duration of spin lock pulse) = 10, 20, 30, 40 ms, with a spin lock B1 amplitude of 500 Hz, slice thickness = 2 mm, FOV = 22 cm, matrix size = 256 × 128, bandwidth = 130 Hz/pixel, echo train length = 4 for a total imaging time of 6 min for four images. The inversion time (TI) was fixed at 860 ms to null the signal of CSF, removing the CSF contribution to T1ρ values. An oblique coronal T1ρ-weighted image of a slice perpendicular to the anterior/posterior commissure (AC/PC) plane was obtained. The slice was chosen to include the head of the hippocampus. Immediately after T1ρ MRI, the entire volume of each subject’s brain was imaged for image segmentation using a T1-weighted 3D volumetric MPRAGE pulse sequence with 124 continuous slices in the coronal plane. The parameters were TR/TE = 3,000 ms/3.5 ms, slice thickness = 1.2 mm, FOV of 24 cm and 192 phase encode steps, and flip angle = 8° for a total imaging time of 10 min.
Data processing
The images were transferred to a G4 PowerBook computer (Apple Corp., Cupertino, CA, USA) and images were processed in the IDL programming language (RSI Cor., Boulder, CO, USA). The signal expression for the T1ρ – weighted MRI is given by the Eq. 1, 2.
| (1) |
where M0 is the thermal equilibrium magnetization.
The T1ρ relaxation time constant is dependent on the amplitude of the spin lock (SL) field, which is reported as a frequency, and typically ranges from zero to a few kilohertz. Equation 1 was linearized and then used to generate T1ρ maps by fitting each pixel’s intensity as a function of TSL time using linear regression. T1ρ was calculated as −1/slope of the straight-line fit. T1ρ of pixels that fitted poorly (R2 < 0.95) were set to zero to remove background and noisy pixels.
Segmentation and automating reporting
The volumetric MPRAGE images were used to automatically report T1ρ values from the hippocampus by a custom written software described before ([5] Neuroimage). Briefly, the volumetric MPRAGE images were used to segment the brain into 92 anatomical structures [5] incorporating all major cortical and sub-cortical regions and coregistered with a standardized template [7]. The template’s labels are then transformed to individual scans by applying the elastic transformation that was found to co-register with the respective T1ρ images. A program written in Matlab was used to automatically record T1ρ values from the right hippocampus (RH) and left hippocampus (LH) onto an excel spreadsheet. The co-registration and the hippocampus segmentation were performed in the presence of an experienced neuroradiologist.
Statistical analyses
Descriptive statistics were performed to calculate the mean value of T1ρ in the RH and LH for different cohorts (control, AD, PD and PDD). Kruskal-Wallis ANOVA was performed followed by post hoc Mann–Whitney U test to compare the T1ρ values among the different cohorts (control, AD, PD and PDD). The Student’s t test was performed between RH and LH T1ρ values for each cohort. Pearson correlations between T1ρ values versus age and between T1ρ versus MMSE score were performed separately for each cohort. A p value of less than 0.05 was considered to be statistically significant. All the statistical computations were performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, USA).
Results
The mean MMSE score in control, AD, PD and PDD were 29.1 ± 5.8, 19.53 ± 5.8, 27.4 ± 3.7, 21.9 ± 3.8, respectively. The average RH and LH T1ρ values in the brain of control, AD, PD and PDD are reported in Table 1. Kruskal–Wallis ANOVA showed significant difference for both RH and LH T1ρ across the four groups (p < 0.001). The Mann–Whitney test showed that both RH and LH T1ρ values were significantly increased in AD compared to control (p = 0.034, p = 0.001) and PD (p < 0.001, p < 0.001) while no significant difference was observed on comparing to PDD (p = 0.539, p = 0.471). In PD, both the RH and LH T1ρ values were significantly decreased relative to controls (p = 0.031, p = 0.027) and PDD (p = 0.004, p = 0.032). Only the RH T1ρ value was significantly decreased (p = 0.043) in controls compared to PDD.
