Abstract
Objectives
Molecular characteristics of hepatitis B virus (HBV), such as genotype and genomic mutations, may contribute to liver-related morbidity and mortality. The association of these characteristics with liver fibrosis severity in sub-Saharan Africa is uncertain. We aimed to characterize molecular HBV features in HIV/HBV co-infected Nigerians and evaluate associations between these characteristics and liver fibrosis severity before and after antiretroviral therapy (ART) initiation.
Methods
HIV/HBV co-infected Nigerians underwent liver fibrosis estimation by transient elastography (TE) prior to and 36 months after ART initiation. Basal core promoter/precore (BCP/PC) and preS1/preS2/S regions of HBV were sequenced from baseline plasma samples. We evaluated associations between HBV mutations and liver fibrosis severity by univariate and multivariable regression.
Results
At baseline, 94 patients underwent TE with median liver stiffness of 6.4 (IQR 4.7–8.7) kPa. Patients were predominantly infected with HBV genotype E (45/46) and HBe-antigen negative (75/94, 79.8%). We identified BCP A1762T/G1764A in 15/35 (43%), PC G1896A in 20/35 (57%), ‘a’ determinant mutations in 12/45 (26.7%), and preS2 deletions in 6/16 (37.5%). PreS2 mutations were associated with advanced fibrosis in multivariable analysis. At follow-up, median liver stiffness was 5.2 (IQR 4.1–6.6) kPa. No HBV molecular characteristics were associated with lack of fibrosis regression, although HIV virologic control, body-mass-index (BMI) and baseline CD4+ T-cell count were associated with a decline in fibrosis stage.
Conclusion
Frequent BCP/PC and preS1/preS2/S mutations were found in ART-naïve HIV/HBV co-infected Nigerians. Median liver stiffness declined after initiation of ART, regardless of pre-ART HBV mutational pattern or virologic characteristics.
Keywords: HIV, HBV, transient elastography, liver fibrosis, ART, Nigeria
Introduction
Hepatitis B virus (HBV) infects an estimated 240 million people worldwide with variable and complex clinical outcomes (1, 2). Some of the highest rates of chronic HBV are seen in sub-Saharan Africa (SSA), where co-infection with human immunodeficiency virus (HIV) is also high. HIV has a detrimental effect on HBV infection, including increased risk of chronicity, liver fibrosis, hepatocellular carcinoma (HCC), and all-cause mortality (3–5). The elements that influence liver fibrosis in HIV/HBV infected patients are complex, including both host- and virus-specific factors. Certain HBV molecular characteristics, including genotype and specific viral mutations, have been associated with more advanced liver fibrosis, HCC, decreased response to therapy and fulminant hepatic failure in both HBV mono-infected and HIV/HBV co-infected patients (6–13).
HBV is a 3.2 kb circular, partially double-stranded DNA virus comprised of four partially overlapping open reading frames. HBV replicates by reverse transcription using a polymerase that lacks proof-reading ability, leading to mutations in the genome and sequence heterogeneity (7). Based on this sequence heterogeneity, HBV has been classified into at least nine genotypes (A-I), which have distinct geographical distributions and variable clinical outcomes (6–9, 14). Similarly, mutations in specific regions of the HBV genome have also been associated with different clinical outcomes. More specifically, mutations in the basal core promoter (BCP) and precore (PC) regions have been found in association with hepatitis B e-antigen (HBeAg) negative chronic hepatitis, cirrhosis and HCC. Mutations in the preS1/preS2/S regions and the overlapping reverse transcriptase region have been associated with HCC, infection despite immunization, failure of detection, and drug resistance (10–13).
