Abstract
The eukaryotic enzyme Bds1 in Saccharomyces cerevisiae is a metallo-β-lactamase-related enzyme evolutionarily originating from bacterial horizontal gene transfer that serves an unknown biological role. Previously, Bds1 was reported to be an alkyl and aryl sulfatase. However, we demonstrate here that Bds1 acts on primary alkyl sulfates (of 6–12 carbon atoms) but not the aryl sulfates p-nitrophenyl sulfate and p-nitrocatechol sulfate. The apparent catalytic rate constant for hydrolysis of the substrate 1-hexyl sulfate by Bds1 is over 100 times lower than that of the reaction catalyzed by its bacterial homolog SdsA1. We show that Bds1 shares a catalytic mechanism with SdsA1 in which the carbon atom of the sulfate ester is the subject of nucleophilic attack, rather than the sulfur atom, resulting in C–O bond lysis. In contrast to SdsA1 and another bacterial homolog with selectivity for secondary alkyl sulfates named Pisa1, Bds1 does not show any substantial activity towards secondary alkyl sulfates. Neither Bds1 nor SdsA1 have any significant activity towards a branched primary alkyl sulfate, primary and secondary steroid sulfates, or phosphate diesters. Therefore, the enzymes homologous to SdsA1 that have been identified and characterized thus far vary in their selectivity towards primary and secondary alkyl sulfates but do not exhibit aryl sulfatase activity.
Keywords: Sulfohydrolase, Sulfatase, Alkyl Sulfate, Aryl Sulfate, Liquid Chromatography-Mass Spectrometry
1. Introduction
Sulfohydrolases are a heterogeneous group of enzymes catalyzing the hydrolysis of sulfate ester linkages in a variety of molecules. In mammals, sulfohydrolases are important for controlling the sulfation state of biomolecules and for the transport of steroids, while in microorganisms sulfohydrolases play a key role in accessing environmental sulfur sources for use in biosynthesis of cysteine, methionine, S-adenosylmethionine, and glutathione and in liberating the remainder of the organic molecule for catabolism or lipid biosynthesis [1–6]. These enzymes can be classified into three groups according to mechanistic considerations [7], and a fourth class of metal-independent sulfatases has also been proposed that may be elucidated by future studies [8]. Class I includes aryl and carbohydrate sulfatases, which act on sulfated steroids and carbohydrates and possess a formylglycine post-translational modification necessary for CO–S bond breakage to yield an alcohol and inorganic sulfate as products [3, 5]. Class II consists of Fe(II) dioxygenases dependent on α-ketoglutarate to oxidatively cleave sulfate esters into aldehydes and inorganic sulfate [2, 9]. The third class comprises sulfohydrolases belonging to the metallo-β-lactamase (MBL) superfamily. SdsA1, a dimeric enzyme secreted from the bacterium Pseudomonas aeruginosa PAO1, is the founding member of this class and has been implicated in the degradation of the man-made detergent sodium dodecyl sulfate [7].
Homologs of the SdsA1 protein exist in a wide range of organisms spanning all the kingdoms of life. While SdsA1 is more reactive towards primary alkyl sulfates than secondary alkyl sulfates, the homolog Pisa1 in Pseudomonas sp. DSM 6611 has been shown to exhibit a strong preference for sec-alkyl sulfates over primary [10, 11]. This Pisa1-catalyzed hydrolysis is highly enantioselective, and mechanistic studies have shown that the reaction catalyzed by both Pisa1 and SdsA1 breaks the sulfate ester C–OS bond, resulting in inversion of stereochemistry at that carbon atom if it is chiral [11]. The crystal structures of both SdsA1 and Pisa1, as well as the Escherichia coli homolog YjcS, have been determined, and a comparison reveals a high degree of similarity in the binuclear Zn(II)-binding site within their active sites [7, 10, 12]. The homolog SdsAP in Pseudomonas sp. S9 has also been described and bears similarity to the other characterized homologs in its ability to hydrolyze alkyl sulfates but not aryl sulfates [13]. An additional homolog of SdsA1, CddY, has been identified in Rhodococcus ruber strain SC1 as part of a six-member gene cluster responsible for oxidation of cyclododecanone, but the specific role of CddY in this metabolism has not been elucidated [14].