Table 1.
The mean (±SD) T1ρ value in hippocampus in the brain of the different cohorts
| Group | Hippocampus T1ρ
|
|
|---|---|---|
| Right | Left | |
| Control | 92.15 ± 2.00 | 90.16 ± 1.82 |
| AD | 99.65 ± 1.98 | 99.53 ± 1.91 |
| PD | 85.68 ± 1.87 | 84.33 ± 2.03 |
| PDD | 102.47 ± 4.66 | 95.33 ± 4.64 |
| Kruskal–Wallis ANOVA (p value) | <0.001 | <0.001 |
The Kruskal–Wallis one way analysis of variance is showing significant difference for T1ρ across the cohorts
AD Alzheimer’s disease, PD Parkinson’s disease, PDD Parkinson’s disease with dementia
In AD, the LH T1ρ value was 10% greater than control, 18% greater than PD and 4% greater than PDD while the RH T1ρ was 8% greater than control, 16% greater than PD and 3% less than PDD. In control, the LH T1ρ was 7% greater than PD and 6% less than PDD while the RH T1ρ was 7% greater than PD and 10% less than PDD. In PDD the LH T1ρ was 13% greater than PD while the RH T1ρ was 20% greater than PD.
In all the cohorts the RH T1ρ value was higher than the LH T1ρ value. In none of the cohorts did this difference reach to the statistically significant level. In control, AD, PD and PDD the RH T1ρ was 2, 0.1, 2, and 8% greater than LH T1ρ.
Figure 1 shows T1ρ maps overlaid on fluid-attenuated T1ρ MR images. Pixels with higher T1ρ value in hippocampus in the brain of AD and PDD individuals are apparent from T1ρ maps (Fig. 1). Figure 2 represents box and whisker plots of T1ρ in different cohorts. In the right hippocampus two outliers are found in the case of AD cohort.
Fig. 1.
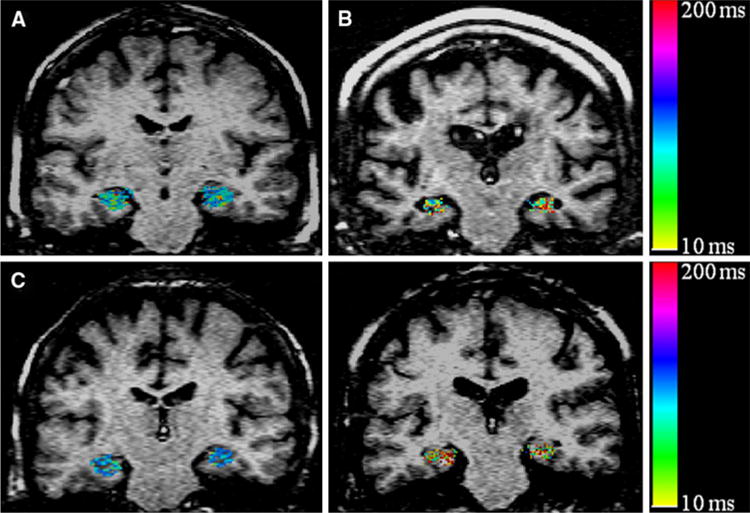
T1ρ maps of the hippocampus region in the brain (in color) overlaid on fluid-attenuated T1ρ MR image of control (77 Y male, a), AD (76 Y male, b), PD (71 Y female, c) and PDD patients (73 Y female, d). Pixels with higher T1ρ (red) are more prominent in the hippocampus of AD and PDD patients. The PD patient shows decrease hippocampus T1ρ. No T1ρ signal from CSF implies that the higher T1ρ values are not due to fluid. AD Alzheimer’s disease, PD Parkinson’s disease, PDD Parkinson’s disease with dementia
Fig. 2.