The majority of studies examining the association between genetic mutations and liver disease have been conducted in Asia/Europe and in patients infected with genotypes (GTs) A-D. There are very few molecular studies of HBV characteristics from Nigeria, despite it having the third highest global prevalence of HBV infection (15). Of those few, most focused on viral evolution and diversity, or described specific mutations related to vaccine- or detection-escape (16–20). More in-depth mutational analysis of HBV circulating in this region is important given the disproportionate mortality burden of viral hepatitis carried by western SSA and predominance of GTE, which to date has been relatively understudied (21). Furthermore, HCC occurs at higher rates and younger ages in this region; identifying potentially unique pathways of HBV disease progression in western SSA is critical to understanding the epidemic and its consequences (22, 23).
The objectives of this study were to: (1) characterize the HBV genotype and prevalence of BCP/PC and preS1/preS2/S mutations in antiretroviral (ART)-naïve HIV/HBV co-infected patients in Jos, Nigeria; and (2) evaluate associations between specific mutations and liver fibrosis severity before and after ART initiation.
Methods
Patient Population and Ethical Considerations
This sub-study was conducted in HIV/HBV patients enrolled in a larger prospective cohort study of liver fibrosis in HIV infected Nigerians before and after ART at the President’s Emergency Plan for AIDS Relief (PEPFAR) / AIDS Prevention Initiative in Nigeria (APIN)-supported Jos University Teaching Hospital (JUTH) HIV Care and Treatment Center (24). All patients were enrolled between July 2011 and February 2012. For this analysis we included all HIV/HBV co-infected adults (≥18 years), defined as ≥ one positive HBsAg (EIA assay, Sysmex). Exclusion criteria were: current/prior ART for the treatment of HIV, HCV seropositivity, HCC, or decompensated liver disease. Patients were recruited and enrolled after written informed consent; the study was approved by the Institutional Review Boards of JUTH and Northwestern University.
Clinical Protocols and Data Collection
Patients were followed prospectively for three years. At study enrollment and annual study visits thereafter patients received a clinical assessment and laboratory studies. Clinical care of all HIV-infected patients at JUTH followed Nigerian and WHO 2010 guidelines (25, 26). ART was provided free of charge by the Nigerian government along with regular monitoring and treatment/prevention of opportunistic infections. In 2012, HIV/HBV co-infected patients were initiated on tenofovir (TDF) plus lamivudine (3TC) or emtricitabine (FTC)-containing regimens at CD4+ T-cell count ≤ 350 cells/mm3 or if evidence of chronic active hepatitis (ALT > 41 IU/ml in men and > 31 IU/ml in women) regardless of CD4 count.
Patient demographic, clinical, laboratory and therapeutic data were collected and entered into a secure computerized database. Data included: gender, age, body mass index (BMI), alcohol use (yes/no), clinical visit dates, ART regimen at initiation and follow up, ART duration, and date of loss to follow up and/or death, hemoglobin g/dl (Hgb), CD4+ T- cell count/mm3, alanine aminotransferase IU/L (ALT), HIV RNA copies/ml (Roche Cobas AmpliPrep TaqMan), HBV DNA IU/ml (Roche Cobas AmpliPrep TaqMan), HBeAg and anti-HBe, Transient elastography (TE) (FibroScan™, Echosens, France) was performed at baseline and at month 36 to assess liver fibrosis. Liver stiffness measurements (LSM) were considered reliable if ten successful measurements were obtained with a success rate > 60%.
HBV Sequencing
Plasma samples obtained from each patient for sequencing were stored at −80 °C. HBV sequencing was conducted at the NUSeq Core Facility at Northwestern University, Chicago, IL. DNA was extracted from 200 μl of plasma using the QIAamp DNA Blood Mini Kit (Qiagen) and eluted in 100 μl of elution butter. Plasma samples with low HBV DNA (< 2000 IU/ml) were ultracentrifuged at 50,000 rpm for 1.5 hours before DNA extraction with a 50 μl elution (27).
