Abstract
Parkinson’s disease (PD) is a progressive neurological disorder and appears to have gender-specific symptoms. Studies have observed a higher frequency for development of PD in male than in female. In the current study, we evaluated the gender-based changes in cortical thickness and structural connectivity in PD patients. With informed consent, 64 PD (43 males and 21 females) patients, and 46 (12 males and 34 females) age-matched controls underwent clinical assessment including MiniMental State Examination (MMSE) and magnetic resonance imaging on a 1.5 Tesla clinical MR scanner. Whole brain high-resolution T1-weighted images were acquired from all subjects and used to measure cortical thickness and structural network connectivity. No significant difference in MMSE score was observed between male and female both in control and PD subjects. Male PD patients showed significantly reduced cortical thickness in multiple brain regions including frontal, parietal, temporal, and occipital lobes as compared with those in female PD patients. The graph theory-based network analysis depicted lower connection strengths, lower clustering coefficients, and altered network hubs in PD male than in PD female. Male-specific cortical thickness changes and altered connectivity in PD patients may derive from behavioral, physiological, environmental, and genetical differences between male and female, and may have significant implications in diagnosing and treating PD among genders.
Keywords: Parkinson’s disease, Magnetic resonance imaging, Brain, Cortical thickness, Structural network connectivity
Introduction
Parkinson’s disease (PD) is a progressive, neurological disorder affecting movements, muscle control, balance, cognitive functions, and consequently affecting the overall quality of life. These symptoms appear to have gender-specific directions in PD patients [19, 21, 31, 38, 45, 47]. Epidemiological studies have shown that male has 1.4–3.7 times higher frequency for developing PD than female [38, 42, 45, 47]. Further, a large meta-analysis study revealed that in any specific time period, approximately twice the number of males suffer from PD than do females [3].
In addition to differences in PD frequency, multiple studies have discovered differences in the clinical and cognitive profile of PD among genders. For example, onset of clinical symptoms of PD appears approximately 2 years earlier in male than in female [1, 21]. Studies have also observed sex-specific pattern in cognitive domains, where male showed more deficits in verbal fluency and recognition of facial emotions, while female depicted higher impairment in visuospatial functions [21, 31]. Additionally, the effect of drugs in terms of tolerability, efficacy, and pharmacokinetics for treating PD has different impacts in male and female [38].
Different genetic components such as gender-specific sex chromosome-linked genes, presence of sex hormones and environmental conditions are hypothesized to distinctly affect PD pathogenesis between male and female. Gene expression profile from post mortem brain of subjects who had been diagnosed with the late-stage idiopathic PD has revealed that the genes implicated in the pathogenesis of PD were up-regulated in male, while genes responsible for neuronal maturation were more pronounced in female [11, 39].
Brain’s structural and functional changes have been widely studied in PD patients using multiple imaging modalities. Magnetic resonance imaging (MRI) is the most commonly used noninvasive imaging modality to study the structural and functional changes in the brain of PD patients. With structural brain MR imaging, atrophy in multiple brain regions including cortical and subcortical structures was observed in PD patients [25, 26, 29, 32, 44]. Studies based on diffusion MR imaging depicted higher brain’s tissue changes in PD patients than in healthy controls [17, 33]. Contradictory findings were observed on cortical thickness analysis in PD patients. While some studies reported reduced cortical thickness in multiple brain sites [29, 32] others reported no significant changes in cortical thickness in PD patients compared with healthy controls [36].
Although, no in vivo information is available showing the gender-based changes in cortical thickness in PD patients, however, studies based on clinical, behavioral and molecular examination clearly observed that female is more protected than male from PD pathology. Gender-based characterization of brain tissue changes in PD patients during the disease progression is crucial for the effective treatment of PD as both male and female have different disease symptoms, and progression and treatment outcomes. In the current study, we investigated gender-based differences in cortical thickness and structural connectivity in PD patients. Our hypothesis was that the difference in PD onset frequency and degree of PD disease severity in male and female might result in differences in cortical thickness and structural network connectivity.
Materials and methods
Participants
Institutional review board committee approved the current study protocol; 64 PD patients (43 males, mean age = 71.2 ± 6.3 years; 21 females, mean age = 69.5 ± 7.0 years), and 46 controls (12 males, mean age = 73.0 ± 10 years; 34 females, mean age = 69.3 ± 10 years) were included in this study. Informed consent was obtained from all subjects before they underwent clinical assessment and whole brain MRI. For cognitive assessment, Mini-Mental State Examination (MMSE) was performed in all subjects. A team of experts including a neurologist, a neuropsychologist, a neurophysiologist and a psychiatrist made the diagnosis for PD as per the UK Parkinson’s Disease Society Brain Bank criteria for Parkinson’s disease [27]. All PD patients met the clinical diagnostic criteria for Parkinson’s disease. The control group consisted of subjects who visited the clinic with subjective complaints, and underwent exactly the same diagnostic work-up as PD patients.
MRI study
MRI was performed on a 1.5-Tesla, Siemens Sonata clinical-scanner (Siemens Medical Systems, Malvern, PA, USA) using a vendor-supplied head coil. Conventional imaging including T1-weighted, T2-weighted and fluid-attenuated-inversion-recovery was performed to examine any gross brain pathology. High-resolution T1-weighted 3D image volumes were acquired using magnetization-prepared rapid acquisition gradient-echo (MPRAGE) pulse sequence covering whole brain with repetition time (TR)/echo time (TE) = 3000 ms/3.5 ms, slice thickness = 1.2 mm, field of view (FOV) of 240 × 240 mm2 and 192 phase encode steps, and flip angle = 8°.
Cortical thicknesses analysis
High-resolution T1-weighted brain images were processed for cortical thickness measurement in all subjects using well-established FreeSurfer pipeline (v 5.3.0). The image-processing methods are described in detail elsewhere [16]. Briefly, non-brain tissue (skull) removal followed by Talairach transformation, intensity normalization, segmentation, tessellation of the gray and white matter boundaries, topology correction, and surface deformation (http://surfer.nmr.mgh.harvard.edu/) were performed. For the quality control assessments all processed data were carefully evaluated to ensure that skull and dura matter were excluded from the analysis. After final processing, gray matter surface maps were smoothed using a Gaussian kernel (full width of half maximum, 15 mm). Vertex-by-vertex general linear model approach was used to evaluate regional cortical thickness changes among different groups using age and gender as covariates in the analysis (ANCOVA; p < 0.05, false discovery rate corrections for multiple comparisons). The statistical parametric maps for regional cortical thickness differences were generated individually for both left and right hemispheres. For structural identification of various brain regions, significant clusters between groups were overlaid onto averaged inflated cortical surface maps.