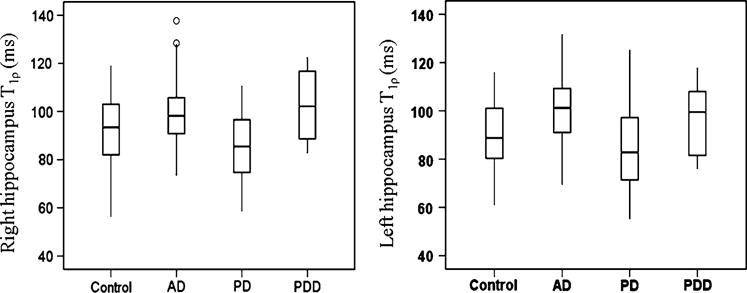
The box and whisker plots show the T1ρ value in right and left hippocampus in the brain of control, AD, PD and PDD cohorts. The right hippocampus T1ρ plot also shows the two outliers (small circle) in case of AD. AD Alzheimer’s disease, PD Parkinson’s disease, PDD Parkinson’s disease with dementia
We analyzed the gender related changes in T1ρ in different cohorts (control, AD, and PD). In all cohorts, both the right and left hippocampus T1ρ values were higher in female compared to the male. However, in none of the cohorts T1ρ difference reach to the statistical significant level. No significant correlation between the T1ρ and age as well as between T1ρ and MMSE scores was observed.
Discussion
In the current study, we found significantly increased T1ρ in the hippocampus in the brain of AD and PDD compared to control and PD. The PD individuals showed significantly decreased T1ρ value compared to controls. Also, no significant difference between the RH T1ρ and LH T1ρ for any of the cohort was noted.
The feasibility of T1ρ in the detection of plaques burden in the mice model of AD has been already reported [3]. Recently, Borthakur et al. [5] have shown increased T1ρ in MTL of AD compared to controls. Further, Haris et al. [10] showed the significantly increased T1ρ in the hippocampus of the MCI and AD compared to controls. All these studies suggested that the presence of AD pathology may contribute to the molecular interactions such as exchanging protons from bulk water with protons associated with slowly tumbling macromolecules in the extracellular space resulting in an increased T1ρ.
Earlier, increased T1ρ has been reported in substantia nigra in the brain of PD patients and suggested the possible role of neuronal loss in increased T1ρ [20]. However, in the current study, we found decreased hippocampus T1ρ in PD even compared to controls. Pathological studies of the brains of patients who died of Parkinson disease have demonstrated increased iron content in the substantia nigra. Because iron accumulation affects the MR signal, attempts have been made to monitor the changes in iron content in patients with Parkinson disease by using MR T2 relaxometry. Here, we suggest that the decreased T1ρ in PD is probably due to proton spin dephasing from iron-induced local field inhomogeneities resulting from the increased iron content in the substantia nigra. No attempt was made to quantify iron content in the hippocampus in the brain of PD and PDD patients; however, such study could further help in understanding the T1ρ mechanism. The Vymazal et al. [30] have shown non-significantly decreased T2 in the substantia nigra. In the current study, we found significantly decreased T1ρ in PD compared to controls suggestive of T1ρ is more sensitive than T2 in detection of the pathological changes. No T2 measurement was performed in the current study as the aim of this study was to define the T1ρ values in the different pathological conditions. However, T1ρ MRI has been previously used to measure T1ρ relaxation time in normal human brain, and showed higher range of values compared to T2.
Brain atrophy rate was significantly increased in PDD compared to PD and control, while no significant difference for brain atrophy was found between PD and control [6]. It has been also shown that in PDD, the degree of hippocampal atrophy may be similar to or even more severe than AD [13]. The number of AD changes has been shown to be higher in PDD patients compared to PD without dementia. Laakso et al. [13] reported that the absolute volume of the hippocampus in PDD was smaller than that of Alzheimer’s disease patients, although not significantly so, while the coexistence of Alzheimer’s disease pathology in the Parkinson’s disease group could not be ruled out. The PDD and AD groups also did not differ in the distribution of the MMSE score, which reflect global cognitive function. We suggest that the increased T1ρ in PDD may be associated with the increased atrophy and high AD related changes.