Two regions of interest within the HBV genome were targeted for amplification and sequencing: the basal core promoter/precore (BCP/PC) region and the complete S/partial P (S/P) region. When unable to successfully amplify or sequence the S/P region, a smaller region of the S (“short S”) was amplified and sequenced to obtain genotype. The BCP/PC region (1742–1900 from EcoR1), S/P region (2535 to 1099 from EcoRI), and, when applicable, the short S region (467 to 704 from EcoRI) were amplified using nested PCRs with primers and protocols as previously described (28–32). Further details regarding primers and reaction conditions are included in the supplementary material.
Traditional capillary sequencing was performed using an ABI 3730 High Throughput DNA Sequencer (Applied Biosystems). The BCP/PC and short S regions were sequenced using second-round PCR primers. The S/P region was sequenced in three overlapping fragments using primers described by Vermeulen, et al., with minor adjustments noted in the supplementary material (30). Forward and reverse sequences were obtained and contigs for the BCP/PC and short S region were created using the Automated Contig Generator Tool (33). The overlapping fragments of the S/P region were assembled using the Fragment Merger Tool (34). Multiple sequence alignment was performed using GeneDoc (35). Neighbor-joining phylogenetic trees with bootstrap support were constructed using Pipeline Tree Mail Tool and visualized by MEGA 7.0 (33, 36). Genotyping was performed by phylogenetic analysis of the complete S/P region or the short S region, as available. DAMBE was used to calculate sequence diversity, and the Mutation Reporter Tool was used to identify mutations at specific loci (33, 37).
Outcomes, Definitions and Statistical Analysis
Baseline clinical characteristics were compared by Mann-Whitney-U for continuous data and Chi-square test for categorical data. The primary outcomes of interest were baseline liver fibrosis severity and change in liver fibrosis between baseline and 36 months. Liver fibrosis was staged based on TE score using validated LSM cut-offs in HIV/HBV co-infected patients: >5.9 kPa (Metavir F2 fibrosis), >7.6 kPa (F3) and >9.4 kPa (F4 fibrosis/cirrhosis) (38). Univariate analyses were performed to assess for any associations between specific HBV mutations and baseline advanced fibrosis (>/= F3) using logistic regression. Multivariable (MV) analyses were performed for characteristics with a p-value < 0.20.
In secondary analyses we evaluated associations between HBV mutations and HBV DNA levels, as well as changes in TE score and HBV DNA after ART. Change in TE score was assessed both as a binary and continuous outcome. The binary outcome was defined as a decline of ≥1 fibrosis stage (yes/no), using the aforementioned TE cut-offs. The percent change in fibrosis was also modeled as a continuous dependent variable. Wilcoxon Signed Rank was used to compare related samples at different time points. A p-value of <0.05 was deemed statistically significant. Calculations were performed using SPSS v22.0 (IBM, Armonk, NY).
Results
Clinical Features of the Cohort
Ninety-four HIV/HBV co-infected patients were enrolled into the parent study. Baseline characteristics are shown in Table 1. Patients had a median age of 32 (IQR 28–40) years, were mostly female (60%) with a median CD4+ T-cell count of 265 (IQR 171–400) cells/mm3. The median log10 HBV DNA was 1.6 (IQR 0–5.5) IU/ml and 19 (20%) participants had reactive HBeAg. Median LSM score was 6.4 (IQR 4.7–8.7) kPa; 21 (22.1%) had LSM >9.4 kPa.
Table 1.