SdsA1 homologs also exist in eukaryotes, though none have been identified in humans, with Bds1 in Saccharomyces cerevisiae being the only eukaryotic class III sulfohydrolase studied to date. The expression of Bds1 has been shown to specifically increase under sulfur-limited (but not carbon-, nitrogen-, or phosphorus-limited) growth conditions [15], suggesting Bds1 may have a role in the assimilation of sulfur from alternative sources or the redistribution of cellular sulfur under sulfur-limited conditions. The BDS1 gene has been previously shown to likely originate from horizontal gene transfer from bacteria to yeast, and the same report includes some characterization of Bds1 activity inferred from a gene deletion mutant, which suggested a possible aryl sulfatase activity for this enzyme [16]. Such an activity is intriguing because it might occur by a different mechanism than the C–OS bond lysis established for SdsA1- and Pisa1-catalyzed alkyl sulfate hydrolysis, since nucleophilic attack on a sulfate ester carbon atom forming an aromatic ring is less favorable.
Here we expressed and purified Bds1, its double mutant D166N, H167A (which converts two amino acids involved in the conserved MBL metal ion-binding motif), and SdsA1 to study their reactivity towards an array of possible substrates, with the goal of better understanding this class of MBL-related enzymes. We determined the chemical mechanism of Bds1-catalyzed sulfate ester hydrolysis, compared the activity of Bds1 to that of its prokaryotic homolog SdsA1, and assessed the activity of Bds1 towards primary 1-alkyl sulfates of varying chain length, a branched-chain primary alkyl sulfate, a secondary alkyl sulfate, aryl sulfates, steroid sulfates, and alkyl phosphates.
2. Materials and methods
2.1 Cloning, expression, and purification of Bds1
The BDS1 gene was amplified using primers that added a sequence encoding a C-terminal His6 tag and inserted into plasmid pRS426 bearing the promoter region of MET25 and terminator region of CYC1. This plasmid was also used as a template for site-directed mutagenesis (using the New England BioLabs Q5 site-directed mutagenesis kit) to create the gene encoding the double mutant D166N, H167A. The sequences of BDS1 and the mutant on each plasmid were confirmed by DNA sequencing.
S. cerevisiae wild type cells (W303-1B, which is MATα ade2-1 can1-100 his3-11,15 leu2-3,112 trp1-1 ura3-1) were transformed with either of these plasmids via the lithium acetate method [17] and the resulting transformant cells cultured overnight at 30°C with shaking in 50 mL of Brent Supplement Mixture synthetic selective medium (Sunrise Science Products) lacking uracil to maintain plasmid selection and containing 2% galactose and 0.1% glucose. These cultures were used to inoculate 1-L cultures in the same medium, which grew overnight to an optical density at 600 nm of 5.0–5.5.
These cells were lysed on ice with a Dounce homogenizer following treatment of the cell walls with dithiothreitol and lyticase [18], and the resulting mixture of soluble proteins was clarified by centrifugation and applied to a Ni-NTA column (Thermo Scientific) equilibrated with 10 mM imidazole in PBS, pH 7.5, with 2 mM PMSF. Lysate was reapplied to the column twice after two ~24 h periods of binding with rotation at 4°C then after a total of ~72 h, the column was washed in two column volumes of similar buffers at 25, 50, 100, and 250 mM imidazole, the latter of which resulted in elution of the Bds1 protein. Purification fractions were analyzed by SDS-PAGE, and the elution fraction with the majority of the desired protein was desalted with Zeba spin columns (Thermo Scientific) and used for subsequent experiments with its total protein concentration determined via the Bradford method (using a Thermo Scientific Coomassie Plus assay kit).
2.2 Expression and purification of SdsA1
A His-tagged version of SdsA1 was expressed in BL21 (DE3) E. coli cells from a plasmid that was a kind gift from Prof. Wolf-Dieter Schubert (University of Pretoria) and the Helmholtz Center for Infection Research. Following transformation by electroporation and selection for colonies on lysogeny broth medium plates with chloramphenicol, cells were cultured in the same medium with shaking (50 mL overnight at 37°C followed by inoculation into 0.5 L at an optical density at 600 nm of 0.2) and grown to an optical density of 0.5. Flasks were cooled to room temperature and SdsA1 expression induced by the addition of 0.4 mM IPTG followed by overnight growth at 25°C with shaking.