Our data suggest that bilateral hippocampus T1ρ changes in AD, PD and PDD compared to the control is consistent with the earlier findings which showed bilateral hippocampus volume loss in AD and PDD. No significant correlation was observed between T1ρ and age in all cohorts suggesting that the change in T1ρ is due to the underlying pathophysiology instead of any age related changes. Further, no significant correlation between MMSE score and T1ρ suggests that cognitive score may not enough sensitive to the tissue pathology.
In our study, as in many of the previous imaging studies, there was considerable overlap between patient groups. We believe this overlap is due to the large variation in the T1ρ values in PDD individuals as also depicted by the graphs. The variation in T1ρ in PDD groups might be due to the small sample size. We did not correlate T1ρ with the atrophy rate as well as hippocampus volume, which may be considered the limitation of the current study. Another potential limitation of our study is the reliance on clinical, rather than autopsy proven, diagnoses.
The two-dimensional T1ρ-weighted MRI pulse sequence was used for the measurement of T1ρ which is limited to a single-slice acquisition since the SL pulse cannot be made slice selective. We have already developed and implemented a three-dimensional T1ρ-weighted pulse sequence on a 1.5-T clinical scanner [31]. T1ρ values measured with this sequence agree with those obtained by a previously validated two-dimensional T1ρ imaging sequence [2]. Furthermore, the 3D images will allow for direct comparison of T1ρ values and brain atrophy rates in several regions of the brain such as the entire hippocampus and entorhinal cortex.
In conclusion, measurement of T1ρ in both AD and PD may contribute to their early diagnosis in the future. Further, measurement of hippocampus T1ρ may help with the early diagnosis of dementia in PD, something that now requires a further investigation by serially monitoring PD patients. In the future, it may important to investigate regional rates of T1ρ changes and the role of serial T1ρ MRI in the assessment of treatments for slowing disease progression.
Acknowledgments
This work was performed at a NIH supported resource center (NIH RR02305) and from a grant from the Pennsylvania State Tobacco Settlement.
Contributor Information
Mohammad Haris, Department of Radiology, Center for Magnetic Resonance and Optical Imaging (CMROI), University of Pennsylvania, B1 Stellar-Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
Anup Singh, Department of Radiology, Center for Magnetic Resonance and Optical Imaging (CMROI), University of Pennsylvania, B1 Stellar-Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
Kejia Cai, Department of Radiology, Center for Magnetic Resonance and Optical Imaging (CMROI), University of Pennsylvania, B1 Stellar-Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
Christos Davatzikos, SBIA, University of Pennsylvania, Philadelphia, PA, USA.
John Q. Trojanowski, Department of Pathology and Lab Medicine, University of Pennsylvania, Philadelphia, PA, USA
Elias R. Melhem, Department of Radiology, University of Pennsylvania, Philadelphia, PA, USA
Christopher M. Clark, Department of Neurology, University of Pennsylvania, Philadelphia, PA, USA
Arijitt Borthakur, Department of Radiology, Center for Magnetic Resonance and Optical Imaging (CMROI), University of Pennsylvania, B1 Stellar-Chance Laboratories, 422 Curie Boulevard, Philadelphia, PA 19104-6100, USA.