Network analysis
The Graph Analysis Toolbox was used for the structural network construction. Pearson correlation coefficient was used to generate interregional regions of interests (ROIs) correlations metrics for both groups. Clustering coefficient (C) and characteristic path length (L) of the network at different densities starting from 0.42 to 0.50 with interval of 0.01 were measured to evaluate the global network topology between groups. The C and L values of both networks were compared with the corresponding mean values of a random graph including same number of nodes, total edges, and degree distribution [30, 41]. The small-world index (SW) was computed as (C/Crand)/(L/Lrand), where Crand and Lrand are the mean C and L of the random network [6]. The characteristic of SW networks, C must be significantly higher than Crand (C/Crand ratio greater than 1), and L should comparable to Lrand [24] (L/Lrand ratio close to 1). The nodal characteristic (betweenness) of the structural networks at threshold density of 0.42 was measured to detect anatomical or functional connections. The network hubs were also identified in each group as nodes with degree at least two standard deviation higher than the mean network degree [5, 24]. Network hub is considered as a crucial regulator of effective information flow in the brain [34]. Clustering coefficient (Gamma) measures the number of connections that exists between the nearest neighbors of a node. The path length (Lambda) defines the number of points required for moving from a given node to another. Generally, the shortest path is considered. The small-world network (Sigma) is characterized by the presence of abundant clustering of connections combined with short average distances between neuronal elements. These networks maximize information processing while minimizing wiring costs, support segregated and integrated information processing, and present resilience against pathology.
Statistical analyses
All the statistical computations were performed using the Statistical Package for Social Sciences (SPSS) version 16.0 (SPSS Inc., Chicago, USA). Subjects’ demographic and MMSE scores were assessed by one way analysis of variance (ANOVA) and Chi square. A p value less than 0.05 was considered statistically significant.
Results
Demographic and cognitive variable
No significant difference in age (p = 0.84) was observed between control and PD patients. The mean MMSE score (control = 29.1 ± 1.1, PD = 27.6 ± 3.2) was significantly decreased in PD patients (p = 0.008). No significant difference in PD disease duration at the time of MRI was observed between male and female patients [male: mean ± SD (3.67 ± 2.17 years); female: mean ± SD (3.69 ± 2.13 years); p value = 0.97]. No gender-based difference in age and mean MMSE score (control; males 29.0 ± 1.1; females, 29.2 ± 1.1; p = 1; PD, males 27.5 ± 3.2; females, 27.8 ± 3.1; p = 0.75) was observed both in PD and control.
Cortical thickness
Significantly lower cortical thickness bilaterally in multiple brain sites including caudal middle frontal, fusiform, inferior parietal, postcentral, rostral middle frontal, superior frontal, superior parietal, superior temporal, supramarginal was observed in PD male than PD female (Table 1; Fig. 1). Unilaterally, decreased cortical thickness was noted in inferior temporal, medial orbitofrontal, and paracentral in the right brain hemisphere, and in caudal middle frontal, lingual, middle temporal, precentral, precuneus in the left brain hemisphere in PD male compared with those in PD female (Table 1; Fig. 1).
Table 1.
Regional cortical thickness difference between PD male and PD female
| Cluster no. | Brain sites | Max t-statistic | Size (mm2) | Tal X | Tal Y | Tal Z | Cortical thickness, mm (mean ± SD)
|
||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PD male | PD female | Control male | Control female | ||||||||
| 1 | L | Postcentral | −6.0326 | 3307.86 | −37.6 | −30.6 | 65.4 | 1.65 ± 0.18 | 1.93 ± 0.19 | 1.68 ± 0.19 | 1.80 ± 0.17 |
| 2 | L | Inferior parietal | −5.7829 | 2373.56 | −46.3 | −55.6 | 24.1 | 2.03 ± 0.17 | 2.30 ± 0.22 | 2.09 ± 0.25 | 2.18 ± 0.22 |
| 3 | L | Superior frontal | −4.0131 | 1122.65 | −6.9 | 51.2 | 34.5 | 2.68 ± 0.22 | 2.93 ± 0.21 | 2.69 ± 0.14 | 2.84 ± 0.19 |
| 4 | L | Superior temporal | −3.9891 | 582.57 | −56.9 | −19 | 1.2 | 2.22 ± 0.30 | 2.52 ± 0.18 | 2.14 ± 0.17 | 2.40 ± 0.27 |
| 5 | L | Rostral middle frontal | −3.6413 | 309.39 | −35.9 | 32.3 | 29.3 | 2.09 ± 0.20 | 2.32 ± 0.21 | 2.25 ± 0.20 | 2.18 ± 0.22 |
| 6 | L | Superior parietal | −3.595 | 394.46 | −24.3 | −61.4 | 50.2 | 1.80 ± 0.18 | 2.00 ± 0.20 | 1.77 ± 0.23 | 1.93 ± 0.22 |
| 7 | L | Caudal middle frontal | −3.1883 | 241.95 | −30.5 | 13.1 | 45 | 2.17 ± 0.21 | 2.37 ± 0.16 | 2.21 ± 0.24 | 2.25 ± 0.21 |
| 8 | L | Superior frontal | −2.9724 | 229.16 | −10 | −6.9 | 61.6 | 2.32 ± 0.24 | 2.58 ± 0.33 | 2.33 ± 0.21 | 2.45 ± 0.27 |
| 9 | L | Precentral | −2.7983 | 144.12 | −22.2 | −22.6 | 68 | 1.97 ± 0.28 | 2.25 ± 0.33 | 1.95 ± 0.30 | 2.21 ± 0.29 |
| 10 | L | Superior frontal | −2.4729 | 40.6 | −20.4 | 4.5 | 52 | 2.14 ± 0.21 | 2.32 ± 0.21 | 2.22 ± 0.15 | 2.21 ± 0.20 |
| 11 | L | Supramarginal | −2.4448 | 22.48 | −42.4 | −26 | 22.5 | 2.27 ± 0.29 | 2.51 ± 0.27 | 2.30 ± 0.18 | 2.42 ± 0.26 |