These cells were lysed by sonication on ice in 50 mM tris-HCl, 10 mM imidazole, pH 7.4, with 2 mM PMSF, which was also the buffer used to equilibrate the Ni-NTA column. Soluble proteins were isolated by centrifugation, loaded onto the Ni-NTA column, and rotated at 4°C for ~72 h. The column was washed three times with two column volumes of 50 mM tris-HCl, 25 mM imidazole, 2 mM PMSF, and 5% glycerol at pH 7.4. SdsA1 was eluted with approximately one column volume of 50 mM tris-HCl, 250 mM imidazole, 2 mM PMSF, and 5% glycerol, pH 7.4, and analyzed by SDS-PAGE and Bradford assay.
2.3 Kinetic analysis and analysis of reactivity by LC-MS
Reactions (40 μL) of 1.0 μM Bds1 and 0.050–1.4 mM hexyl sulfate in 10 mM tris-HCl, pH 7.4, were prepared in HPLC vials with low-volume inserts and incubated at 25°C. Vials were frozen at various time points in kinetics experiments to stop the reaction and subsequently thawed, mixed, and analyzed immediately by LC-MS to measure the remaining substrate concentration. Reactions (80 μL) of 0.080–1.0 μM SdsA1 and 0.025–0.40 mM hexyl sulfate were set up similarly except reactions were analyzed immediately without freezing due to the faster reaction rate. To determine reactivity of different substrates (at 0.50 mM), reactions were carried out for a longer period of time (50–140 h) without freezing. The commercially available aryl sulfatase from P. vulgata (Sigma-Aldrich) was used as a control in some experiments, as was the Bds1 mutant bearing the D166N, H167A amino acid conversions.
Reactions were analyzed with a LC-MS instrument coupling a Dionex UltiMate 3000 HPLC and a LTQ Velos Pro linear ion trap MS. HPLC separation was carried out with 10-μL injection volume using a Thermo Scientific Acclaim Surfactant column (2.1 × 150 mm, 5 μm) in isocratic mixtures of 40 mM ammonium formate (pH 5.0, Fisher Scientific Optima grade) and acetonitrile (BDH, Chromanorm LC-MS grade) at 0.5 mL/min. flow rate with detection of substrate by ESI-MS in negative ion mode. The peak area for the chromatographic peak bearing the exact mass of the substrate was measured and converted to substrate concentration using a standard curve for each substrate analyzed under the same conditions as the reaction samples. Hyperbolic fitting for kinetic experiments was performed in Kaleidagraph 4.1 (Synergy Software), which was also used to determine statistical significance of results via one-way ANOVA with Bonferroni’s posthoc test.
3. Results and discussion
3.1 Mechanism of Bds1-catalyzed hydrolysis of primary 1-alkyl sulfates
To examine enzyme reactivity, we used LC-MS to monitor substrate disappearance and GC-MS to detect alcohol hydrolysis products extracted from reaction mixtures. We found that Bds1 hydrolyzes primary 1-alkyl sulfates bearing alkyl chains of 6–10 and 12 carbon atoms, while Bds1 has little reactivity towards similar molecules of 14, 16, and 18 carbon atoms. In contrast, SdsA1 hydrolyzed all of these substrates. The D166N, H167A Bds1 mutant was unreactive towards any of these substrates, suggesting that the mutant is inactive, as expected from disruption of the conserved Zn2+-binding site.
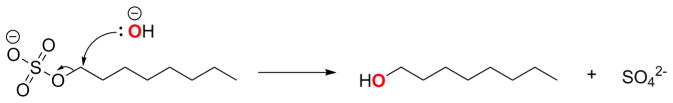
To investigate the mechanism by which Bds1 catalyzes hydrolysis of these primary alkyl sulfates, we analyzed the mass of the octanol product in reactions of 1-octyl sulfate with Bds1 and SdsA1 in normal isotopic water versus 18O-enriched water. Like SdsA1, Bds1 incorporated the labeled oxygen into the octanol product. This result suggests that Bds1 functions by the same hydrolysis mechanism previously observed for SdsA1 [11] involving C–O bond cleavage rather than S–O bond cleavage (Fig. 1).
Fig. 1.
Proposed mechanism of Bds1-catalyzed alkyl sulfate hydrolysis by nucleophilic attack of an exchangeable water molecule (presumed to be activated in the Bds1 active site to formally become a hydroxide ion) on the carbon atom of the sulfate ester linkage, showing incorporation of labelled oxygen (bold/red) into the alcohol product.