References
- 1.Aronen HJ, Ramadan UA, Peltonen TK, Markkola AT, Tanttu JI, Jääskeläinen J, Häkkinen AM, Sepponen R. 3D spin-lock imaging of human gliomas. Magn Reson Imaging. 1999;17:1001–1010. doi: 10.1016/s0730-725x(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 2.Borthakur A, Wheaton AJ, Gougoutas AJ, Akella SV, Regatte RR, Charagundla SR, Reddy R. In vivo measurement of T1rho dispersion in the human brain at 1.5 Tesla. J Magn Reson Imaging. 2004;19:403–409. doi: 10.1002/jmri.20016. [DOI] [PubMed] [Google Scholar]
- 3.Borthakur A, Gur T, Wheaton AJ, Corbo M, Trojanowski JQ, Lee VM, Reddy R. In vivo measurement of plaque burden in a mouse model of Alzheimer’s disease. J Magn Reson Imaging. 2006;24:1011–1017. doi: 10.1002/jmri.20751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borthakur A, Mellon E, Niyogi S, Witschey W, Kneeland JB, Reddy R. Sodium and T1rho MRI for molecular and diagnostic imaging of articular cartilage. NMR Biomed. 2006;19:781–821. doi: 10.1002/nbm.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthakur A, Sochor M, Davatzikos C, Trojanowski JQ, Clark CM. T1rho MRI of Alzheimer’s disease. Neuroimage. 2008;41:1199–1205. doi: 10.1016/j.neuroimage.2008.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burton EJ, McKeith IG, Burn DJ, Williams ED, O’Brien JT. Cerebral atrophy in Parkinson’s disease with and without dementia: a comparison with Alzheimer’s disease, dementia with Lewy bodies and controls. Brain. 2004;127:791–800. doi: 10.1093/brain/awh088. [DOI] [PubMed] [Google Scholar]
- 7.Davatzikos C, Fan Y, Wu X, Shen D, Resnick SM. Detection of prodromal Alzheimer’s disease via pattern classification of magnetic resonance imaging. Neurobiol Aging. 2008;29:514–523. doi: 10.1016/j.neurobiolaging.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Firbank MJ, Harrison RM, O’Brien JT. A comprehensive review of proton magnetic resonance spectroscopy studies in dementia and Parkinson’s disease. Dement Geriatr Cogn Disord. 2002;14:64–76. doi: 10.1159/000064927. [DOI] [PubMed] [Google Scholar]
- 9.Gibb WRG, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haris M, McArdle E, Fenty M, Singh A, Davatzikos C, Trojanowski JQ, Melhem ER, Clark CM, Borthakur A. Early marker for Alzheimer’s disease: hippocampus T1rho estimation. J Magn Reson Imaging. 2009;29:1008–1012. doi: 10.1002/jmri.21735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ito K, Nagano-Saito A, Kato T, Arahata Y, Nakamura A, Kawasumi Y, Hatano K, Abe Y, Yamada T, Kachi T, Brooks DJ. Striatal and extrastriatal dysfunction in Parkinson’s disease with dementia: a 6-[18F]fluoro-L-dopa PET study. Brain. 2002;125:1358–1365. doi: 10.1093/brain/awf134. [DOI] [PubMed] [Google Scholar]
- 12.Johnson KA, Holman BL, Mueller SP, Rosen TJ, English R, Nagel JS, Growdon JH. Single photon emission computed tomography in Alzheimer’s disease. Abnormal iofetamine I 123 uptake reflects dementia severity. Arch Neurol. 1988;45:392–396. doi: 10.1001/archneur.1988.00520280038013. [DOI] [PubMed] [Google Scholar]
- 13.Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 14.Mäkelä HI, De Vita E, Gröhn OH, Kettunen MI, Kavec M, Lythgoe M, Garwood M, Ordidge R, Kauppinen RA. B0 dependence of the on-resonance longitudinal relaxation time in the rotating frame (T1rho) in protein phantoms and rat brain in vivo. Magn Reson Med. 2004;51:4–8. doi: 10.1002/mrm.10669. [DOI] [PubMed] [Google Scholar]
- 15.Matsui H, Udaka F, Miyoshi T, Hara N, Tamura A, Oda M, Kubori T, Nishinaka K, Kameyama M. N-isopropyl-p-123I iodoamphetamine single photon emission computed tomography study of Parkinson’s disease with dementia. Intern Med. 2005;44:1046–1050. doi: 10.2169/internalmedicine.44.1046. [DOI] [PubMed] [Google Scholar]
