Abstract
Background
A randomized controlled study (RCT) recently showed that short-term heart rate variability (HRV) biofeedback in addition to standard rehabilitation care for alcohol dependence can reduce craving, anxiety and improve cardiovascular autonomic function. In this one-year follow-up study we aimed to explore whether completion of 2-week HRV-Biofeedback training is associated with long-term abstinence. Furthermore, we sought to identify potential predictors of post-treatment abstinence.
Methods
We conducted a survey on abstinence in patients with alcohol dependence 1 year after completion of an RCT comparing HRV-biofeedback in addition to inpatient rehabilitation treatment alone (controls). Abstinence rates were compared and analysed for association with demographic data as well as psychometric and autonomic cardiac assessment before and after completion of the biofeedback training using bivariate and multivariate regression analyses.
Results
Out of 48 patients who participated in the RCT, 27 patients (9 females, ages 42.9 ± 8.6, mean ± SD) completed our one-year follow-up. When including in the analysis only patients who completed follow-up, the rate of abstinence tended to be higher in patients who underwent HRV-biofeedback 1 year earlier compared to those who received rehabilitative treatment alone (66.7% vs 50%, p = ns). This non-significant trend was also observed in the intention-to-treat analysis where patients who did not participate in the follow-up were assumed to have relapsed (46,7% biofeedback vs. 33.3% controls, p = ns). Neither cardiac autonomic function nor psychometric variables were associated with abstinence 1 year after HRV-biofeedback.
Conclusion
Our follow-up study provide a first indication of possible increase in long-term abstinence after HRV-biofeedback for alcohol dependence in addition to rehabilitation.
Trial registration
The original randomized controlled trial was registered in the German Clinical Trials Register (DRKS00004618). This one-year follow-up survey has not been registered.
Keywords: HRV, Heart rate variability, Biofeedback, Autonomic, Abstinence, Alcohol addiction, Craving, Rehabilitation, Survey, Relapse
Background
Alcohol dependence is a major global public health problem which affects 5.1% of the global population and causes up to 3.3 million deaths per year, the majority of those being related to cardiovascular diseases [1]. Although integrative multimodal acute and rehabilitative treatment regimens have been widely established to reduce the disease burden related to alcohol dependence, malcompliance and low rates of adherence to treatment are frequently compromising success of these therapies [2, 3]. Moreover, even after completion of rehabilitation relapse poses a major problem. Major predictors of post-treatment relapse have been identified in large observational studies, including substance use patterns prior to treatment, psychiatric comorbidities, social and psychological characteristics as well as craving [4–9].
We recently showed in a randomized controlled study that heart rate variability (HRV) biofeedback in addition to standard rehabilitation care for alcohol dependence can reduce craving and anxiety more effectively than rehabilitative treatment alone [10]. In this study, we also observed improvement in cardiac autonomic and neurovascular function in patients undergoing biofeedback possibly mediated by counterbalancing a chronic shift toward an increased sympathetic and decreased parasympathetic tone. However, our short-term follow-up data did not answer the question whether the observed improvements in psychometric and cardiac autonomic endpoints translate into reduced risk of post-treatment relapse.
In this follow-up study we aimed to determine the relapse rate 1 year after HRV-biofeedback training and integrative inpatient rehabilitative treatment in order to explore potential treatment effects and acquire first long-term data that could form a basis for confirmatory research in large study populations. Furthermore, we sought to identify predictors of abstinence following combined rehabilitative and HRV-biofeedback treatment.