| 12 | L | Superior parietal | −2.4134 | 78.28 | −18.1 | −80.8 | 39.7 | 1.87 ± 0.19 | 2.04 ± 0.26 | 1.92 ± 0.17 | 1.98 ± 0.21 |
| 13 | L | Precuneus | −2.4055 | 25.96 | −4.4 | −61.7 | 18.9 | 2.32 ± 0.22 | 2.51 ± 0.20 | 2.33 ± 0.25 | 2.34 ± 0.23 |
| 14 | L | Caudal middle frontal | −2.3645 | 24.97 | −40.1 | 9.7 | 48.2 | 2.22 ± 0.19 | 2.38 ± 0.21 | 2.25 ± 0.18 | 2.35 ± 0.18 |
| 15 | L | Fusiform | −2.3456 | 32.43 | −32.6 | −78.5 | −11.1 | 1.97 ± 0.17 | 2.11 ± 0.17 | 1.20 ± 0.18 | 2.04 ± 0.21 |
| 16 | L | Middle temporal | −2.3429 | 27.65 | −61.2 | −51.5 | 1.6 | 2.46 ± 0.23 | 2.65 ± 0.21 | 2.51 ± 0.19 | 2.57 ± 0.26 |
| 17 | L | Lingual | −2.321 | 36.63 | −17.3 | −69.9 | −10.2 | 1.81 ± 0.16 | 1.95 ± 0.18 | 1.80 ± 0.12 | 1.85 ± 0.21 |
| 1 | R | Fusiform | −7.2254 | 4027.7 | 38.5 | −66.5 | −15.3 | 2.19 ± 0.19 | 2.51 ± 0.20 | 2.27 ± 0.14 | 2.36 ± 0.19 |
| 2 | R | Caudal middle frontal | −4.8082 | 1618.82 | 29.7 | 8.8 | 48.9 | 2.19 ± 0.15 | 2.43 ± 0.25 | 2.25 ± 0.13 | 2.29 ± 0.21 |
| 3 | R | Postcentral | −4.5131 | 1814.9 | 39.1 | −29.9 | 64 | 1.65 ± 0.14 | 1.84 ± 0.19 | 1.71 ± 0.16 | 1.72 ± 0.17 |
| 4 | R | Inferior parietal | −4.1342 | 341.95 | 42.8 | −61.9 | 45.5 | 2.09 ± 0.17 | 2.30 ± 0.23 | 2.12 ± 0.27 | 2.27 ± 0.22 |
| 5 | R | Inferior parietal | −3.6082 | 633.4 | 49.9 | −50.4 | 8.7 | 2.28 ± 0.19 | 2.48 ± 0.20 | 2.28 ± 0.17 | 2.32 ± 0.21 |
| 6 | R | Postcentral | −3.5816 | 335.79 | 59.9 | −8.9 | 31.3 | 1.78 ± 0.20 | 1.99 ± 0.17 | 1.85 ± 0.17 | 1.90 ± 0.22 |
| 7 | R | Inferior parietal | −3.3508 | 451.44 | 42.9 | −78 | 21 | 2.24 ± 0.22 | 2.47 ± 0.24 | 2.25 ± 0.16 | 2.46 ± 0.23 |
| 8 | R | Supramarginal | −3.1256 | 120.92 | 56 | −37.2 | 32 | 2.16 ± 0.23 | 2.39 ± 0.25 | 2.23 ± 0.20 | 2.25 ± 0.18 |
| 9 | R | Superior parietal | −2.9378 | 392.02 | 29.7 | −43.8 | 54.6 | 1.76 ± 0.19 | 1.94 ± 0.20 | 1.77 ± 0.21 | 1.89 ± 0.17 |
| 10 | R | Superior temporal | −2.8605 | 302.2 | 65.3 | −20.5 | 4.2 | 2.52 ± 0.20 | 2.71 ± 0.19 | 2.54 ± 0.18 | 2.62 ± 0.22 |
| 11 | R | Supramarginal | −2.808 | 178.39 | 46 | −37.6 | 41.4 | 2.02 ± 0.18 | 2.22 ± 0.26 | 2.08 ± 0.29 | 2.15 ± 0.18 |
| 12 | R | Superior frontal | −2.6901 | 165 | 7.8 | 56.9 | 25.8 | 2.60 ± 0.23 | 2.81 ± 0.24 | 2.62 ± 0.16 | 2.70 ± 0.22 |
| 13 | R | Superior parietal | −2.5623 | 96.16 | 21.5 | −58.9 | 63.8 | 1.97 ± 0.24 | 2.17 ± 0.23 | 2.01 ± 0.25 | 2.09 ± 0.21 |
| 14 | R | Superior parietal | −2.5585 | 269.61 | 18.2 | −75.9 | 43.5 | 1.88 ± 0.17 | 2.03 ± 0.20 | 1.93 ± 0.19 | 1.20 ± 0.20 |
| 15 | R | Inferior temporal | −2.4346 | 119.6 | 53.7 | −28 | −20.9 | 2.56 ± 0.23 | 2.76 ± 0.26 | 2.53 ± 0.27 | 2.64 ± 0.26 |
| 16 | R | Rostral middle frontal | −2.3588 | 43.53 | 19.6 | 53.1 | 22.8 | 2.26 ± 0.17 | 2.41 ± 0.20 | 2.30 ± 0.15 | 2.42 ± 0.20 |
| 17 | R | Medial orbitofrontal | −2.3268 | 28.73 | 7.1 | 48.1 | −20.8 | 2.25 ± 0.26 | 2.47 ± 0.28 | 2.22 ± 0.21 | 2.33 ± 0.20 |
| 18 | R | Paracentral | −2.2724 | 16.75 | 13.2 | −25.6 | 47 | 2.04 ± 0.22 | 2.21 ± 0.19 | 2.13 ± 0.22 | 2.07 ± 0.15 |
| 19 | R | Inferior parietal | −2.2692 | 24.24 | 44.9 | −67 | 27.7 | 2.09 ± 0.18 | 2.29 ± 0.32 | 2.15 ± 0.25 | 2.26 ± 0.20 |
Each area consists of adjacent voxels showing a significant group difference; some brain structures have more than one area of change. The magnitude of the peak (t statistic) in each area and its Talairach coordinates (a standardized common brain space) are listed together with size (in normalized space) and the mean (SD) of cortical thickness at specific vertex for PD male and PD female. Cortical thickness of control male and female on same vertex is also shown
L left hemispheres, R right hemispheres
Fig. 1.
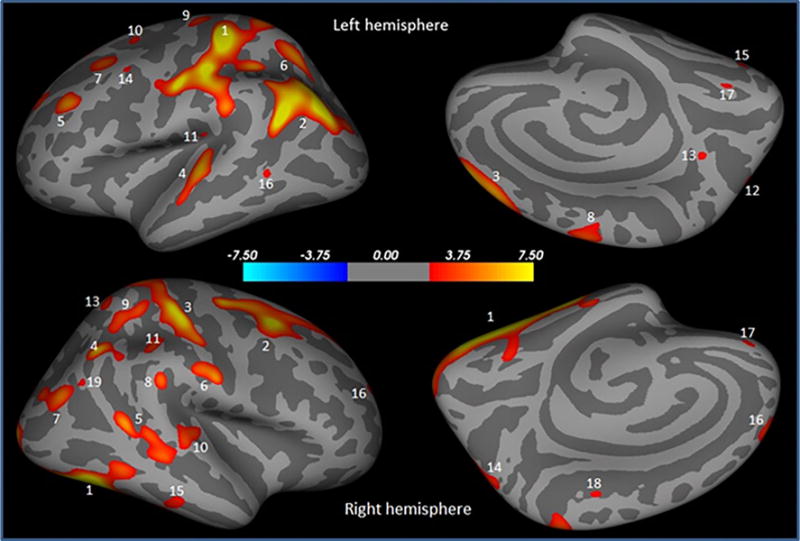
Brain regions showing significantly reduced cortical thickness in PD male compared to PD female overlaid onto inflated pial surface. These regions are postcentral (1), inferior parietal (2), superior frontal (3), superior temporal (4), rostral middle frontal (5), superior parietal (6), caudal middle frontal (7), precentral (8), superior frontal (9), superior frontal (10), supramarginal (11), superior parietal (12), precuneus (13), caudal middle frontal (14), fusiform (15), middle temporal (16), lingual (17) in the left hemisphere, and fusiform (1), caudal middle frontal (2), postcentral (3), inferior parietal (4), inferior parietal (5), postcentral (6), inferior parietal (7), supramarginal (8), superior parietal (9), superior temporal (10), supramarginal (11), superior frontal (12), superior parietal (13), superior parietal (14), inferior temporal (15), rostral middle frontal (16), medial orbitofrontal (17), paracentral (18), and inferior parietal (19) in the right hemisphere
PD male showed significantly decreased cortical thickness bilaterally in inferior parietal, precentral, rostral middle frontal, superior parietal, supramarginal (Table 2; Fig. 2), and unilaterally in fusiform, middle temporal (Table 2; Fig. 2) in the right hemisphere, and in inferior temporal, parsorbitalis, postcentral, superior frontal in the left hemisphere as compared with control female (Table 2; Fig. 2). No significant difference in cortical thickness was observed between control female versus PD female, control male versus control female, and control male versus PD male.