- 16.Matsui H, Nishinaka K, Oda M, Niikawa H, Kubori T, Udaka F. Dementia in Parkinson’s disease: diffusion tensor imaging. Acta Neurol Scand. 2007;116:177–181. doi: 10.1111/j.1600-0404.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- 17.McGeer PL, Kamo H, Harrop R, Li DK, Tuokko H, McGeer EG, Adam MJ, Ammann W, Beattie BL, Calne DB, et al. Positron emission tomography in patients with clinically diagnosed Alzheimer’s disease. CMAJ. 1986;134:597–607. [PMC free article] [PubMed] [Google Scholar]
- 18.McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 19.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of health and human services task force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 20.Michaeli S, Liimatainen T, Rydeen CE, Kotz CM, Nixon JP, Hanson T, Tuite PJ. T(1rho) and T(2rho) MRI in the evaluation of Parkinson’s disease. J Neurol. 2010 doi: 10.1007/s00415-009-5446-2. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med. 2003;348:1356–1364. doi: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 22.O’Brien J, Barber B. Neuroimaging in dementia and depression. Adv Psychiatr Treat. 2000;6:109–119. [Google Scholar]
- 23.Padovani A, Costanzi C, Gilberti N, Borroni B. Parkinson’s disease and dementia. Neurol Sci. 2006;1:S40–S43. doi: 10.1007/s10072-006-0546-6. [DOI] [PubMed] [Google Scholar]
- 24.Regatte RR, Akella SV, Wheaton AJ, Lech G, Borthakur A, Kneeland JB, Reddy R. 3D-T1rho-relaxation mapping of articular cartilage: in vivo assessment of early degenerative changes in symptomatic osteoarthritic subjects. Acad Radiol. 2004;11:741–749. doi: 10.1016/j.acra.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 25.Santyr GE. MR imaging of the breast imaging and tissue characterization without intravenous contrast. Magn Reson Imaging Clin N Am. 1994;2:673–690. [PubMed] [Google Scholar]
- 26.Senjem ML, Gunter JL, Shiung MM, Petersen RC, Jack CR., Jr Comparison of different methodological implementations of voxel-based morphometry in neurodegenerative disease. Neuroimage. 2005;26:600–608. doi: 10.1016/j.neuroimage.2005.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stebbins GT, Murphy CM. Diffusion tensor imaging in Alzheimer’s disease and mild cognitive impairment. Behav Neurol. 2009;21:39–49. doi: 10.3233/BEN-2009-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Summerfield C, Junqué C, Tolosa E, Salgado-Pineda P, Gómez-Ansón B, Martí MJ, Pastor P, Ramírez-Ruíz B, Mercader J. Structural brain changes in Parkinson disease with dementia: a voxel-based morphometry study. Arch Neurol. 2005;62:281–285. doi: 10.1001/archneur.62.2.281. [DOI] [PubMed] [Google Scholar]
- 29.Tam CW, Burton EJ, McKeith IG, Burn DJ, O’Brien JT. Temporal lobe atrophy on MRI in Parkinson disease with dementia: a comparison with Alzheimer disease and dementia with Lewy bodies. Neurology. 2005;64:861–865. doi: 10.1212/01.WNL.0000153070.82309.D4. [DOI] [PubMed] [Google Scholar]
- 30.Vymazal J, Righini A, Brooks RA, Canesi M, Mariani C, Leonardi M, Pezzoli G. T1 and T2 in the brain of healthy subjects, patients with Parkinson disease, and patients with multiple system atrophy: relation to iron content. Radiology. 1999;211:489–495. doi: 10.1148/radiology.211.2.r99ma53489. [DOI] [PubMed] [Google Scholar]
- 31.Witschey WR, Borthakur A, Elliott MA, Fenty M, Sochor MA, Wang C, Reddy R. T1rho-prepared balanced steady-state free precession for rapid 3D T1rho-weighted MRI. J Magn Reson Imaging. 2008;28:744–754. doi: 10.1002/jmri.21444. [DOI] [PMC free article] [PubMed] [Google Scholar]