Methods
Study design and population
This is a one-year follow-up survey study after a randomized controlled trial on the effects of HRV-biofeedback on cardiac autonomic function assessed via time and frequency domain parameters of HRV, autonomic neurovascular function assessed via laser Doppler flowmetry of cutaneous blood flow after sympathetic stimulation as well as craving, anxiety and depressive symptoms evaluated using psychometric tests. These techniques were reported in detail elsewhere [10]. Briefly, male and female patients undergoing inpatient rehabilitation treatment for alcohol use disorder received either HRV-biofeedback in addition to standard rehabilitative care or standard rehabilitative care only. The study intervention comprised application of a validated HRV-biofeedback system (StressPilot™; BioSign, Ottenhofen, Germany) with continuous measurement and real-time visualization of HRV. Study subjects were instructed to breathe at a given frequency of six cycles per minute to increase the parasympathetic tone and thereby HRV. Patients in the HRV-biofeedback group underwent three 20-min sessions of HRV-biofeedback training per week over 2 weeks whereas control patients did not undergo biofeedback. Psychometric testing, and assessment of neurovascular and autonomic cardiac function were undertaken before the beginning of the first biofeedback session, immediately after completion of the last biofeedback session as well as 3 and 6 weeks afterwards.
To perform a one-year follow-up assessment of abstinence, study participants were contacted by mail 1 year after discharge from rehabilitative therapy after having given written permission to be contacted during inpatient treatment. They were asked to answer a standard questionnaire about their social situation, alcohol and drugs consumption, as well as need for further institutional treatment in the period of 12 months after discharge. Abstinence from alcohol and drugs was considered no consumption during 12 months after discharge from our clinic. We extracted demographic characteristics in subjects who had undergone HRV-biofeedback in addition to rehabilitation 1 year earlier and those who had received rehabilitative treatment alone from the original dataset.
Comparison of abstinence rates
Abstinence rates 1 year after discharge from the rehabilitative therapy were evaluated applying standard criteria of the German Society for Addiction Research and Addiction Treatment [11]. Individuals who have not consumed any alcohol since discharge from the inpatient treatment were considered abstinent whereas any alcohol consumption post-rehabilitation was defined relapse. In a first analysis we included all patients who participated in the original study protocol and returned our survey 1 year later to compare rates of abstinence in patients that had been allocated to the HRV-biofeedback group and those who had undergone rehabilitative treatment alone during the RCT. We then went on and repeated the comparative analysis applying an intention-to-treat approach, including the entire study population of the RCT irrespective of whether they have participated in the one-year follow-up. In this more conservative analysis, non-responders were considered to have relapsed.
Analysis of factors related to abstinence
In order to define individual factors related to abstinence we compared patients who reported being abstinent 1 year post-intervention and those who reported relapse in terms of demographic characteristics as well as psychometric measurements and parameters of the autonomic cardiac function. These analyses were performed in patients who completed follow-up. We included in our analyses psychometric scores at baseline and those obtained immediately following the final HRV-biofeedback training session (post-biofeedback or post-observation period for patients in the control group). Psychometric scores were obtained using the Obsessive Compulsive Drinking Scale, as measure of craving as well as the subscales Anxiety and Depression from the Symptom Checklist-90, as measures of anxiety and depression. Measures of the cardiac autonomic function comprised the time-domain parameter coefficient of variation of R-R intervals (CVNN) and frequency-domain parameters high frequency (HF), low frequency (LF) and total power (TP) at baseline and immediately post-biofeedback or observation period. In order to improve our understanding of the association between applied treatments, and sustained abstinence, this analysis was undertaken in both the biofeedback and the control group.
We then went on to identify specific predictors of abstinence after rehabilitative inpatient care by relating baseline data extracted from the original dataset of the randomized controlled trial to data from the present survey. We conducted these analyses separately for control patients and those who had received HRV-biofeedback. In order to identify any specific changes in biological or psychological characteristics due to HRV-biofeedback which relate to sustained abstinence, analyses were repeated using data on patient characteristics and outcomes obtained immediately after completion of the biofeedback intervention, the duration of the control period, respectively.