Baseline Characteristics
| Baseline Characteristic | Total HIV/HBV Cohort (N=94) | Subset Available for HBV Sequencinga (N=68) | Subset Unavailable for HBV Sequencinga (N=26) | p-valuea |
|---|---|---|---|---|
| Age | 32 (28–40) | 32 (28–40) | 35 (30–43) | 0.296 |
| Female | 56 (59.6%) | 42 (61.8%) | 14 (53.8%) | 0.484 |
| BMI, kg/m2 | 23.4 (20–26) | 23.8 (20.2–26) | 22.5 (19.4–26.8) | 0.302 |
| EtOH Use, Y | 25 (26.6%) | 20 (29.4%) | 5 (19.2%) | 0.318 |
| CD4+ T-cell/mm3 | 265 (167–401) | 265 (201–444) | 251 (126–340) | 0.267 |
| CD4+ T-cell ≤ 200 cells/mm3 | 27 (28.7%) | 17 (25%) | 10 (38.5%) | 0.197 |
| Log10 HIV RNA, copies/ml | 4.8 (4.2–5.4) | 4.8 (4.3–5.4) | 4.8 (4.1–5.3) | 0.355 |
| Log10 HBV DNA, IU/ml | 1.6 (0–5.5) | 1.6 (0–4.9) | 1.6 (0–6.7) | 0.266 |
| HBV DNA <2000 IU/ml | 63 (67%) | 48 (70.6%) | 15 (57.7%) | 0.234 |
| ALT, IU/ml | 24 (17–39) | 24 (15–39) | 26 (19–39) | 0.269 |
| HBeAgb Reactive | 19 (20.2%) | 13 (19.1%) | 6 (25%) | 0.541 |
| Liver Stiffness, kPa | 6.4 (4.7–8.7) | 6.1 (4.6–7.9) | 6.4 (4.7–9.8) | 0.627 |
| Cirrhosisc | 21 (22.3%) | 14 (20.6%) | 7 (26.9%) | 0.510 |
| Initiated ARTd | 81 (86.2%) | 57 (83.8%) | 24 (92.3%) | 0.286 |
Abbreviations: HIV, human immunodeficiency virus; HBV, hepatitis B virus; BMI, body-mass-index; EtOH, alcohol; ALT, alanine transaminase; HBeAg, hepatitis B e-antigen; ART, antiretroviral therapy
Continuous variables reported as median (IQR), categorical variables reported as number (%)
P-value comparing those patients with samples available for sequencing and those without samples available using Chi-Square for categorical variables and Mann-Whitney-U for continuous variables
Includes 5 samples with concurrent HBeAg and anti-HBe
Cirrhosis defined by baseline liver stiffness measurement ≥ 9.4 kPa
ART started after baseline liver stiffness measurement
After enrollment, 82 (86.3%) patients initiated antiretroviral therapy (ART), primarily TDF-based (83.8%). Median follow up during the study was 31 (IQR 28–33) months; 72/94 patients received a follow-up TE. Median ART duration between TE scans was 27.5 (IQR 19–31) months. Over the study period, median liver stiffness declined from 6.4 (IQR 4.7–8.7) kPa to 5.2 (IQR 4.1–6.6) kPa.
HBV Genotype, Phylogenetic Analysis and Sequence Diversity
Sixty-nine of 94 patients had frozen, banked plasma samples available for amplification and sequencing. There were no significant differences in patient characteristics with and without banked specimens (Table 1).
Forty-six patient samples were successfully genotyped based on sequencing of the complete or partial S region; we were unable to amplify the S region from 21 samples and did not obtain adequate sequencing data from two samples.
The complete S/partial P (‘S/P,’ nt 2535–1099) was successfully sequenced from 17 patients. Shorter S regions were successfully sequenced in an additional 29 patients: S region without preS1 or preS2 (nt 155–835) in four patients and the “short S” segment (nt 467–704, encompassing amino acids 105–184 of the S region) in 25 patients. These S region sequences were genotyped by phylogeny. Forty-five of the sequences belonged to genotype E (GTE), and the remaining one sequence belonged to genotype D (GTD). The 17 S/P sequences are shown in a neighbor-joining phylogram, rooted on genotype F, in Figure 1. One sequence (JU120057) appears to be genotype D3, while the remaining 16 sequences belong to genotype E.
Fig. 1. Phylogenetic Relationships of Complete S/partial P Region (nt 2848-1418 from the EcoR1 site).