Table 2.
Regional cortical thickness difference between PD male and control female
| Cluster no. | Brain sites | Max t-statistic | Size (mm2) | Tal X | Tal Y | Tal Z | Cortical thickness; mm (mean ± SD)
|
||
|---|---|---|---|---|---|---|---|---|---|
| Control female | PD male | ||||||||
| 1 | L | Pars orbitalis | 5.1623 | 440.75 | −42.1 | 45.4 | −9.4 | 2.65 ± 0.18 | 2.41 ± 0.23 |
| 2 | L | Inferior parietal | 5.1616 | 571.19 | −36.5 | −66.5 | 43.8 | 2.19 ± 0.17 | 1.99 ± 0.18 |
| 3 | L | Supramarginal | 4.6329 | 352.31 | −57.4 | −48.4 | 33.1 | 2.61 ± 0.19 | 2.39 ± 0.23 |
| 4 | L | Inferior parietal | 4.4719 | 758.65 | −41.2 | −74.7 | 27.9 | 2.44 ± 0.26 | 2.20 ± 0.19 |
| 5 | L | Postcentral | 3.6049 | 98.38 | −39.3 | −32.6 | 63.4 | 1.96 ± 0.19 | 1.77 ± 0.21 |
| 6 | L | Superior parietal | 3.3874 | 86.72 | −20 | −65.2 | 54.1 | 2.11 ± 0.24 | 1.92 ± 0.22 |
| 7 | L | Supramarginal | 3.3605 | 126.42 | −50.8 | −52.8 | 20.2 | 2.30 ± 0.26 | 2.11 ± 0.17 |
| 8 | L | Rostral middle frontal | 3.3191 | 81.32 | −34 | 30.7 | 31 | 2.26 ± 0.20 | 2.09 ± 0.19 |
| 9 | L | Precentral | 3.1038 | 55.47 | −20.4 | −23 | 68.3 | 2.18 ± 0.30 | 1.94 ± 0.28 |
| 10 | L | Superior frontal | 3.0241 | 43.22 | −7.4 | 29 | 49.8 | 2.80 ± 0.19 | 2.64 ± 0.21 |
| 11 | L | Inferior temporal | 2.8718 | 43.93 | −56.4 | −36 | −16.1 | 2.71 ± 0.29 | 2.48 ± 0.28 |
| 12 | L | Superior frontal | 2.8017 | 0.86 | −7.1 | 53 | 33.7 | 2.84 ± 0.20 | 2.67 ± 0.21 |
| 1 | R | Inferior parietal | 5.052 | 1142.64 | 40.3 | −65.9 | 46.3 | 2.33 ± 0.20 | 2.12 ± 0.18 |
| 2 | R | Middle temporal | 4.4132 | 712.77 | 53.7 | −54.5 | −2.6 | 2.57 ± 0.21 | 2.32 ± 0.26 |
| 3 | R | Fusiform | 3.9129 | 638.73 | 39.8 | −66.1 | −14.8 | 2.35 ± 0.19 | 2.16 ± 0.20 |
| 4 | R | Rostral middle frontal | 3.2135 | 62.23 | 20.1 | 53.1 | 22.9 | 2.41 ± 0.20 | 2.25 ± 0.17 |
| 5 | R | Superior parietal | 3.1849 | 67.08 | 14.1 | −59.4 | 62.8 | 2.10 ± 0.19 | 1.92 ± 0.24 |
| 6 | R | Precentral | 3.1614 | 59.88 | 33.8 | −19.3 | 66.3 | 2.40 ± 0.32 | 2.13 ± 0.30 |
| 7 | R | Supramarginal | 2.9318 | 20.04 | 54.2 | −42.9 | 39 | 2.39 ± 0.23 | 2.22 ± 0.19 |
Each area consists of adjacent voxels showing a significant group difference; some brain structures have more than one area of change. The magnitude of the peak (t statistic) in each area and its Talairach coordinates (a standardized common brain space) are listed together with size (in normalized space) and the mean (SD) of cortical thickness for control female and PD male
L left hemispheres, R right hemispheres
Fig. 2.
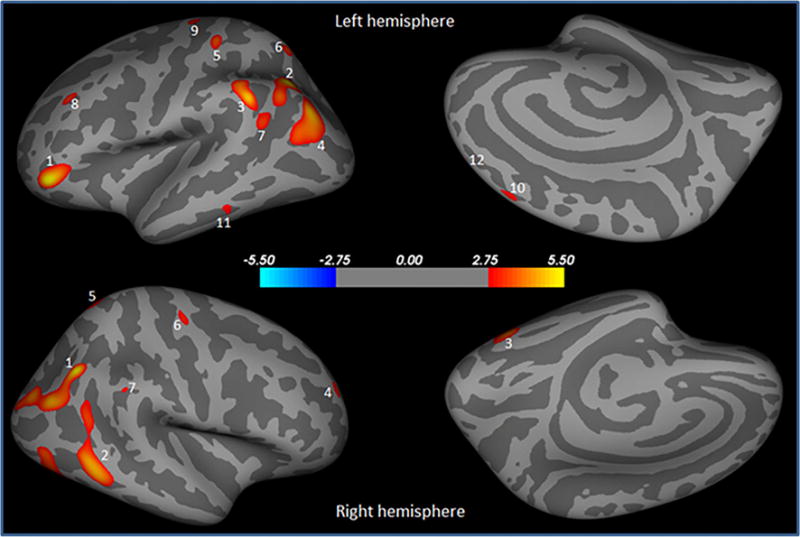
Brain regions showing significantly reduced cortical thickness in PD male compared to control female overlaid onto inflated pial surface. These areas are pars orbitalis (1), inferior parietal (2), supramarginal (3), inferior parietal (4), postcentral (5), superior parietal (6), supramarginal (7), rostral middle frontal (8), precentral (9), superior frontal (10), inferior temporal (11), superior frontal (12) in the left hemisphere, and inferior parietal (1), middle temporal (2), fusiform (3), rostral middle frontal (4), superior parietal (5), precentral (6), and supramarginal (7) in the right hemisphere
Network analysis
Both PD male and female showed widespread positive and negative interregional ROIs correlations (Fig. 3). The correlation strength was lower in PD male (0.40 ± 0.09) than in PD female (0.43 ± 0.11); however, the difference was not statistically significant (p = 0.12). A trend of lower clustering coefficients [gamma, PD male (1.116), PD female (1.135), p = 0.67), and (sigma, PD male (1.046), PD female (1.122); p = 0.53)], and higher path length [lambda, PD male (1.067), PD female (1.012); p = 0.48)] was observed in PD male than PD female (Fig. 4). The normalized gamma was greater than 1 across a wide range of densities in both PD male and PD female (Fig. 4a), while the normalized lambda in both groups was close to 1 (Fig. 4b, c). The cortical correlation network followed an SW property across a wide range of densities in both PD male and PD female.
