Abstract
Phascolarctobacterium can produce short-chain fatty acids, including acetate and propionate, and can be associated with the metabolic state and mood of the host. The present study investigated the colonization characteristics of Phascolarctobacterium faecium in healthy individuals <1–80 years old in Southern China. A total of 150 fresh fecal samples were collected, and bacterial DNA was isolated from these samples for quantitative polymerase chain reaction analysis. Phascolarctobacterium faecium demonstrated a high colonization rate and abundant colonization in the human gastrointestinal tract. The colonization rate varied between 43.33–93.33%, and the abundance of Phascolarctobacterium faecium ranged between 3.22–5.76 log cells g-1 (<1 years old) and 3.06–9.33 log cells g-1 (>1 year old). The permillage of Phascolarctobacterium faecium in total bacteria ranged between 0.004–1.479. There was presence of Phascolarctobacterium faecium-like bacteria in younger individuals with a gradual increase in the number of bacteria maintained at a high level with increasing ages (between 1 and 60 years old), but with a decrease in elderly individuals (>60 years old). The results of the present study demonstrated that Phascolarctobacterium faecium is abundantly colonized in the human gastrointestinal tract.
Keywords: Phascolarctobacterium faecium, real-time polymerase chain reaction, colonization rate, age-related distribution, gut microbiota
Introduction
The human gut microbiota is composed of 400–500 species of microbes. However, the molecular classification of operational taxonomic units (OTUs) indicate the presence of more than 1,000 OTUs (OTUs, equivalent to species) in the gut of each in different societies, and that the number of OTUs increases with age (1,2). The gene pool of the microbial habitants of the gut is extremely diverse and considerably larger than the gene pool of the host, which determines a number of metabolic capacities of the bacteria that are essential for the survival of these organisms in the gut (1,3,4).
In recent years, high throughput sequencing technologies have revealed the correlation between gut microbiota and the host. Phascolarctobacterium was found to be a substantial acetate/propionate-producer that could be dramatically increased by berberine and metformin. This in turn may contribute to the beneficial effects of the two drugs on the host (5). Moreover, Phascolarctobacterium was found to be positively correlated to the positive mood of the human (6). An increasing number of studies proposed that Phascolarctobacterium faecium (P. faecium) exerted beneficial effects on the host, including rat model of nonalcoholic fatty liver (7).
P. faecium, which utilizes succinate and produces propionate, was first purified from koala feces in 1992. P. faecium are obligate anaerobic, Gram-negative, non-spore-forming, non-motile, asaccharolytic, and belonging to firmicutes (8). Although uncultured colonies closely related to P. faecium were frequently detected in samples from the human gastrointestinal tract, isolation of Phascolarctobacterium from the human gastrointestinal tract and the expansion of the culture are not yet described in the literature (9), thereby limiting the functional studies and clinical applications. In addition, the gut microbiota can be affected by aging and diet habit. For example, the elderly subjects as compared to the adult population show a reduction in the diversity of the microbiota, characterized by a considerable interindividual variability, with lower numbers of firmicutes (10). The bacterial composition can also be affected by the diet. Human gut bacterial communities like P. faecium are altered by the addition of cruciferous vegetables to a regulated fruit- and vegetable-free diet (11). P. faecium may have undergone a widespread distribution in the human gastrointestinal tract. As the intestinal microbiota changes during aging, presumably, the level of intestinal P. faecium may be different during the human life. Thus, the detection of the presence of this bacterium requires an in-depth investigation.
Therefore, we have attempted to isolate P. faecium bacteria from human feces and investigated the distribution of P. faecium-like bacteria in human feces using real-time PCR analysis with species-specific primers.