3.2 Kinetics of Bds1-catalyzed hydrolysis of 1-hexyl sulfate
We compared the reaction kinetics for Bds1- and SdsA1-catalyzed hydrolysis of a primary 1-alkyl sulfate by monitoring the decrease in substrate concentration over the course of the reaction via LC-MS. Although it does not have a particularly high specificity constant, 1-hexyl sulfate was chosen as the substrate for this comparison because its slower turnover rate with a higher KM than substrates like 1-octyl sulfate makes it more conducive to analysis at measurable substrate concentrations and because it did not cause noticeable enzyme inhibition (which we observed for both Bds1 and SdsA1 at relatively low concentrations of 1-octyl sulfate and which has been observed for Pisa1 [10] and SdsAP [13]).
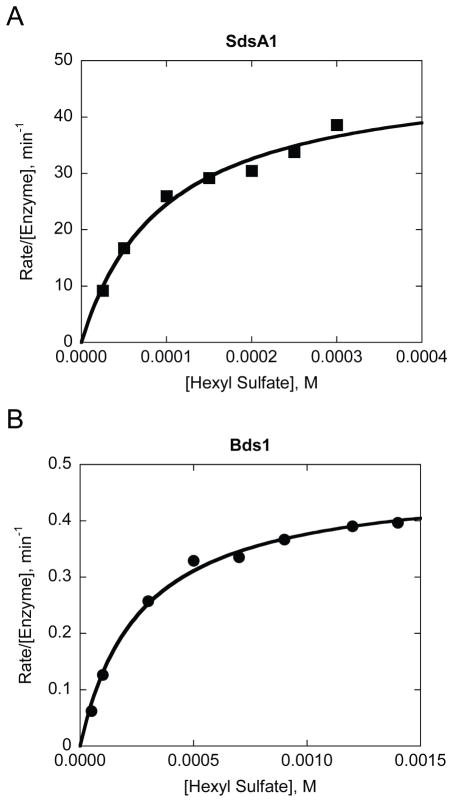
As shown in Fig. 2, the apparent KM values for Bds1 and SdsA1 are somewhat comparable (110 ± 17 μM for SdsA1 and 300 ± 115 μM for Bds1), while the SdsA1-catalyzed reaction occurs with a value of kcat over 100-fold higher than that of the Bds1-catalyzed reaction (64 ± 27 min−1 for SdsA1 versus 0.4 ± 0.1 min−1 for Bds1). This result suggests that the active site of Bds1 is less tuned towards sulfate ester hydrolysis than the active site of SdsA1, possibly due to subtle structural and/or electrostatic differences. One potential implication of this result is that primary alkyl sulfates may not be the biologically relevant substrates for Bds1. Another possibility is that while the ability to hydrolyze such substrates may have conferred an evolutionary advantage to S. cerevisiae (e.g., by liberating inorganic sulfate for use in sulfur-limited conditions), the reaction rate itself may not have undergone stringent selection.
Fig. 2.
Hydrolysis of 1-hexyl sulfate by Bds1 occurs with a similar apparent KM but over 100-fold lower kcat compared to SdsA1-catalyzed hydrolysis of this substrate. Reaction kinetics are shown for A) SdsA1 (0.16 μM; squares) and B) Bds1 (1.0 μM; circles) at 25°C in 10 mM tris-HCl, pH 7.4. Data are fit with hyperbolas from which the Michaelis-Menten parameters kcat and KM were determined. Data are shown for one experiment with each enzyme, representative of three independent biological replicates.
3.3 Reactivity of Bds1 towards aryl sulfates
To study the effect of over-expressing or deleting the BDS1 gene in vivo on the ability of S. cerevisiae to utilize various sulfate sources for growth, we made the Δbds1 yeast deletion mutant and used it in growth tests on solid medium plates supplemented with various sulfate sources. Cells with WT levels of Bds1 and over-expressed Bds1 grew more robustly on octyl sulfate plates than cells lacking Bds1 or expressing the D166N, H167A catalytic mutant. Additionally, cells over-expressing BDS1 were able to grow more robustly on plates bearing dodecyl sulfate. In contrast, over-expression of BDS1 did not enhance growth on plates supplemented with p-nitrophenyl sulfate or p-nitrocatechol sulfate (Supplementary Fig. A.4).
The enhanced growth observed on octyl sulfate and dodecyl sulfate when BDS1 is over-expressed is consistent with a previous report [16]. However, that report also suggested that the presence of Bds1 enabled p-nitrocatechol sulfate hydrolysis because a slight increase in cell density of WT cells compared to Δbds1 cells cultured in the presence of this aryl sulfate was observed and because those WT cells had an increased optical density at 516 nm, where at high pH the hydrolysis product p-nitrocatechol absorbs light and where cells also scatter light [16].