Fig. 3.
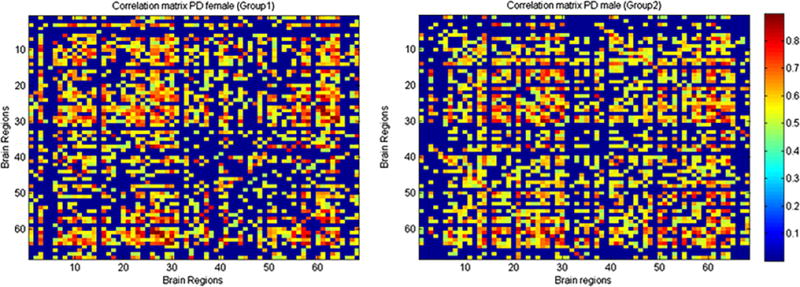
Inter-regional cortical brain regions correlation matrix of PD female and PD male. Warm colour showed connected regions. These regions are—bankssts, caudal anterior cingulate, caudal middle frontal, cuneus, entorhinal, fusiform, inferior parietal, inferior temporal, isthmus cingulate, lateral occipital, lateral orbitofrontal, lingual, medial orbitofrontal, middle temporal, parahippocampal, paracentral, pars opercularis, pars orbitalis, pars triangularis, perical-carine, postcentral, posterior cingulate, precentral, precuneus, rostral anterior cingulate, rostral middle frontal, superior frontal, superior parietal, superior temporal, supramarginal, frontal pole, temporal pole, transverse temporal, insula both in the left and right hemisphere
Fig. 4.
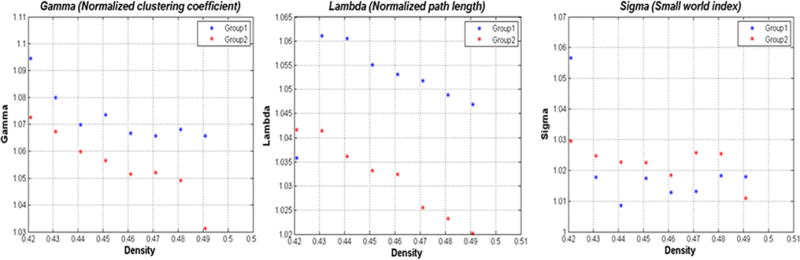
Changes in global network properties as a function of network densities in PD female and PD male (a–c). Both networks follow a small-world organization across the range of densities
Lower nodal betweenness was observed in left caudal middle frontal, left rostral middle frontal, and right parahippocampal in PD male than in PD female (Fig. 5). On the basis of nodal betweenness, the network hubs were identified in left inferior temporal, left rostral anterior cingulate, right fusiform, and right isthmus cingulate area in PD male, while in PD female, network hubs were in left rostral middle frontal, right parahippocampal, and right superior temporal regions (Fig. 6).
Fig. 5.
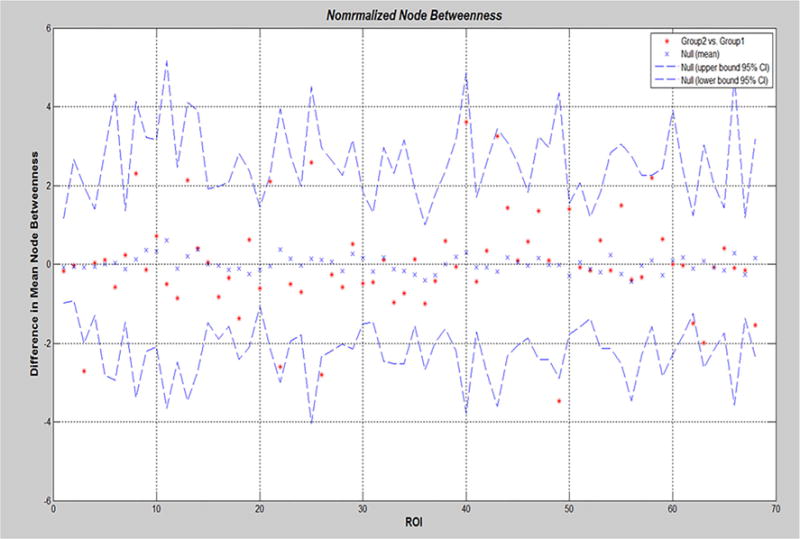
Mean node betweenness differences in regional network topology between PD female (group 1) and PD male (group 2). PD male showed lower nodal betweenness in left caudal middle frontal, left rostral middle frontal, and right parahippocampal than PD female
Fig. 6.
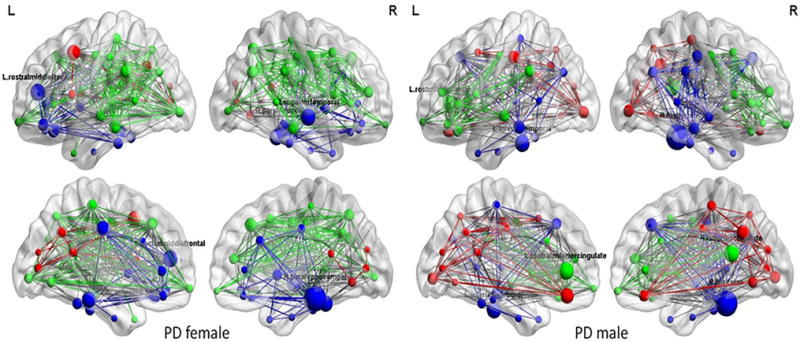
Structural correlation networks and hubs overlaid on ICBM152 brain template of PD female and PD male. Color lines indicate connections (edge), and spheres represent regions (node). The radius of the spheres is proportional to the nodal betweenness
Discussion
Studies have shown that impaired cognition and dementia in PD patients are associated with the structural and functional brain changes [2, 9, 14, 20, 25]. However, no study so far has defined cortical thickness changes among PD genders. In the current study, we observed gender-based changes in cortical thickness and structural network connectivity in PD patients, where male PD patients are more affected than female PD patients suggestive of higher brain’s tissues’ impairment in male PD.