Materials and methods
Isolation of strains of P. faecium
Fecal samples were freshly collected from healthy Chinese females (age, median 25 years old) and serially diluted in pre-reduced PBS (0.15 M NaCl, 0.15 M sodium phosphate, 0.05 M phosphate-buffered saline; pH 7.0) from 10−1 to 10−5g and immediately transferred into an anaerobic glove box containing 80% N2, 10% CO2, and 10% H2. 100 µl fecal diluents were then inoculated to the chocolate plate (Detgerm Microbiology Technology, Guangzhou, China) and incubated at 37°C for 5 days. Single pinpoint colonies were selected and regrown on chocolate plates. This step was repeated until pure cultures were obtained. Finally, two strains have been achieved with pure cultures. 16S rDNA from each was PCR amplified using the universal primers 27f(59-AGA GTT TGA TCC TGG CT-39) and 1492r(59-GGT TAC CTT GTT ACG ACT T-39) (12). The 16S rDNA gene sequences of the strains were submitted to GenBank, and similar sequences were searched for in the public databases using the BLAST algorithm. The sequence similarity was analyzed with the EzTaxon-e-server (13). MEGA5.0 software was used to construct the phylogenetic tree by the neighbor-joining method (14,15).
Fecal sample collection
Fecal samples from healthy infant subjects aged <1 year old (n=30), 1–10 years old (n=30), 10–30 years old (n=30), 30–60 years old (n=30), and 60–80 years old (n=30), were collected and DNA extracted as described below. All the individuals we tested were in southern China, who consumed high proportion of carbohydrate and a moderate amount of fat, protein and vegetables daily in general.
DNA extraction from human fecal samples
Total DNA was extracted from the fecal samples by a QIAamp Fast DNA Stool Mini kit (Hilden, Germany), following the manufacturer's instructions.
Species-specific primer design
Two specific primers were designed from the variable regions of the 16S RNA gene sequence of P. faecium. The GenBank program of NCBI (BLAST) showed that both the primers were specific to the P. faecium. The primers PF1 (5′GGC GGC TTA ATA AGT CGA GC3′) and PF2 (5′CGT TCG CTA CCC TGG CTT TC3′) amplified a 200 bp amplicon.
Primers for examining the species specificity
Primers were purchased from Sangon Biotech Co., Ltd (Shanghai, China). The specificity was further confirmed by the DNA extracted from different species, comprising four phyla: (1) Actinobacteria: 2 species of Propionibacterium, 3 of Micrococcus and 2 of Nocardia. (2) Bacteroidetes: 5 Bacteroides, 2 Flavobacterium and 3 Sphingobacterium. (3) Firmicutes: 10 Bacillus, 5 Staphylococcus, 6 Enterococcus, 8 Lactobacillus, 4 Clostridium and 2 Listeria. (4) Proteobacteria: 2 Neisseriales, 8 Burkholderia, 10 Salmonella, 1 Pseudomonas. These cultures were provided by The Clinical Laboratory Culture Collection of Zhujiang Hospital. The DNA extract of the above strains was utilized in real-time PCR. The results did not reveal any amplified signal from all these strains. DNA electrophoresis post-PCR substantiate the specificity of the primers (Fig. 1).
Figure 1.

P. faecium specific primer confirmation by PCR. Lanes 1 and 2 were clinically-isolated P. faecium (strain 1 and strain 2, respectively), lane 3: Propionibacterium, lane 4: Micrococcus, lane 5:Nocardia, lane 6:Bacteroides, lane 7: Flavobacterium, lane 8: Sphingobacterium, lane 9: Bacillus, lane 10: Staphylococcus, lane 11: Enterococcus, lane 12: Lactobacillus, lane 13: Clostridium, lane 14: Listeria, lane 15: Neisseriales, lane 16: Burkholderia, lane 17: Salmonella, lane 18: Pseudomonas, lane 19: negative control, lane M: marker.
Real-time PCR on P. faecium
The real-time PCR standard curve of P. faecium was built using SYBR® Select Master Mix (#44729, Life Technologies, Grand Island, NY, USA), following the manufacturer's protocol, in an ABI vii7 real-time PCR System (Applied Biosystems, USA). The DNA samples were pre-denatured at 95°C for 2 min (first stage) and then amplified with 40 cycles at 95°C for 15 sec, at 58°C for 30 sec, at 72°C for 60 sec, and the final extension at 72°C for 5 min. The fluorescent product was detected in the last step of each cycle. To distinguish the target PCR product from the non-target product, melting curve analysis was performed after amplification using the default settings of the ABI software from 60 to 95°C.