To determine whether the purified Bds1 enzyme is capable of hydrolyzing p-nitrocatechol sulfate to produce p-nitrocatechol, we monitored the absorption spectrum of p-nitrocatechol sulfate over 120 h in the presence of Bds1, the D166N, H167A Bds1 catalytic mutant, and a commercially available aryl sulfatase from P. vulgata. Only the latter aryl sulfatase showed evidence of reaction, producing isosbestic points as the substrate reacted and the p-nitrocatechol product was formed (Supplementary Fig. A.5).
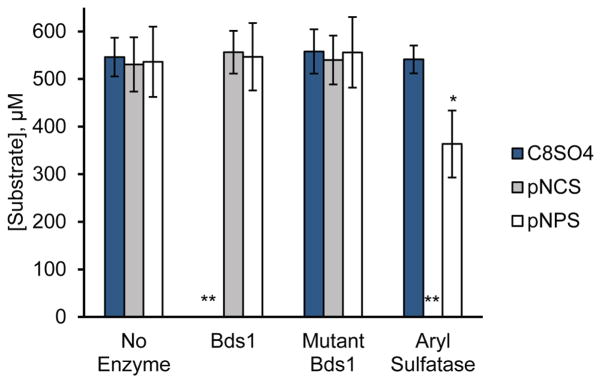
We confirmed these spectroscopic results by LC-MS, as shown in Fig. 3. Using this technique, we did not observe any decrease in substrate concentration for p-nitrocatechol sulfate or p-nitrophenyl sulfate upon incubation with Bds1 for ~50 h. Under the same conditions, the concentrations of both these aromatic substrates significantly decreased in the presence of the P. vulgata aryl sulfatase, and Bds1 catalyzed the complete reaction of 1-octyl sulfate. Thus, aryl sulfates do not appear to be substrates for Bds1.
Fig. 3.
Bds1 hydrolyzes primary alkyl but not aryl sulfates. The substrate concentration remaining after ~50 h reaction of 500 μM substrate with 1.0 μM enzyme is shown for the substrates 1-octyl sulfate (C8SO4), p-nitrocatechol sulfate (pNCS), and p-nitrophenyl sulfate (pNPS) in the absence of enzyme (No Enzyme) or in the presence of Bds1, the catalytic mutant Bds1(D166N, H167A) (Mutant Bds1), or 74 mg L−1 aryl sulfatase from Patella vulgata (Aryl Sulfatase) in 10 mM tris-HCl, pH 7.4. Substrate concentrations following reaction were measured by LC-MS. Bars and error bars indicate the average and standard deviation of three independent biological replicates, each analyzed with three technical replicates. *p<0.0001 compared to the same substrate in the presence of Bds1 or the Bds1 catalytic mutant or in the absence of enzyme. **Substrate levels at the end of these reactions were below the limit of detection, i.e., less than 1 μM substrate.
In light of its homology with and mechanistic similarity to SdsA1 and Pisa1, it is unsurprising that Bds1 lacks aryl sulfatase activity. As a class, aryl sulfatases employ nucleophilic attack on the sulfur atom of the sulfate ester, rather than the carbon atom, and they contain a conserved cysteine or serine within a consensus motif needed for active site post-translational modification [3, 5]. In contrast, Bds1 does not have such a motif and instead resembles the alkyl sulfatases SdsA1 (with which it shares 46% identity) and Pisa1 (with which Bds1 shares 49% identity), harboring the conserved Zn(II)-binding residues and the histidine residue hypothesized to protonate the sulfate to create a better leaving group for the reaction (H299 in Bds1, H306 in SdsA1, and H317 in Pisa1). Carbon nucleophilic attack on aromatic substrates (SNAr) would likely require a different active site arrangement than that of SdsA1, which is at least partially optimized for carbon attack on aliphatic substrates.