There are contradictory findings on gender-based clinical and behavioral differences among PD patients [19, 31, 38, 42, 47]. Indeed most of the studies have observed gender-based differences in PD symptoms’ appearance and frequency [19, 21, 45, 47]. In male, symptoms appear earlier than in female with tremor as a primary indication, while in female the initial signs are bradykinesia and rigidity [21]. Gender-based rapid eye movement (REM) sleep behavior disorder (RBD) was examined in PD patients, which depicted higher prevalence of RBD in male than in female [49]. A cross-sectional study over 24,402 PD patients showed that male PD patients have more wandering, verbal and physical abusiveness, and inappropriate behavior, whereas female PD patients have more depression; moreover, there were gender-based differences with respect to pharmacologic therapies, where most of the male PD patients were on antipsychotic drugs, while female PD patients received antidepressants [19].
The current study observed gender-based differences in regional cortical thickness in multiple brain sites depicting lower cortical thickness in PD male than in PD female. Since these regions are primarily involved in critical roles including autonomic, cognitive, affective, language, and visual functions we anticipate more behavioral and brain functional changes in PD male. For example, the medial orbitofrontal cortex, which plays a crucial role in sensory integration, decision-making, showed significantly decreased cortical thickness in PD male than in PD female. The other brain areas with decreased cortical thickness in PD male include temporal, frontal, occipital, and parietal, and caudal region, and changes in these regions may responsible for the gender-specific differences in clinical, cognitive and behavioral profiles in PD patients. Gender-based changes in gray matter were previously measured in Alzheimer’s disease (AD), where male AD patients showed higher gray matter loss in anterior cingulate than did female AD patients [4], whereas, based on the cortical thickness analyses, no gender-based difference in AD patients was observed [37]. Other studies on patients with temporal lobe epilepsy and schizophrenia depicted lower cortical thickness in female than in male patients [18, 22]. We suggest that the differential disease pathology in PD affects the male more than it does the female.
PD male showed lower structural network correlations than PD female due to altered cortical thickness. Structural network in both PD male and PD female showed SW topology, which is consistent with the previous studies on other pathologies including Alzheimer’s disease, schizophrenia, mild cognitive impairment, and epilepsy [8, 23, 46, 48]. We did not observe any significant difference for the normalized clustering coefficient, and normalized path length between PD male and PD female.
A node with high betweenness is present at the intersection of many short paths and may control the information flow. In a brain structural network, a node with high betweenness has the potential to participate in large number of functional interactions [40]. In the current study, the nodal betweenness in PD male was significantly altered than in PD female suggestive of decreased structural brain connectivity, which may contribute to the differential behavioral and brain’s functional changes in PD male. PD male showed alerted hubs compared to PD female. Although there has been no gender-based study on the hub locations in PD patients, we suggest that altered location of hubs in PD male might be due to change in the regional cortical thickness.
The various effects of PD disease pathology on the brain structural organization and network connectivity among genders can be well explained on the basis of involvement of the sex hormones and genes in neurodevelopment and regulation of the brain’s functional activities. Sex hormones are shown to be a very important factor in gender-based differentiation of structural and functional changes in the brain. The most important sex hormone in the female is estrogen; especially 17β-estradiol has been thoroughly studied in relation to their neuroprotective effects [10]. Several studies have observed neuroprotective effect of estrogen on dopamine systems and resulting reduction of risk for PD in female. Other studies have suggested that the estrogen-based hormone therapy relieves PD symptoms when given in the early stage of the disease, and PD symptoms may deteriorate when the treatment is discontinued [7, 15, 28, 35]. The genes involved in the pathogenesis of PD including α-synuclein and PINK-1 are shown to be overexpressed in male, while the genes responsible for the neuronal maturation and signal transduction are overexpressed in female [11, 39]. Recent study measured higher level of α-synuclein in plasma of male PD than female PD [12]. Further, they observed that increased plasma α-synuclein level correlates with cognitive impairment, hallucinations, psychosis, apathy and sleep disorders in PD patients [12]. We suggest that higher expression of genes involved in PD pathogenesis in male and neuroprotective effects of sex hormones in female might be a possible explanation for the lower cortical thickness in male PD than female PD.
Most of the patients included in the current study were on anti-Parkinson drugs treatment at the time of MRI study. However, this study does not evaluate any effect of the treatment on cortical thickness in PD patients, while the effect of anti-Parkinson drugs on cortical thickness has been reported previously. It is found that PD patients with impulse control disorder (ICD) showed increased cortical thickness in limbic regions compared with PD patients without ICD treated for the equal daily dose of levodopa [43]. Similarly, in another study, PD patients with long term treatment who developed levodopa-induced-dyskinesia (LID) showed higher cortical thickness in the inferior frontal sulcus than PD patients without LID [13].
It is important to mention some limitations of the current study including absence of clinical and neurocognitive profiles’ correlation with cortical thickness changes. Nevertheless, the current study provides an in vivo imaging clinical biomarker to evaluate the gender-based changes in cortical thickness, which may help to improve the clinical management of PD patients.
Conclusions
Gender-based differences on regional cortical thickness appeared in PD patients, where male PD patients are more affected than female PD patients suggestive of greater brain tissue changes in male PD than in female PD. These male-specific brain tissue changes as reflected by decreased cortical thickness in multiple brain regions may derive from physiological, genetical and environmental differences between male and female and may have significant implications in diagnosing and treating PD among genders. The findings also highlight the need for gender-specific medications for better clinical management of PD patients.
Acknowledgments
This work was performed at a NIH supported resource center (NIH RR02305) and from a grant from the Pennsylvania State Tobacco Settlement (SAP4100027296).
Abbreviations
- PD
Parkinson’s disease
- MRI
Magnetic resonance imaging
- MMSE
Mini-Mental State Examination
- MPRAGE
Magnetization-prepared rapid acquisition gradient-echo
- TR
Repetition time
- TE
Echo time
- FOV
Field of view
- ANCOVA
Analysis of covariance
- ROIs
Regions of interests
- C
Clustering coefficient
- L
Characteristic path length
- SW
Small-world index
- ANOVA
Analysis of variance
- RBD
Rapid eye movement sleep behavior disorder
Footnotes
Compliance with ethical standards
Conflicts of interest: The authors declare no conflict of interest.
Ethical standard: All human studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All persons gave their informed consent prior to their inclusion in the study.