Statistical analysis
Statistical analyses were performed using SPSS ® 21 (IBM, Armonk, NY, USA). Dichotomous data were compared using Ӽ2 test in patients who completed follow-up. Continuous data on study population characteristics were compared between patients who had received HRV-biofeedback in addition to rehabilitation and those who had undergone rehabilitative treatment only using Student’s t–test or Mann-Whitney U test, according to distribution. The same tests were used to compare study population characteristics and post-biofeedback outcomes between patients who reported abstinence after 1 year and those who had relapse. These analyses were first undertaken in those patients that have participated in the follow-up assessment excluding those that have not returned the survey questionnaire. The same tests were then applied to undertake an intention-to-treat analysis in the entire study population, considering those who have not participated in the one-year follow-up to have relapsed. Comparisons between patients with and without achievement of on-year abstinence were performed separately for the biofeedback and the control group. Alpha level for statistical significance was set to 0.05. Bivariate logistic regression analyses were undertaken to identify possible predictor variables among demographic and outcome variables with respect to one-year abstinence in patients who completed follow-up. Those variables that emerged as predictors of abstinence were included in multivariate models with adjustment for demographic characteristics. Unstandardized B-coefficients (ß) and p-values were computed.
Results
Demographic characteristics
Among 48 study participants who have been contacted by mail, 27 responded and completed the questionnaire (56.2%; 15 biofeedback and 12 control patients, 18 males and 9 females, ages 42.9 ± 8.6, mean ± SD). Demographic characteristics of patients who completed follow-up are shown in Table 1. Within this population, there were no differences in age, gender, tobacco use, neuropathy and number of cases of liver disease between patients who have undergone biofeedback in addition to rehabilitative treatment and those who have undergone rehabilitation only. (Table 1) Demographic characteristics of the study population which consists of all patients included in the intention-to-treat analysis are reported elsewhere and have been additionally amended to Table 1 [10].
Table 1.
Demographic Characteristics
| Patients who completed follow-up | Intention-to-treat | |||||
|---|---|---|---|---|---|---|
| HRV Biofeedback (n = 15) |
Control (n = 12) |
p-value | HRV Biofeedback (n = 24) |
Control (n = 24) |
p-value | |
| Age (years) | 41.2 ± 8 | 43.6 ± 9 | 0.74 | 40 ± 7 | 44 ± 8 | 0.06 |
| Gender (%) | 53.3 m, 46.7 f | 83.3 m, 16.7 f | 0.09 | 70.8 m, 29.2 f | 70.8 m, 29.2 f | 0.09 |
| Smoking (%) | 80 | 75 | 0.75 | 79 | 75 | 0.36 |
| Comorbidities | ||||||
| • Neuropathy (%) | 26.7 | 0 | 0.05 | 33.3 | 16.7 | 0.09 |
| • Hepatic steatosis (%) | 20 | 50 | 0.05 | 20.8 | 37.5 | 0.07 |
Analyses of demographic data showed no differences in size, weight, gender, tobacco use and comorbidities between patients who have undergone HRV-biofeedback one year earlier and those who have received rehabilitation only. This was true when both, only patients who completed follow-up or all participants of the interventional randomized controlled trial (Intention-to-treat) were included in the analyses
m male, f female
Abstinence
When including only patients who completed follow-up in the analyses, abstinence rates tended to be higher in those who had undergone HRV-biofeedback in addition to rehabilitation compared to those who received rehabilitative treatment alone. Similarly, the intention-to-treat analysis, where patients who have not completed follow-up were considered to have relapsed, also showed a trend toward higher abstinence rates among patients who had undergone HRV-biofeedback than those who underwent rehabilitation alone (Fig. 1).
Fig. 1.
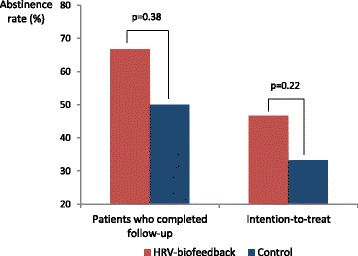
Abstinence rate one year after discharge from the rehabilitative therapy. The bar graph shows the rates of one-year abstinence in each study arm (HRV-biofeedback and control). Results are separately displayed for patients who completed follow-up and the entire population (intention-to-treat). A non-significant trend toward increased abstinence one year post-HRV-biofeedback (red bars) compared with control patients who have not undergone the intervention (blue bars) was observed when only complete cases were included. This was also true in the intention-to-treat analysis where patients who have not completed follow-up were considered to have relapsed was performed
Characteristics of abstinent patients vs. relapse patients
In order to identify factors important to abstinence we compared demographic characteristics as well as outcome data on psychometric tests and autonomic cardiac function at baseline and immediately after completion of the RCT between patients who showed abstinence 1 year after completion of the trial and those who had relapse. As shown in Table 2, none of the included study population characteristics were different between abstinent patients and those who had relapse. This was true for both, the biofeedback group and the control group.