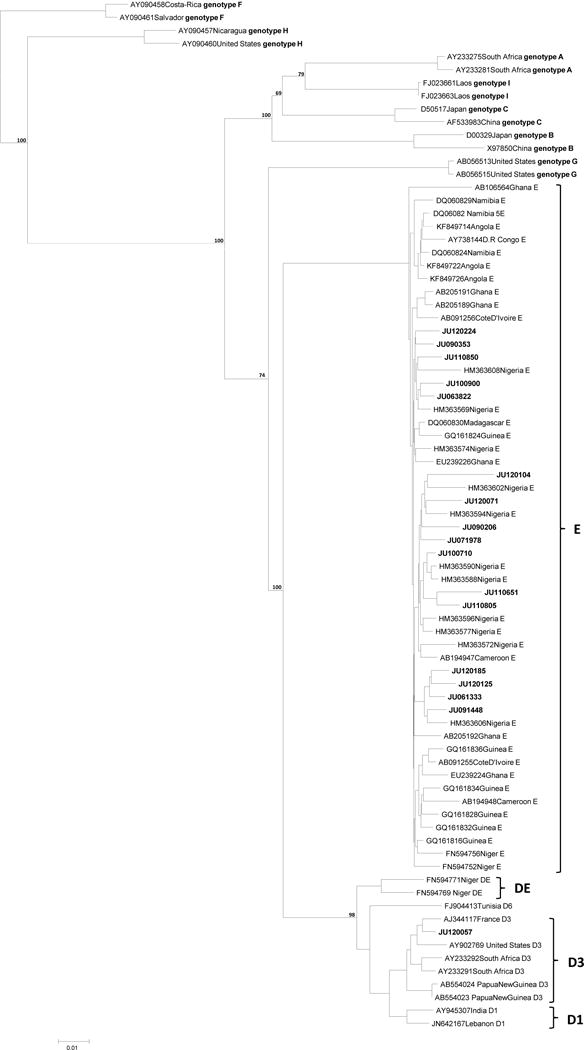
A rooted phylogenetic tree of 17 complete S/ partial polymerase sequences (2535- 1099 from EcoR1) of HBV isolated from HBV/HIV co-infected Nigerian individuals. The 17 isolates sequenced in this study are noted by the prefix JU and bold font. These were compared using neighbour-joining with 62 HBV reference sequences, labeled with accession numbers and country of origin. Bootstrap statistical analysis was performed using 1000 datasets, indicated as percentages on the nodes.
The intragroup divergence of the 16 genotype E sequences was 2.23% (± 1.07). Nucleotide diversity of the individual sequences, as compared to a genotype E consensus sequence, was strongly associated with age (β=0.582, p=0.018). There were no associations between sequence diversity and HIV RNA, CD4+ T-cell count and HBV DNA level in univariate analyses.
Sequencing Results
PreS1 and preS2 were sequenced in 17 patients, and further analyzed in the 16 GTE patients. There were no preS1 start codon mutations, pre-S1 deletions, or preS2 start codon mutations. Six (37.5%) of the samples had preS2 deletions, ranging from 1 to 11 amino acids in length. One patient had a preS2 glycine insertion after the third amino acid and one patient had the F22S mutation.
The S region, including the ‘a’ determinant (amino acids 124–147), was sequenced in 42 GTE samples. Overall, 12 (26.7%) GTE patients had at least one mutation in the ‘a’ determinant region. The most frequent mutations in this region were sT131P (4/45) and sS140L (3/45). One well described vaccine-escape mutation, sG145R, occurred in two patients. Outside of the ‘a’ determinant, common mutations occurred at residues 164 (sE164G in 5/46 and sE164A in 1/46), 110 (sI110L in 5/46) and 184 (sA184V in 2/46).
The complete or partial reverse transcriptase (RT) region of the Pol gene was analyzed in all 47 patients. No clinically significant mutations associated with drug resistance were identified.
The BCP/PC region (nt 1742–1900) was successfully sequenced in 35 patients. Fifteen (43%) had the double A1762T/G1764A mutation (“TA mutation”) in the BCP region; an additional patient had only G1764A. Six patients had C1766G and four patients had T1753C. Twenty patients (57%) had PC G1896A mutations and 12 (34%) had PC G1899A mutations.