References
- 1.Alves G, Muller B, Herlofson K, HogenEsch I, Telstad W, Aarsland D, Tysnes OB, Larsen JP, Norwegian ParkWest study g Incidence of Parkinson’s disease in Norway: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2009;80:851–857. doi: 10.1136/jnnp.2008.168211. [DOI] [PubMed] [Google Scholar]
- 2.Amboni M, Tessitore A, Esposito F, Santangelo G, Picillo M, Vitale C, Giordano A, Erro R, de Micco R, Corbo D, Tedeschi G, Barone P. Resting-state functional connectivity associated with mild cognitive impairment in Parkinson’s disease. J Neurol. 2015;262:425–434. doi: 10.1007/s00415-014-7591-5. [DOI] [PubMed] [Google Scholar]
- 3.Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D. Parkinson’s disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology. 2000;55:1358–1363. doi: 10.1212/wnl.55.9.1358. [DOI] [PubMed] [Google Scholar]
- 4.Ballmaier M, O’Brien JT, Burton EJ, Thompson PM, Rex DE, Narr KL, McKeith IG, DeLuca H, Toga AW. Comparing gray matter loss profiles between dementia with Lewy bodies and Alzheimer’s disease using cortical pattern matching: diagnosis and gender effects. Neuroimage. 2004;23:325–335. doi: 10.1016/j.neuroimage.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 5.Bassett DS, Bullmore E, Verchinski BA, Mattay VS, Weinberger DR, Meyer-Lindenberg A. Hierarchical organization of human cortical networks in health and schizophrenia. J Neurosci: Off J Soc Neurosci. 2008;28:9239–9248. doi: 10.1523/JNEUROSCI.1929-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett DS, Meyer-Lindenberg A, Achard S, Duke T, Bullmore E. Adaptive reconfiguration of fractal small-world human brain functional networks. Proc Natl Acad Sci USA. 2006;103:19518–19523. doi: 10.1073/pnas.0606005103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benedetti MD, Maraganore DM, Bower JH, McDonnell SK, Peterson BJ, Ahlskog JE, Schaid DJ, Rocca WA. Hysterectomy, menopause, and estrogen use preceding Parkinson’s disease: an exploratory case-control study. Mov Disord: Off J Mov Disord Soc. 2001;16:830–837. doi: 10.1002/mds.1170. [DOI] [PubMed] [Google Scholar]
- 8.Bernhardt BC, Chen Z, He Y, Evans AC, Bernasconi N. Graph-theoretical analysis reveals disrupted small-world organization of cortical thickness correlation networks in temporal lobe epilepsy. Cereb Cortex. 2011;21:2147–2157. doi: 10.1093/cercor/bhq291. [DOI] [PubMed] [Google Scholar]
- 9.Beyer MK, Bronnick KS, Hwang KS, Bergsland N, Tysnes OB, Larsen JP, Thompson PM, Somme JH, Apostolova LG. Verbal memory is associated with structural hippocampal changes in newly diagnosed Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2013;84:23–28. doi: 10.1136/jnnp-2012-303054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourque M, Dluzen DE, Di Paolo T. Neuroprotective actions of sex steroids in Parkinson’s disease. Front Neuroendocrinol. 2009;30:142–157. doi: 10.1016/j.yfrne.2009.04.014. [DOI] [PubMed] [Google Scholar]
- 11.Cantuti-Castelvetri I, Keller-McGandy C, Bouzou B, Asteris G, Clark TW, Frosch MP, Standaert DG. Effects of gender on nigral gene expression and parkinson disease. Neurobiol Dis. 2007;26:606–614. doi: 10.1016/j.nbd.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caranci G, Piscopo P, Rivabene R, Traficante A, Riozzi B, Castellano AE, Ruggieri S, Vanacore N, Confaloni A. Gender differences in Parkinson’s disease: focus on plasma alpha-synuclein. J Neural Transm. 2013;120:1209–1215. doi: 10.1007/s00702-013-0972-6. [DOI] [PubMed] [Google Scholar]
- 13.Cerasa A, Morelli M, Augimeri A, Salsone M, Novellino F, Gioia MC, Arabia G, Quattrone A. Prefrontal thickening in PD with levodopa-induced dyskinesias: new evidence from cortical thickness measurement. Parkinsonism Relat Disord. 2013;19:123–125. doi: 10.1016/j.parkreldis.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 14.Christopher L, Strafella AP. Neuroimaging of brain changes associated with cognitive impairment in Parkinson’s disease. J Neuropsychol. 2013;7:225–240. doi: 10.1111/jnp.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Currie LJ, Harrison MB, Trugman JM, Bennett JP, Wooten GF. Postmenopausal estrogen use affects risk for Parkinson disease. Arch Neurol. 2004;61:886–888. doi: 10.1001/archneur.61.6.886. [DOI] [PubMed] [Google Scholar]
- 16.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- 17.Deng B, Zhang Y, Wang L, Peng K, Han L, Nie K, Yang H, Zhang L, Wang J. Diffusion tensor imaging reveals white matter changes associated with cognitive status in patients with Parkinson’s disease. Am J Alzheimer’s Dis Other Dement. 2013;28:154–164. doi: 10.1177/1533317512470207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Doherty MJ, Rostad SW, Kraemer DL, Vossler DG, Haltiner AM. Neocortical gliosis in temporal lobe epilepsy: gender-based differences. Epilepsia. 2007;48:1455–1459. doi: 10.1111/j.1528-1167.2007.01046.x. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez HH, Lapane KL, Ott BR, Friedman JH. Gender differences in the frequency and treatment of behavior problems in Parkinson’s disease. SAGE Study Group. Systematic Assessment and Geriatric drug use via Epidemiology. Mov Disord: Off J Mov Disord Soc. 2000;15:490–496. [PubMed] [Google Scholar]
- 20.Filoteo JV, Reed JD, Litvan I, Harrington DL. Volumetric correlates of cognitive functioning in nondemented patients with Parkinson’s disease. Mov Disord: Off J Mov Disord Soc. 2014;29:360–367. doi: 10.1002/mds.25633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haaxma CA, Bloem BR, Borm GF, Oyen WJ, Leenders KL, Eshuis S, Booij J, Dluzen DE, Horstink MW. Gender differences in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:819–824. doi: 10.1136/jnnp.2006.103788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hamilton LS, Narr KL, Luders E, Szeszko PR, Thompson PM, Bilder RM, Toga AW. Asymmetries of cortical thickness: effects of handedness, sex, and schizophrenia. Neuroreport. 2007;18:1427–1431. doi: 10.1097/WNR.0b013e3282e9a5a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He Y, Dagher A, Chen Z, Charil A, Zijdenbos A, Worsley K, Evans A. Impaired small-world efficiency in structural cortical networks in multiple sclerosis associated with white matter lesion load. Brain: J Neurol. 2009;132:3366–3379. doi: 10.1093/brain/awp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosseini SM, Hoeft F, Kesler SR. GAT: a graph-theoretical analysis toolbox for analyzing between-group differences in large-scale structural and functional brain networks. PLoS One. 2012;7:e40709. doi: 10.1371/journal.pone.0040709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hu MT, White SJ, Chaudhuri KR, Morris RG, Bydder GM, Brooks DJ. Correlating rates of cerebral atrophy in Parkinson’s disease with measures of cognitive decline. J Neural Transm. 2001;108:571–580. doi: 10.1007/s007020170057. [DOI] [PubMed] [Google Scholar]