Table 2.
Characteristics comparison between abstinent and relapsed patients from HRV-biofeedback and control groups
| HRV-biofeedback | p-value | Control | p-value | |||
|---|---|---|---|---|---|---|
| Abstinent (n=10) |
Relapse (n=5) |
Abstinent (n=6) |
Relapse (n=6) |
|||
| Demographic factors | ||||||
| Age (years) | 44.0±6.0 | 39.8±9,3 | 0.37 | 44.0±5.3 | 43.3±12.7 | 0.9 |
| Gender (%) | 80 m, 20 f | 40 m, 60 f | 0.13 | 83.3 m, 16.7 f | 83.3 m, 16.7 f | 1.0 |
| Smoking (%) | 80 | 80 | 1.0 | 83.3 | 66.7 | 0.5 |
| Psychometric characteristics | ||||||
| Craving at baseline | 15.2±13.1 | 11.9±11.3 | 0.62 | 10.0±5.4 | 10.2±1.8 | 0.94 |
| Craving post-intervention | 10.0±10.0 | 8.8±8.8 | 0.85 | 6.5±4.9 | 5.5±5.4 | 1.0 |
| Anxiety at baseline | 6.0±3.8 | 6.2±5.2 | 0.94 | 5.8±5.2 | 3.2±3.2 | 0.31 |
| Anxiety post-intervention | 4.0±3.7 | 5.1±7.3 | 1.0 | 3.8±2.9 | 3.2±3.9 | 0.58 |
| Depression at baseline | 9.4±7.3 | 11.3±9.0 | 0.69 | 9.5±7.2 | 8.2±5.8 | 0.73 |
| Depression post-intervention | 5.4±5.1 | 10.5±10.8 | 0.37 | 6.7±5.5 | 6.5±6.3 | 0.81 |
| Heart rate variability | ||||||
| CVNN at baseline (%) | 3.5±1.1 | 5.1±2.1 | 0.15 | 3.6±0.7 | 4.9±1.6 | 0.11 |
| CVNN post-intervention (%) | 4.5±1.21 | 5.5±1.7 | 0.27 | 3.5±1.3 | 5.4±2.7 | 0.16 |
| HF at baseline (ms2) | 222.8±258.8 | 606.6±1331.2 | 0.2 | 193.1±182.5 | 310.4±312.3 | 0.81 |
| HF post-intervention (ms2) | 93.4±101.5 | 405.9±400.9 | 0.07 | 86.2±42.3 | 1074.8±1589.1 | 0.31 |
| LF at baseline (ms2) | 229.9±122.7 | 681.96±811.4 | 0.16 | 260.6±216.0 | 674.9±665.8 | 0.18 |
| LF post-intervention (ms2) | 546.5±409.5 | 615.82±573.9 | 0.85 | 343.9±317.2 | 1246.2±2444.4 | 0.81 |
| TP at baseline (ms2) | 915.8±659.7 | 2332.2±2475.8 | 0.16 | 1255.3±1331.8 | 1425.4±1248.9 | 1.0 |
| TP post-intervention (ms2) | 1138.3±597.7 | 1945.7±1773.6 | 0.44 | 713.9±539.3 | 2480.8±4048.6 | 0.39 |
Analyses revealed no differences between abstinent patients and those who had relapse in any of the included demographic, psychometric or HRV parameters. Among patients who completed follow-up, this was true both in between patients who have undergone HRV-biofeedback one year earlier and those who have received rehabilitation only. “Craving” refers to the Obsessive Compulsive Drinking Scale score. “Anxiety” and “Depression” refer to scores of subscales Anxiety and Depression from the Symptom Checklist-90
CVNN coefficient of variation of R-R intervals, HF high frequency, LF low frequency, TP total power
Logistic regression analyses
None of the assessed demographic and outcome variables were associated with abstinence 1 year after the HRV-biofeedback intervention. (Table 3) The same was true for control patients who underwent rehabilitation care alone. As bivariate models did not reveal any significant associations, no multivariate models were built.