The standard curve was created using 10-fold serial dilutions of P. faecium pure culture DNA corresponding to 103 to 109 cells/reaction, determined microscopically by DAPI staining method (16). The bacterial concentration of each sample was estimated by comparing the threshold cycle (CT) values obtained from the standard curve. All the samples were analyzed in triplicate. A linear correlation between the cell counts and the CT values (r2=0.998) was observed when the number of cells/reaction mixture was between 103 and 109. The melting curve analysis of the amplicons obtained by real-time PCR generated a distinct peak at 82°C in the 16S rRNA gene of P. faecium.
Statistical analysis
The data were expressed as median with interquartile ranges in the parentheses. Man-Whitney U test was used to estimate the amount of P. faecium in feces, whereas the χ2 test was used to analyze the colonization rate in various stages of age (P<0.05 was considered to indicate a statistically significant difference). All data were analyzed by SPSS20.0 software (IBM SPSS, Armonk, NY, USA).
Results
Successful isolation of P. faecium from human feces
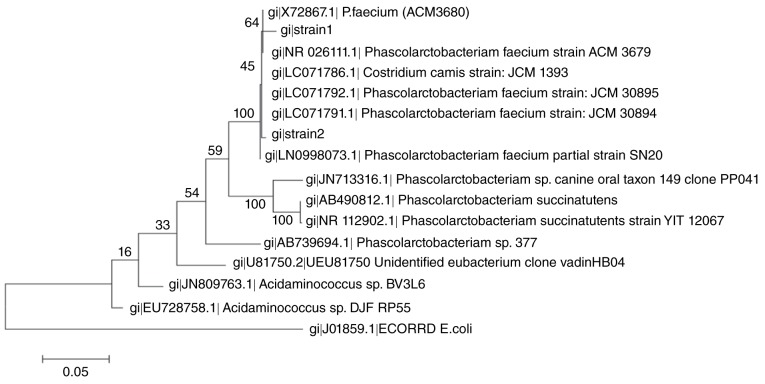
P. faecium was successfully isolated from the human gut. The 16S rDNA sequence indicated that the strain belonged to the originally-isolated strain from koala feces, with 99% similarity. The phylogenetic tree showed that the position of these two clinically isolated strains among the selected clones or strains belonged to Phascolarctobacterium (Fig. 2).
Figure 2.
Phylogenetic tree showing the position of the two clinically isolated P. faecium strains (strain1 and strain2) among the selected clones or strains belonging to Phascolarctobacterium. The tree, which was rooted using Escherichia coli as the outgroup, was generated by the neighbor-joining method.
P. faecium have a high colonization rate in gastrointestinal tract
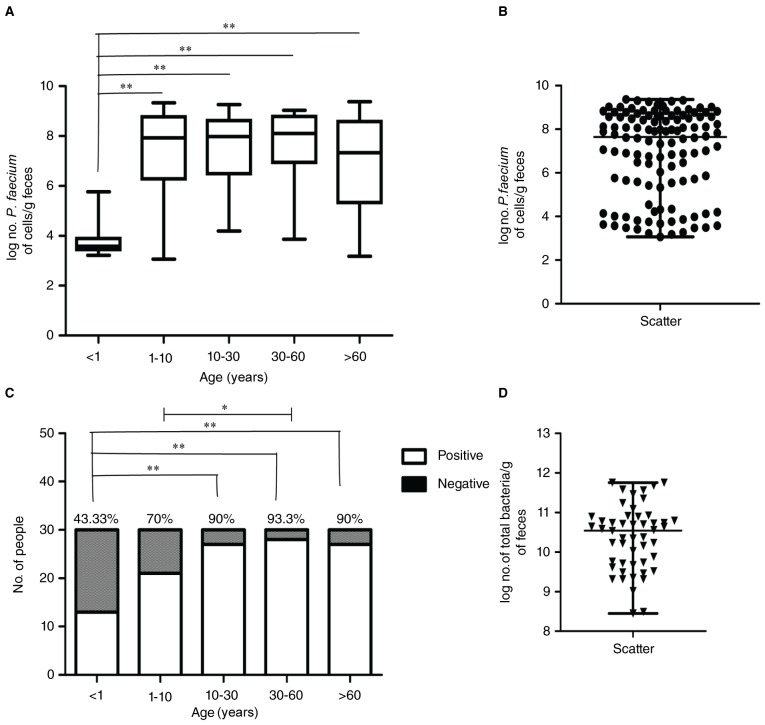
As presented in Fig. 3, a P. faecium colonization characteristic analysis was conducted. The colonization rate was calculated by the number of P. faecium colonized samples/total number of samples in the same age stage. Five different age stages presented different colonization rates. The highest colonization rate in the five age stages was found in individuals aged 30–60 years old (93.33%). Individuals <1 year old showed the lowest colonization rate (43.33%). The colonization rate increased quickly from infant to youth (70%), 1–10 years old. Adults aged 10–30 years old exhibited a similar colonization rate as compared to the elder group (90%). The statistical results by χ2 test were represented in Fig. 3C. The colonization rate in infants is significantly low to those aged 10–30 years old, 30–60 year-old adults, and elders (P<0.01). On the other hand, youth aged 1–10y also exhibits a significantly low colonization rate as compared to adults aged 30–60y (P<0.05) (Fig. 3C).