- 26.Laakso MP, Partanen K, Riekkinen P, Lehtovirta M, Helkala EL, Hallikainen M, Hanninen T, Vainio P, Soininen H. Hippocampal volumes in Alzheimer’s disease, Parkinson’s disease with and without dementia, and in vascular dementia: an MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 27.Litvan I, Bhatia KP, Burn DJ, Goetz CG, Lang AE, McKeith I, Quinn N, Sethi KD, Shults C, Wenning GK, Movement Disorders Society Scientific Issues C Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord: Off J Mov Disord Soc. 2003;18:467–486. doi: 10.1002/mds.10459. [DOI] [PubMed] [Google Scholar]
- 28.Liu B, Dluzen DE. Oestrogen and nigrostriatal dopaminergic neurodegeneration: animal models and clinical reports of Parkinson’s disease. Clin Exp Pharmacol Physiol. 2007;34:555–565. doi: 10.1111/j.1440-1681.2007.04616.x. [DOI] [PubMed] [Google Scholar]
- 29.Mak E, Bergsland N, Dwyer MG, Zivadinov R, Kandiah N. Subcortical atrophy is associated with cognitive impairment in mild Parkinson disease: a combined investigation of volumetric changes, cortical thickness, and vertex-based shape analysis. AJNR Am J Neuroradiol. 2014;35:2257–2264. doi: 10.3174/ajnr.A4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maslov S, Sneppen K. Specificity and stability in topology of protein networks. Science. 2002;296:910–913. doi: 10.1126/science.1065103. [DOI] [PubMed] [Google Scholar]
- 31.Miller IN, Cronin-Golomb A. Gender differences in Parkinson’s disease: clinical characteristics and cognition. Mov Disord: Off J Mov Disord Soc. 2010;25:2695–2703. doi: 10.1002/mds.23388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pereira JB, Ibarretxe-Bilbao N, Marti MJ, Compta Y, Junque C, Bargallo N, Tolosa E. Assessment of cortical degeneration in patients with Parkinson’s disease by voxel-based morphometry, cortical folding, and cortical thickness. Hum Brain Mapp. 2012;33:2521–2534. doi: 10.1002/hbm.21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, Brumback B, Okun MS, Mareci TH, Bowers D. Gray and white matter contributions to cognitive frontostriatal deficits in non-demented Parkinson’s disease. PLoS One. 2016;11:e0147332. doi: 10.1371/journal.pone.0147332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52:1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 35.Sandyk R. Estrogens and the pathophysiology of Parkinson’s disease. Int J Neurosci. 1989;45:119–122. doi: 10.3109/00207458908986223. [DOI] [PubMed] [Google Scholar]
- 36.Segura B, Baggio HC, Marti MJ, Valldeoriola F, Compta Y, Garcia-Diaz AI, Vendrell P, Bargallo N, Tolosa E, Junque C. Cortical thinning associated with mild cognitive impairment in Parkinson’s disease. Mov Disord: Off J Mov Disord Soc. 2014;29:1495–1503. doi: 10.1002/mds.25982. [DOI] [PubMed] [Google Scholar]
- 37.Seo SW, Im K, Lee JM, Kim ST, Ahn HJ, Go SM, Kim SH, Na DL. Effects of demographic factors on cortical thickness in Alzheimer’s disease. Neurobiol Aging. 2011;32:200–209. doi: 10.1016/j.neurobiolaging.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 38.Shulman LM, Bhat V. Gender disparities in Parkinson’s disease. Expert Rev Neurother. 2006;6:407–416. doi: 10.1586/14737175.6.3.407. [DOI] [PubMed] [Google Scholar]
- 39.Simunovic F, Yi M, Wang Y, Stephens R, Sonntag KC. Evidence for gender-specific transcriptional profiles of nigral dopamine neurons in Parkinson disease. PLoS One. 2010;5:e8856. doi: 10.1371/journal.pone.0008856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sporns O. Networks of the Brain. MIT Press; Cambridge: 2011. [Google Scholar]
- 41.Sporns O, Zwi JD. The small world of the cerebral cortex. Neuroinformatics. 2004;2:145–162. doi: 10.1385/NI:2:2:145. [DOI] [PubMed] [Google Scholar]
- 42.Swerdlow RH, Parker WD, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Wooten GF. Gender ratio differences between Parkinson’s disease patients and their affected relatives. Parkinsonism Relat Disord. 2001;7:129–133. doi: 10.1016/s1353-8020(00)00029-8. [DOI] [PubMed] [Google Scholar]
- 43.Tessitore A, Santangelo G, De Micco R, Vitale C, Giordano A, Raimo S, Corbo D, Amboni M, Barone P, Tedeschi G. Cortical thickness changes in patients with Parkinson’s disease and impulse control disorders. Parkinsonism Relat Disord. 2016;24:119–125. doi: 10.1016/j.parkreldis.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 44.Tinaz S, Courtney MG, Stern CE. Focal cortical and subcortical atrophy in early Parkinson’s disease. Mov Disord: Off J Mov Disord Soc. 2011;26:436–441. doi: 10.1002/mds.23453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, Nelson LM. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 46.van den Heuvel MP, Mandl RC, Stam CJ, Kahn RS, Hulshoff Pol HE. Aberrant frontal and temporal complex network structure in schizophrenia: a graph theoretical analysis. J Neurosci: Off J Soc Neurosci. 2010;30:15915–15926. doi: 10.1523/JNEUROSCI.2874-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wooten GF, Currie LJ, Bovbjerg VE, Lee JK, Patrie J. Are men at greater risk for Parkinson’s disease than women? J Neurol Neurosurg Psychiatry. 2004;75:637–639. doi: 10.1136/jnnp.2003.020982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yao Z, Zhang Y, Lin L, Zhou Y, Xu C, Jiang T, Alzheimer’s Disease Neuroimaging I Abnormal cortical networks in mild cognitive impairment and Alzheimer’s disease. PLoS Comput Biol. 2010;6:e1001006. doi: 10.1371/journal.pcbi.1001006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoritaka A, Ohizumi H, Tanaka S, Hattori N. Parkinson’s disease with and without REM sleep behaviour disorder: are there any clinical differences? Eur Neurol. 2009;61:164–170. doi: 10.1159/000189269. [DOI] [PubMed] [Google Scholar]