Table 3.
Bivariate linear regression analyses
| HRV-biofeedback (n=15) |
Control (n=12) |
|||
|---|---|---|---|---|
| Unstandardized B coefficient |
p- value | Unstandardized B coefficient |
p-value | |
| Demographic factors | ||||
| Age | 0.07 | 0.35 | <0.01 | 0.89 |
| Gender | 1.79 | 0.16 | 0.00 | 1.00 |
| Smoking | 0.67 | 0.32 | 0.20 | 0.78 |
| Psychometric characteristics | ||||
| Craving at baseline | 0.02 | 0.59 | -0.01 | 0.93 |
| Craving post-intervention | 0.01 | 0.79 | 0.04 | 0.71 |
| Anxiety at baseline | -0.10 | 0.93 | 0.16 | 0.29 |
| Anxiety post-intervention | -0.03 | 0.73 | 0.06 | 0.79 |
| Depression at baseline | -0.03 | 0.66 | 0.03 | 0.70 |
| Depression post-intervention | -0.08 | 0.33 | >0.01 | 0.95 |
| Heart rate variability | ||||
| CVNN at baseline | -0.65 | 0.17 | -1.07 | 0.16 |
| CVNN post-intervention | -0.54 | 0.26 | -0,79 | 0.21 |
| HF at baseline | <-0.01 | 0.26 | <-0.01 | 0.41 |
| HF post-intervention | <-0.01 | 0.26 | <-0.01 | 0.30 |
| LF at baseline | <-0.01 | 0.23 | <-0.01 | 0.22 |
| LF post-intervention | 0.00 | 0.79 | <-0.01 | 0.48 |
| TP at baseline | 0 | 0.46 | <0.01 | 0.75 |
| TP post-intervention | <0.01 | 0.79 | <-0.01 | 0.32 |
Regression analyses in patients who completed follow-up did not reveal any predictors of abstinence among demographic, psychometric or HRV parameters both in patients who have been treated with HRV-biofeedback and controls. “Craving” refers to the Obsessive Compulsive Drinking Scale score. “Anxiety” and “Depression” refer to scores of subscales Anxiety and Depression from the Symptom Checklist-90
CVNN coefficient of variation of R-R intervals, HF high frequency, LF low frequency, TP total power
Discussion
In this follow-up study 1 year after a randomized controlled trial of HRV-biofeedback in patients with alcohol addiction, we observed a tendency toward higher rates of long-term abstinence in the interventional study arm when compared with patients of the control arm that have not undergone HRV-biofeedback. Viewed in conjunction with our previous observation of improvement in cardiac autonomic and neurovascular function and reduction of craving and anxiety after HRV-biofeedback in the same study population, these findings warrant follow-up research to confirm this trend in a larger study population and assess the neurophysiological mechanisms whereby HRV-biofeedback might alter long-term abstinence [10]. Although our study was not powered to show significant group differences 1 year post-treatment, our data might contribute to generating the hypothesis that HRV-biofeedback has beneficial effects on long-term abstinence.
A recent randomized clinical trial had confirmed that HRV-biofeedback as adjuvant therapy leads to reduced craving in patients with addiction to alcohol and drugs [12]. Similar to our previous randomized controlled trial, this investigation has been able to show a trend toward improvement of autonomic cardiac function following the intervention. While long-term abstinence has not been assessed in this study, the observed weak short-term effect on HRV might offer an explanation why in our study changes in abstinence 1 year post-intervention did not reach statistical significance. In both studies, the duration and frequency of HRV-biofeedback training was limited, corresponding to 6 treatment sessions over a period of 2 weeks in our RCT and 3 sessions during 3 weeks in the work of Eddie et al. This relatively short treatment regimen might have been insufficient to achieve an improvement in autonomic cardiac function which influences craving, and thereby chance of relapse, to a degree which translates into long-term abstinence beyond the duration of the intervention. Although our RCT showed that at follow up 3 weeks post-intervention, HRV was already decreasing toward baseline, the reduction in craving was sustained until the last follow up 6 weeks post-intervention. In fact, treatment with HRV-biofeedback can improve psychometric measures such as subjective fatigue independent from changes in autonomic cardiac function [13]. Taken together, this might implicate that HRV-biofeedback treatment has to be applied more often and over a longer time period to achieve a sustained effect on craving and thereby abstinence. However, dose-response studies in larger study populations seem necessary to elucidate the therapeutic potential of this treatment to improve outcomes of rehabilitation for alcohol addiction.