Factors Associated with Baseline Liver Fibrosis Severity, HBV DNA, and HBeAg
PreS2 insertion-deletion mutants (‘indels’) were significantly associated with advanced fibrosis/cirrhosis (LSM > 7.6 kPa) in the univariate (UV) analysis (Table 2). This relationship was strongest with five patients who had preS2 indels between preS2 amino acid 1–25, in the B-cell epitope (p=0.017, data not shown). There was no relationship between preS2 indels and patient age, HBV DNA level, HBeAg status, HIV RNA level or CD4+ T-cell count. The relationship between preS2 mutations and liver fibrosis severity was statistically significant in the multivariable analysis, controlling for age in the final model, albeit with a p-value of 0.047 (Table 2). There were no statistically significant associations between the presence of ‘a’ determinant mutations and baseline liver fibrosis severity or HBV DNA.
Table 2.
Predictors of Baseline Advanced Fibrosis (LSM >7.6 kPa) by Univariate and Multivariable Logistic Regression
| Advanced Fibrosis (N=28) | No Advanced Fibrosis (N=67) | Univariate Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Data Totalb | N (%) or Median (IQR) | ORc (95% CI) | p-value | ORc (95% CI) | p-value | |
| Age, years | 95 | 41 (29.5–44.5) | 32 (28–26) | 1.06 (1.01–1.11) | 0.011 | 1.18 (0.95–1.74) | 0.126 |
| Female | 95 | 13 (46.4%) | 43 (64.2%) | 0.46 (0.19–1.14) | 0.094 | – | – |
| BMI, kg/m2 | 95 | 22.5 (20.3–24.6) | 23.6 (20–26.7) | 0.96 (0.87–1.05) | 0.336 | ||
| EtOH Use, yes | 95 | 13 (46.4%) | 12 (17.9%) | 3.90 (1.48–10.3) | 0.006 | – | – |
| CD4 < 200 cells/mm3 | 95 | 9 (32.1%) | 19 (28.4%) | 1.26 (0.43–3.3) | 0.634 | ||
| Log10 HIV RNA, copies/ml | 95 | 4.6 (4.1–5.3) | 4.8 (4.3–5.4) | 0.88 (0.49–1.59) | 0.674 | ||
| Log10 HBV DNA, IU/ml | 95 | 4.4 (0–6.7) | 0 (0–2.8) | 1.24 (1.06–1.44) | 0.006 | – | – |
| HBeAgd Reactive | 93 | 9 (33.3%) | 10 (15.2%) | 2.75 (0.97–7.83) | 0.058 | – | – |
| BCP A1762T/G1764G | 35 | 6 (50%) | 10 (62.5%) | 1.3 (0.32–5.27) | 0.713 | ||
| BCP C1766G | 35 | 3 (35%) | 3 (13%) | 2.22 (0.37–13.2) | 0.292 | ||
| PC G1896A | 35 | 7 (58.3%) | 13 (56.5%) | 1.17 (0.28–4.91) | 0.380 | ||
| A1762T/G1764A + G1896A | 35 | 3 (35%) | 4 (17.4%) | 1.58 (0.29–8.62) | 0.595 | ||
| PreS2 InDels | 16 | 6 (66.7%) | 1 (14.3%) | 20 (1.68–238) | 0.036 | 37 (1.01–1312) | 0.047 |
| S I110L | 45 | 2 (14.3%) | 3 (9.7%) | 1.56 (0.23–10.53) | 0.651 | ||
| S E164G/A | 45 | 1 (7.1%) | 5 (16.1%) | 0.40 (0.04–3.79) | 0.424 | ||
| ‘A’ Determinant Mutations | 45 | 3 (21.4%) | 9 (29%) | 0.67 (0.15–2.97) | 0.595 | ||
Abbreviations: LSM, liver stiffness measurement; kPa, kilopascal; BMI, body-mass-index; EtOH, alcohol; CD4+, CD4+ T-cell count; HIV, human immunodeficiency virus; HBV, hepatitis B virus; BCP, basal core promoter; PC, precore; indel, insertion-deletion mutant
The multivariate model assessed covariates with p<0.20 in univariate model via backward stepwise regression – in MV column indicates covariates that were assessed and removed from final model due to non-significance
Total number of patients with valid data for each variable. Values < 95 are representative of missing data, or insufficient sample / inability to sequence the patient sample
For all continuous variables, ORs are measured per unit increment
Includes 5 patients with concurrent HBeAg and anti-HBe
We saw a higher frequency of the 1762/1764 “TA mutation” and a lower frequency of the G1896A mutation in patients with HBV DNA ≥ 20,000 IU/ml, but these differences were not statistically significant (data not shown). There were no associations between any BCP/PC mutants and baseline liver fibrosis severity, HBV DNA, patient age, HIV RNA or CD4+T-cell counts.