3.4 Reactivity of Bds1 towards secondary alkyl sulfates, branched primary alkyl sulfates, steroid sulfates, and alkyl phosphates
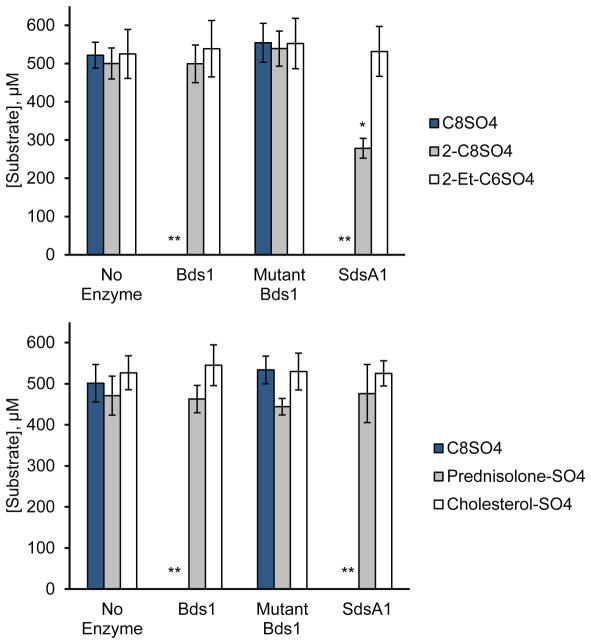
Both SdsA1 and Pisa1 [10, 11], as well as other bacterial sulfatases [19–21], are capable of hydrolyzing at least one enantiomer of the sec-alkyl sulfate rac-2-octyl sulfate. Some bacterial sulfatases are capable of hydrolyzing branched-chain primary alkyl sulfates, including those bearing 2-alkyl substituents [2, 22], and steroid sulfatases are important in human brain tissue to hydrolyze cholesterol sulfate and other sterol/steroid sulfates, rendering them biologically active [1]. To determine whether these structurally diverse sulfates are substrates of Bds1, we compared Bds1 reactivity after 95–120 h with the three structural isomers 1-octyl sulfate, rac-2-octyl sulfate, and 2-ethylhexyl sulfate and with primary and secondary steroid sulfates.
As observed for the homolog Pisa1 [11, 23], SdsA1 appears to react with high enantiomeric selectivity towards the chiral substrate rac-2-octyl sulfate, as only half of the racemic starting material is depleted. However, Bds1 shows no substantial reactivity towards this sec-alkyl sulfate (Fig. 4). Thus, Pisa1 preferentially hydrolyzes sec-alkyl sulfates, SdsA1 can hydrolyze both primary and secondary yet prefers prim-alkyl sulfates [11], and Bds1 hydrolyzes only prim-alkyl sulfates, suggesting subtle active site structural differences in Bds1 that limit its substrate tolerance. Neither Bds1 nor SdsA1 shows significant activity towards the branched primary alkyl sulfate 2-ethylhexyl sulfate, the primary steroid sulfate prednisolone-21-sulfate, or the secondary steroid sulfate cholesterol-3-sulfate (Fig. 4).
Fig. 4.
Bds1 is not active towards a secondary alkyl sulfate, a primary branched-chain alkyl sulfate, or a primary or secondary steroid sulfate. The substrate concentration remaining after 95–120 h reaction of 500 μM substrate with 1.0 μM enzyme is shown for the substrates 1-octyl sulfate (C8SO4), rac-2-octyl sulfate (2-C8SO4), 2-ethylhexyl sulfate (2-Et-C6SO4), prednisolone-21-sulfate (Prednisolone-SO4), and cholesterol-3-sulfate (Cholesterol-SO4) in the absence of enzyme (No Enzyme) or in the presence of Bds1, the catalytic mutant Bds1(D166N, H167A) (Mutant Bds1), or SdsA1 in 10 mM tris-HCl, pH 7.4. Substrate concentrations following reaction were measured by LC-MS. Bars and error bars indicate the average and standard deviation of three independent biological replicates, each analyzed with three technical replicates. *p<0.0001 compared to the same substrate in the presence of Bds1 or the Bds1 catalytic mutant or in the absence of enzyme. **Substrate levels at the end of these reactions were below the limit of detection, i.e., less than 1 μM substrate.
We also investigated phosphate diesters as potential biologically relevant substrates, since phosphate diesters bear the same −1 charge as the sulfate group and are biologically widespread. However, no reactivity of SdsA1 [7] or Bds1 towards dibutyl phosphate, miltefosine, or bis(p-nitrophenyl) phosphate was observed (Supplementary Fig. A.7).
In summary, the SdsA1 homologs that have been identified and characterized to date vary in their selectivity towards primary and secondary alkyl sulfates but do not exhibit aryl sulfatase activity, whereas the uncharacterized homolog CddY does not seem to be involved in sulfate assimilation. One intriguing possibility is that the MBL scaffold of these enzymes might allow the evolution of different functionalities with minimal changes, therefore acting as an evolutionary hotspot.