Moreover, the underlying mechanism whereby improvement of the autonomic cardiac function leads to alleviation of craving, and possibly changes in abstinence, needs to be elucidated. The observed effect of HRV-biofeedback on autonomic cardiac function and craving might be explained by the physiological mechanism of action of this technique. HRV-biofeedback is a behavioral intervention that targets enhancement of the beat-to-beat fluctuations of the heart rate (HRV) due paced breathing. Physiologically, the heart rate is determined by the intrinsic sinoatrial node discharge rate as well its autonomic alteration mediated by cardiac sympathetic and parasympathetic activity [14]. The preganglionic sympathetic and parasympathetic outflow is determined by the central autonomic network (CAN), a functional unit of the central nerve system which regulates adaptive visceromotor and behavioral responses to internal and environmental stimuli [15]. Interestingly, there is an overlap between the CAN and the anterior executive region (AER), another functional network of brain centers which is responsible of assessing the motivational content of internal and external stimuli and regulating context-dependent behaviors. Cerebral centers that are part of both CAN and AER comprise the insular, anterior cingulate and prefrontal cortices as well as the amygdala and the periaquaductal gray [16]. The functional overlap of these centers viewed in conjunction with the previous observation of reduced HRV in several mental disorders support the concept of HRV constituting an index of individual self-regulation and psychological flexibility [17, 18]. Although the exact neurophysiological interaction between CAN and AER is poorly elucidated, their common capacity of altering HRV might explain why improvement of HRV could also lead to stabilization of craving and anxiety in alcohol dependent patients. Interestingly, decrease in high frequency HRV, a spectral analysis based parameter of parasympathetic activity, has been shown to predict alcohol craving independent of age, anxiety and levels of alcohol consumption. This suggests that impaired cardiac parasympathetic function is associated with increased measures of craving [19]. Furthermore, HRV reactivity to cue-exposure has been pointed as a predictor of relapse after treatment independent of therapeutic regime and after controlling for the severity of the alcohol dependence [20]. In fact, neuroimaging studies showed that patients with alcohol use disorder exposed to alcohol-related cues which induce craving, i.e. alcohol print advertisements or images (or the taste or smell) of their favorite alcoholic beverage, present activation of ventral striatum, anterior cingulate and ventromedial prefrontal cortices [21].
Our study is limited by a low return rate of the mailed questionnaires. Therefore, the observed differences in abstinence rates between HRV-biofeedback treated and control patients might not have reached statistical significance due to a type two error. In the intent-to-treat analysis, statistical power might have been compromised by the conservative approach of imputing missing data, where all patients who have not completed follow-up were considered to have relapsed. This limitation might be overcome by increasing the sample size in future studies. Alternatively, it might be necessary to increase the frequency and duration of the HRV-biofeedback treatment sessions to translate the observed trend into a significant long-term difference.
We did not assess the amount of past-year drinking, therefore we cannot comment on possible associations between treatment with HRV-biofeedback and the severity of relapse. Furthermore, patients weren’t asked to continue paced breathing after the intervention and therefore we haven’t assessed the rate of those still practicing the breathing technique after discharge. This might further explain why in our study observed trends of increase in abstinence have not reached statistical significance.
Taken together, our study forms a basis for a long-term investigation of HRV-biofeedback in patients with alcohol addiction which should include a larger sample size and a dose-response protocol to identify the optimal regimen and achieve a sustained improvement in craving and autonomic function. This, in turn, might translate into improved long-term abstinence.