Figure 3.
P. faecium colonization characteristic analysis. (A) Log no. P. faecium of cells g−1 feces from five different age stages; significant differences were determined by χ2 test. (B) The scatter spot distribution of log no. P. faecium of cells g−1 feces. (C) No. of positive and negative individuals and colonization rate in five different age stages; significant differences were determined by Mann-Whitney U test. (D) The scatter spot distribution of log No. of total bacteria g−1. Microbial data were expressed as median, with interquartile ranges in parentheses as non-normal distribution. *means significant differences P<0.05 **means significant differences P<0.01.
P. faecium abundantly colonized the gastrointestinal tract
The values ranged from 3.22–5.76 log cells g−1and 3.06–9.33 log cells g−1in samples from infants (age<1y) to youth (1y<age<10y). The highest level was presented in adults aged between 30–60y, ranging from 3.86–9.03 log cells g−1. Adults aged from 10–30y and elders reveal values, 4.20–9.26 and 3.18–9.37, respectively. Remarkably, the concentration of P. faecium cells in the fecal samples from infants was significantly less (P<0.01) as compared to the other sample groups determined by a Mann-Whitney U test (Table I, Fig. 3A and B).
Table I.
No. of P. faecium-like bacteria/g of feces and percentage in total bacteria
| Group (years) | No. of P. faecium colonized sample/total No. of sample | log no. of P. faecium cells/g feces (a) | log no. of total bacteria cells/g feces (a) | % relative to the total no. of cells |
|---|---|---|---|---|
| <1 | 13/30 (43.33%) | 3.78 (3.22–5.76)b | 9.14 (8.47–10.92) | 0.004 |
| 1 to 10 | 21/30 (70%) | 7.30 (3.06–9.33) | 10.32 (9.46–10.91) | 0.955 |
| 10 to 30 | 27/30 (90%) | 7.55 (4.20–9.26) | 10.64 (10.35–10.93) | 0.812 |
| 30 to 60 | 28/30 (93.33%) | 7.68 (3.86–9.03) | 10.51 (10.16–10.76) | 1.479 |
| >60 | 27/30 (90%) | 6.82 (3.18–9.37) | 10.56 (9.722–10.96) | 0.195 |
No., number.
Due to a non-normal distribution, microbial data were expressed as median, with interquartile ranges in parentheses.
significant differences (P<0.01) in fecal samples were determined between age <1y and the other different age stages, by Man-Whitney U test.
P. faecium-like bacteria were present at a high level in total bacterial population
Total bacteria in samples ranged from 9.14–10.64 log cells g−1 in the fecal samples (Table I, Fig. 3D). The relative abundance of P. faecium in total bacteria (‰) was calculated by the amount of P. faecium/ the amount of total bacteria*1000. Each sample was calculated separately and their means in each group were presented in Table I. The permillage of P. faecium in total bacteria ranged from 0.004–1.479. This data revealed a high colonization status of P. faecium in the gastrointestinal tract (Table I).