Supplementary Material
Bds1 hydrolyzes sulfate esters via a C–O bond-breaking catalytic mechanism
Bds1 acts on primary alkyl but not branched-chain, steroid, or aryl sulfates
In contrast to two bacterial homologs, Bds1 is not a secondary alkyl sulfatase
Acknowledgments
We thank Drs. Stephanie M. Boussert, Wendy C. Cory, and Frederick J. Heldrich for generously sharing resources and their expertise and Dr. Wolf-Dieter Schubert (University of Pretoria) and the Helmholtz Center for Infection Research for the SdsA1 pBRR22bll plasmid. We also thank Nathan Adamson, James Holt, Avery Zierk, Corinne Shea, William Zierenberg, Noah Denman, Catherine Smith, and Taylor Devaney for previous work on SdsA1. This work was supported by the National Institutes of Health [grant numbers 5 P20 RR016461 and 8 P20 GM103499], the National Science Foundation [grant number CHE-1229559], the College of Charleston [Summer Undergraduate Research with Faculty grants], the Howard Hughes Medical Institute [grant number 52007537 through the Pre-college & Undergraduate Science Education Program], and Research Corporation for Science Advancement [Cottrell College Science Awards numbers 22490 to M.F. and 22643 to J.L.F.]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A. Supplementary data
Supplementary data include 1) a sequence alignment of Bds1 with homologous proteins; 2) structures for potential substrates tested; 3) SDS-PAGE of purification fractions; 4) growth tests of WT cells and Δbds1 cells expressing Bds1, the Bds1 catalytic mutant, or vector control on medium supplemented with various sulfate sources; 5) visible absorption spectra of p-nitrocatechol sulfate over time in the presence of Bds1, the Bds1 catalytic mutant, and a commercially available aryl sulfatase; 6) mass spectra for 18O isotope experiments; and 7) analysis of reactions with phosphate diesters.
Footnotes
Conflict of interest
The authors declare that they have no conflicts of interest with the contents of this article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Iwamori M, Moser HW, Kishimoto Y. Steroid sulfatase in brain: comparison of sulfohydrolase activities for various steroid sulfates in normal and pathological brains, including the various forms of metachromatic leukodystrophy. J Neurochem. 1976;27:1389–1395. doi: 10.1111/j.1471-4159.1976.tb02620.x. [DOI] [PubMed] [Google Scholar]
- 2.Kahnert A, Kertesz MA. Characterization of a sulfur-regulated oxygenative alkylsulfatase from Pseudomonas putida S-313. J Biol Chem. 2000;275:31661–31667. doi: 10.1074/jbc.M005820200. [DOI] [PubMed] [Google Scholar]
- 3.Kertesz MA. Riding the sulfur cycle--metabolism of sulfonates and sulfate esters in gram-negative bacteria. FEMS Microbiol Rev. 2000;24:135–175. doi: 10.1016/S0168-6445(99)00033-9. [DOI] [PubMed] [Google Scholar]
- 4.Thomas OR, White GF. Metabolic pathway for the biodegradation of sodium dodecyl sulfate by Pseudomonas sp. C12B. Biotechnol Appl Biochem. 1989;11:318–327. [PubMed] [Google Scholar]
- 5.Toesch M, Schober M, Faber K. Microbial alkyl- and aryl-sulfatases: mechanism, occurrence, screening and stereoselectivities. Appl Microbiol Biotechnol. 2014;98:1485–1496. doi: 10.1007/s00253-013-5438-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas D, Surdin-Kerjan Y. Metabolism of sulfur amino acids in Saccharomyces cerevisiae. Microbiol Mol Biol Rev. 1997;61:503–532. doi: 10.1128/mmbr.61.4.503-532.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hagelueken G, Adams TM, Wiehlmann L, Widow U, Kolmar H, Tummler B, Heinz DW, Schubert WD. The crystal structure of SdsA1, an alkylsulfatase from Pseudomonas aeruginosa, defines a third class of sulfatases. Proc Natl Acad Sci U S A. 2006;103:7631–7636. doi: 10.1073/pnas.0510501103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gadler P, Faber K. New enzymes for biotransformations: microbial alkyl sulfatases displaying stereo- and enantioselectivity. Trends Biotechnol. 2007;25:83–88. doi: 10.1016/j.tibtech.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 9.Muller I, Kahnert A, Pape T, Sheldrick GM, Meyer-Klaucke W, Dierks T, Kertesz M, Uson I. Crystal structure of the alkylsulfatase AtsK: insights into the catalytic mechanism of the Fe(II) alpha-ketoglutarate-dependent dioxygenase superfamily. Biochemistry. 2004;43:3075–3088. doi: 10.1021/bi035752v. [DOI] [PubMed] [Google Scholar]