Conclusions
Our data suggest that HRV-biofeedback might contribute to long-term abstinence when applied in addition to rehabilitation care. Since the trends observed in this follow-up study did not reach statistical significance, further research is warranted to confirm this hypothesis in a larger study population.
Acknowledgements
The authors thank Mr. Frank Judenfeind for his technical assistance in conducting the study. Additionally, the authors extend their sincere appreciation to Dr. Günther Freier and Dr. Ralph Deymann for their general support. We acknowledge support by the German Research Foundation and the Open Access Publication Funds of the Technische Universität Dresden.
Funding
The publication fee was covered by the Open Access Publication Funds of the Technische Universität Dresden. No additional funding was received for this study.
Availability of data and materials
The data for the current study will not be provided as participants were assured at the time of informed consent that only researchers involved in the project would have access to their individual data.
Abbreviations
- AER
Anterior executive region
- CAN
Central autonomic network
- CVNN
Coefficient of variation of R-R intervals
- f
female
- HF
High frequency
- HRV
Heart rate variability
- LF
Low frequency
- m
male
- ns
not significant
- RCT
Randomized controlled trial
- SD
Standard deviation
- TP
Total power
- vs
versus
Authors’ contributions
AIP, MS and TS have made substantial contributions to conception of the study; AIP and TS have drafted the statistical analysis plan and have analyzed the data; BMW, KB, KW and MS have made substantial contributions to interpretation of the data; AIP has drafted the first version of the manuscript; BMW, KB, KW, MS and TS have made substantial contributions to reviewing the manuscript for intellectual content; All authors read and approved the final manuscript.
Ethics approval and consent to participate
The original trial was approved by our institutional review board (Ethikkommission an der Technischen Universität Dresden; IRB number: EK118042010). Written and oral informed consent was obtained from each participant prior to this study. This one-year follow up survey was part of our institutional protocol. Each subject provided written informed consent on the use of these data for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ana Isabel Penzlin, Email: Ana.Penzlin@ppcr.org.
Kristian Barlinn, Email: Kristian.Barlinn@uniklinikum-dresden.de.
Ben Min-Woo Illigens, Email: Illigens@bidmc.harvard.edu.
Kerstin Weidner, Email: Kerstin.Weidner@uniklinikum-dresden.de.
Martin Siepmann, Email: Martin.Siepmann@uniklinikum-dresden.de.
Timo Siepmann, Phone: +49-351-458-17094, Email: timo.siepmann@uniklinikum-dresden.de.
References
- 1.World Health Organization. Global status report on alcohol and health-2014. Geneva: World Health Organization; 2014. http://www.who.int/substance_abuse/publications/global_alcohol_report/en/.
- 2.Preuss UW, Zimmermann J, Schultz G, Watzke A, Schmidt P, Löhnert B, et al. Risk profiles of treatment noncompletion for inpatients and outpatients undergoing alcohol disorder rehabilitation treatment. Subst Abuse Rehabil. 2012;3:35–42. doi: 10.2147/SAR.S24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Newham R, Russell C, Davies JB. Planned and unplanned discharge from alcohol services in Scotland, 2004-2008. Alcohol Alcohol. 2010;45(1):64–69. doi: 10.1093/alcalc/agp081. [DOI] [PubMed] [Google Scholar]
- 4.McKay JR, Weiss RV. A review of temporal effects and outcome predictors in substance abuse treatment studies with long-term follow ups: preliminary results and methodological issues. Eval Rev. 2001;25(2):113–161. doi: 10.1177/0193841X0102500202. [DOI] [PubMed] [Google Scholar]