Age distribution of P. faecium-like bacteria
P. faecium-like bacteria appeared in early-life and increased in adult stage followed by a decrease in the elderly age. According to the colonization rate, value, and permillage of total bacteria, P. faecium-like bacteria appeared in early-life, increased in adult age, and then subsequently decreased in the elder age. This was a common bacteria colonized in the gastrointestinal tract.
Discussion
The lumen of the human gastronintestinal tract contains trillions of commensal bacteria, which are difficult to cultivate. P. faecium was first purified from koala feces. Since then, additional reports of P. faecium have been lacking. We firstly isolated some strains of P. faecium from the human gastrointestinal tract. The isolation of the genus of Phascolarctobacterium from human feces was a herculean task as a tip-size colony had to be harvested from Columbia Blood Agar plate after approximately one week.
Culture-dependent techniques, which are a beneficial way to elucidate the gut bacterial repertoire, can be used only for a limited range of bacteria in the human gut (17). Thus, improving the culture-dependent methods for the isolation of P. faecium from feces remains a key challenge in microbiology.
In the current study, we found that this species not only has a high colonization rate but is also abundantly colonized in the human gastrointestinal tract. This may suggest its critical role in the dynamic balance of the gut microbiota. The colonization rate ranged from 43.33 to 93.33% and the amounts ranged from 3.22–5.76 log cells g−1 and 3.06–9.33 log cells g−1, according to different age stages. This confirmed that aging was a crucial factor for the dynamic balance of the gut flora. Age distribution showed that P. faecium-like bacteria were present in the early-life, and the number increased rapidly in people aged 1–60 years old, the number decreased in elders (>60 years old). This phenomenon may be attributed to the diet habit. A previous study demonstrated that rats fed with high-fat diet appeared to have a higher amount of the short chain fatty acid producers, including P. faecium (18). Besides, Phascolarctobacterium spp. specialise in the utilisation of succinate produced by other bacteria (8). In parallel, the abundance of Bacteroides and Parabacteroides, both major producers of succinate, increased by high fat diet and was positively correlated with body weight (19). The elderly and individuals aged less than 1 year consumed relative less fat and have a relative low body weight, this may resulted in Bacteroides and Parabacteroides decreased, and the available succinate for P. faecium was decreased. Physical exercise was another factor accounting for Phascolarctobacterium decrease in the elderly. Rats with a low capacity for running that were fed an acute high-fat diet appeared to decrease the amount of Phascolarctobacterium and be more susceptible to nonalcoholic fatty liver disease (7). Compared with the young, the elderly population have less physical exercise, which may be another reason for Phascolarctobacterium decrease.
Of note, individuals in these two age stages (<1 year and >60 years old, respectively) are more susceptible to digestive and metabolic diseases, which are likely linked to the less amount of P. faecium. P. faecium-like bacteria are also present at a high percentage in total bacteria. The permillage of P. faecium in total bacteria ranged from 0.004 to 1.479. In addition, P. faecium was firstly isolated from koala, an animal fed on poisonous eucalyptus leaves, it may have link with the detoxication of gut microbiota. Thus, P. faecium may exert a beneficial role in the human gastrointestinal tract. However, further investigation is imperative to delve into the functional role of these strains in the human gastrointestinal tract.
Herein, we demonstrate, for the first time, the corroboration and development of a quantitative real-time PCR for the detection and quantification of bacteria related to P. faecium. Our results demonstrate that P. faecium is present and colonizes the gastrointestinal tract in early-life and develops to a high level in healthy adults followed by a decrease in elders. Further studies are a prerequisite to exploring the role of P. faecium in the microbiota development and its role in human health.
Acknowledgments
The present study was supported by grants from the National Natural Science Foundation of China (No. 81441066), the Natural Science Foundation of Guangdong Province (S2013010014850), the Guangdong Provincial Department of Science and Technology (2015A020213002), and Guangzhou Kangze Medical Science and Technology Co., Ltd fund (201511).