- 10.Knaus T, Schober M, Kepplinger B, Faccinelli M, Pitzer J, Faber K, Macheroux P, Wagner U. Structure and mechanism of an inverting alkylsulfatase from Pseudomonas sp. DSM6611 specific for secondary alkyl sulfates. FEBS J. 2012;279:4374–4384. doi: 10.1111/febs.12027. [DOI] [PubMed] [Google Scholar]
- 11.Schober M, Gadler P, Knaus T, Kayer H, Birner-Grunberger R, Gully C, Macheroux P, Wagner U, Faber K. A stereoselective inverting sec-alkylsulfatase for the deracemization of sec-alcohols. Org Lett. 2011;13:4296–4299. doi: 10.1021/ol201635y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liang Y, Gao Z, Dong Y, Liu Q. Structural and functional analysis show that the Escherichia coli uncharacterized protein YjcS is likely an alkylsulfatase. Protein Sci. 2014;23:1442–1450. doi: 10.1002/pro.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Long M, Ruan L, Li F, Yu Z, Xu X. Heterologous expression and characterization of a recombinant thermostable alkylsulfatase (sdsAP) Extremophiles. 2011;15:293–301. doi: 10.1007/s00792-011-0357-4. [DOI] [PubMed] [Google Scholar]
- 14.Kostichka K, Thomas SM, Gibson KJ, Nagarajan V, Cheng Q. Cloning and characterization of a gene cluster for cyclododecanone oxidation in Rhodococcus ruber SC1. J Bacteriol. 2001;183:6478–6486. doi: 10.1128/JB.183.21.6478-6486.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boer VM, de Winde JH, Pronk JT, Piper MD. The genome-wide transcriptional responses of Saccharomyces cerevisiae grown on glucose in aerobic chemostat cultures limited for carbon, nitrogen, phosphorus, or sulfur. J Biol Chem. 2003;278:3265–3274. doi: 10.1074/jbc.M209759200. [DOI] [PubMed] [Google Scholar]
- 16.Hall C, Brachat S, Dietrich FS. Contribution of horizontal gene transfer to the evolution of Saccharomyces cerevisiae. Eukaryot Cell. 2005;4:1102–1115. doi: 10.1128/EC.4.6.1102-1115.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gietz RD, Schiestl RH. High-efficiency yeast transformation using the LiAc/SS carrier DNA/PEG method. Nat Protoc. 2007;2:31–34. doi: 10.1038/nprot.2007.13. [DOI] [PubMed] [Google Scholar]
- 18.Diekert K, de Kroone AI, Kispal G, Lill R. Isolation and subfractionation of mitochondria from the yeast Saccharomyces cerevisiae. Methods Cell Biol. 2001;65:37–51. doi: 10.1016/s0091-679x(01)65003-9. [DOI] [PubMed] [Google Scholar]
- 19.Matcham GW, Dodgson KS. Purification, properties and cellular localization of the stereospecific CS2 secondary alkylsulphohydrolase of Comamonas terrigena. Biochem J. 1977;167:723–729. doi: 10.1042/bj1670723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pogorevc M, Faber K. Purification and characterization of an inverting stereo- and enantioselective sec-alkylsulfatase from the gram-positive bacterium Rhodococcus ruber DSM 44541. Appl Environ Microbiol. 2003;69:2810–2815. doi: 10.1128/AEM.69.5.2810-2815.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw DJ, Dodgson KS, White GF. Substrate specificity and other properties of the inducible S3 secondary alkylsulphohydrolase purified from the detergent-degrading bacterium Pseudomonas C12B. Biochem J. 1980;187:181–190. doi: 10.1042/bj1870181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ellis AJ, Hales SG, Ur-Rehman NG, White GF. Novel alkylsulfatases required for biodegradation of the branched primary alkyl sulfate surfactant 2-butyloctyl sulfate. Appl Environ Microbiol. 2002;68:31–36. doi: 10.1128/AEM.68.1.31-36.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schober M, Knaus T, Toesch M, Macheroux P, Wagner U, FK The Substrate Spectrum of the Inverting sec-Alkylsulfatase Pisa1. Adv Synth Catal. 2002;354:1737–1742. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.