- 5.McKay JT, Foltz C, Stephens RC, Leahy PJ, Crowley EM, Kissin W. Predictors of alcohol and crack cocaine use outcomes over a 3-year follow up in treatment seekers. J Subst Abus Treat. 2005;28(Suppl 1):S73–S82. doi: 10.1016/j.jsat.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Rose GL, Skelly JM, Badger GJ, Ferraro TA, Helzer JE. Efficacy of automated telephone continuing care following outpatient therapy for alcohol dependence. Addict Behav. 2015;41:223–231. doi: 10.1016/j.addbeh.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schellekens AF, de Jong CA, Buitelaar JK, Verkes RJ. Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. Eur Psychiatry. 2015;30(1):128–136. doi: 10.1016/j.eurpsy.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 8.Oslin D, Cary M, Slaymaker V, Colleran C, Blow F. Daily ratings measures of alcohol craving during an inpatient stay define subtypes of alcohol addiction that predict subsequent risk for resumption of drinking. Drug Alcohol Depend. 2009;103(4):131–136. doi: 10.1016/j.drugalcdep.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneekloth T, Biernacka J, Hall-Flavin D, et al. Alcohol craving as a predictor of relapse. Am J Addict. 2012;21:20–26. doi: 10.1111/j.1521-0391.2012.00297.x. [DOI] [PubMed] [Google Scholar]
- 10.Penzlin AI, Siepmann T, Illigens BM, Weidner K, Siepmann M. Heart rate variability biofeedback in patients with alcohol dependence: a randomized controlled study. Neuropsychiatr Dis Treat. 2015;11:2619–2627. doi: 10.2147/NDT.S84798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.German Society for Addiction Research and Therapy (Deutsche Gesellschaft für Suchtforschung und -therapie, DG-Sucht) Documentation standards III for the evaluation of treatment of addicts (Dokumentationsstandards III für die evaluation der Behandlung von Abhängigen) Sucht. 2001;47(2):3–94. [Google Scholar]
- 12.Eddie D, Kim C, Lehrer P, Deneke E, Bates M. A pilot study of brief heart rate variability biofeedback to reduce craving in young adult men receiving inpatient treatment for substance use disorders. Appl Psychophysiol Biofeedback. 2014;39(3-4):181–192. doi: 10.1007/s10484-014-9251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Windthorst P, Mazurak N, Kuske M, Hipp A, Giel KE, Enck P, et al. Heart rate variability biofeedback therapy and graded exercise training in management of chronic fatigue syndrome: an exploratory pilot study. J Psychosom Res. 2017;93:6–13. doi: 10.1016/j.jpsychores.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 14.Berntson GG, Bigger JT, Jr, Eckberg DL, Grossman P, Kaufmann PG, Malik M, et al. Heart rate variability: origins, methods, and interpretive caveats. Psychophysiology. 1997;34(6):623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 15.Benarroch E. The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc. 1993;68:988–1001. doi: 10.1016/S0025-6196(12)62272-1. [DOI] [PubMed] [Google Scholar]
- 16.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behavior. Brain. 1993;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 17.Thayer J, Hansen A, Saus-Rose E, Johnsen B. Heart rate variability, prefrontal neural function, and cognitive performance: the neurovisceral integration perspective on self-regulation, adaptation, and health. Ann Behav Med. 2009;37:141–153. doi: 10.1007/s12160-009-9101-z. [DOI] [PubMed] [Google Scholar]
- 18.Kemp A, Quintana D, Felmingham K, Matthews S, Jelinek H. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: implications for cardiovascular risk. PLoS One. 2012;7:e30777. doi: 10.1371/journal.pone.0030777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quintana D, Guastella A, McGregor I, Hickie I, Kemp A. Heart rate variability predicts alcohol craving in alcohol dependent outpatients: further evidence for HRV as a psychophysiological marker of self-regulation. Drug Alcohol Depend. 2013;132(1-2):395–398. doi: 10.1016/j.drugalcdep.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Garland EL, Franken IHA, Howard MO. Cue-elicited heart rate variability and Attentional bias predict alcohol relapse following treatment. Psychopharmacology. 2012;222(1):17–26. doi: 10.1007/s00213-011-2618-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schacht JP, Anton RF, Myrick H. Functional neuroimaging studies of alcohol cue reactivity: a quantitative meta-analysis and systematic review. Addict Biol. 2013;18(1):121–133. doi: 10.1111/j.1369-1600.2012.00464.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data for the current study will not be provided as participants were assured at the time of informed consent that only researchers involved in the project would have access to their individual data.