Glossary
Abbreviations
- SCFA
short-chain fatty acids
- P. faecium
Phascolarctobacterium faecium
- OTUs
operational taxonomic units
- CT
threshold cycle
- PCR
polymerase chain reaction
References
- 1.Ramakrishna BS. Role of the gut microbiota in human nutrition and metabolism. J Gastroenterol Hepatol. 2013;28(Suppl 4):S9–S17. doi: 10.1111/jgh.12294. [DOI] [PubMed] [Google Scholar]
- 2.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, Magris M, Hidalgo G, Baldassano RN, Anokhin AP, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–227. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shanahan F. The colonic microbiota in health and disease. Curr Opin Gastroenterol. 2013;29:49–54. doi: 10.1097/MOG.0b013e32835a3493. [DOI] [PubMed] [Google Scholar]
- 4.Goodman AL, Gordon JI. Our unindicted coconspirators: Human metabolism from a microbial perspective. Cell Metab. 2010;12:111–116. doi: 10.1016/j.cmet.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Zhao Y, Xu J, Xue Z, Zhang M, Pang X, Zhang X, Zhao L. Modulation of gut microbiota by berberine and metformin during the treatment of high-fat diet-induced obesity in rats. Sci Rep. 2015;5:14405. doi: 10.1038/srep14405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Su Q, Xie B, Duan L, Zhao W, Hu D, Wu R, Liu H. Gut microbes in correlation with mood: Case study in a closed experimental human life support system. Neurogastroenterol Motil. 2016;28:1233–1240. doi: 10.1111/nmo.12822. [DOI] [PubMed] [Google Scholar]
- 7.Panasevich MR, Morris EM, Chintapalli SV, Wankhade UD, Shankar K, Britton SL, Koch LG, Thyfault JP, Rector RS. Gut microbiota are linked to increased susceptibility to hepatic steatosis in low-aerobic-capacity rats fed an acute high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2016;311:G166–G179. doi: 10.1152/ajpgi.00065.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dot TD, Osawa R, Stackebrandt E. Phascolarctobacterium faecium gen. nov, spec. nov., a Novel Taxon of the Sporomusa Group of Bacteria. Syst Appl Microbiol. 1993;16:380–384. doi: 10.1016/S0723-2020(11)80269-9. [DOI] [Google Scholar]
- 9.Watanabe Y, Nagai F, Morotomi M. Characterization of Phascolarctobacterium succinatutens sp. nov., an asaccharolytic, succinate-utilizing bacterium isolated from human feces. Appl Environ Microbiol. 2012;78:511–518. doi: 10.1128/AEM.06035-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rondanelli M, Giacosa A, Faliva MA, Perna S, Allieri F, Castellazzi AM. Review on microbiota and effectiveness of probiotics use in older. World J Clin Cases. 2015;3:156–162. doi: 10.12998/wjcc.v3.i2.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li F, Hullar MAJ, Schwarz Y, Lampe JW. Human gut bacterial communities are altered by addition of cruciferous vegetables to a controlled fruit- and vegetable-free diet. J Nutr. 2009;139:1685–1691. doi: 10.3945/jn.109.108191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jordan EM, Thompson FL, Zhang XH, Li Y, Vancanneyt M, Kroppenstedt RM, Priest FG, Austin B. Sneathiella chinensis gen. nov., sp. nov., a novel marine alphaproteobacterium isolated from coastal sediment in Qingdao, China. Int J Syst Evol Microbiol. 2007;57:114–121. doi: 10.1099/ijs.0.64478-0. [DOI] [PubMed] [Google Scholar]
- 13.Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol. 2012;62:716–721. doi: 10.1099/ijs.0.038075-0. [DOI] [PubMed] [Google Scholar]
- 14.Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 15.Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morikawa K, Yanagida M. Visualization of individual DNA molecules in solution by light microscopy: DAPI staining method. J Biochem. 1981;89:693–696. doi: 10.1093/oxfordjournals.jbchem.a133247. [DOI] [PubMed] [Google Scholar]
- 17.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, Gill SR, Nelson KE, Relman DA. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–1638. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, Morris MJ. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10:e126931. doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lecomte V, Kaakoush NO, Maloney CA, Raipuria M, Huinao KD, Mitchell HM, Morris MJ. Changes in gut microbiota in rats fed a high fat diet correlate with obesity-associated metabolic parameters. PLoS One. 2015;10:e126931. doi: 10.1371/journal.pone.0126931. [DOI] [PMC free article] [PubMed] [Google Scholar]