The majority of the BCP/PC mutations were found in HBeAg-negative patients [BCP “TA”: 12/30 eAg (−), 3/5 eAg (+); PC G1896A: 19/30 eAg (−), 1/5 eAg (+)], although analysis is limited by very low number of eAg+ sequences (n=5) and this was not statistically significant. Notably, all three HBeAg (+) patients with the double TA mutation, including one patient also with G1896A, experienced HBeAg seroconversion over the study period.
Factors Associated with Fibrosis Change and HBV Virologic Control after ART
The percentage of HIV/HBV patients with cirrhosis declined from approximately 22% (21/95) to 11% (8/72). A total of 52 patients (72%) patients experienced a decline in LSM, and 29 (40%) decreased by at least one fibrosis stage. Conversely, 8/72 (11%) patients had increases in liver stiffness that classified them into a higher fibrosis stage. HBV DNA levels decreased from a median of 1.6 log10 IU/ml to 0 log10 IU/ml. Fifty (69.4%) patients achieved an undetectable HBV DNA level (<20 IU/ml) at the three-year follow-up period; for comparison 51 (70.8%) achieved an undetectable HIV RNA (<200 copies/ml).
Features associated with a lack of decline in fibrosis stage are shown in Table 3. The MV model, after controlling for baseline liver stiffness, identified three main factors associated with stable/progressive liver fibrosis after ART initiation: BMI, baseline CD4+ T-cell count and virologic control of HIV RNA at follow-up. There were no significant associations between specific HBV mutations and fibrosis change. Results were similar when fibrosis change was measured as a continuous outcome. No HBV mutations were associated with a detectable HBV DNA (≥20 IU/ml) at the 36-month follow-up in UV or MV analyses.
Table 3.
Factors Associated with Stable or Increased Fibrosis Stage by Univariate and Multivariable Logistic Regression
| Stable/Increased Fibrosis Stage (N=43) | Decreased Fibrosis Stage (N=29) | Univariate Analysis | Multivariable Analysisa | ||||
|---|---|---|---|---|---|---|---|
| Characteristic | Data Totalb | N (%) or median (IQR) | ORc (95% CI) | p-value | aORc (95% CI) | p-value | |
| Age, years | 72 | 32 (28–38) | 35 (29–42) | 0.98 (0.94–1.03) | 0.447 | ||
| Female | 72 | 24 (55.8%) | 20 (69%) | 0.57 (0.21–1.53) | 0.247 | ||
| BMI, kg/m2 | 72 | 24.7 (23–28.8) | 21.9 (19.5–24.4) | 1.23 (1.08–1.41) | 0.003 | 1.35 (1.13–1.62) | 0.001 |
| EtOH Use, yes | 72 | 11 (25.6%) | 8 (27.6%) | 0.90 (0.31–2.61) | 0.850 | ||
| Baseline log10 HBV DNA, IU/ml | 72 | 1.5 (0–2.3) | 3 (0–5.5) | 0.85 (0.72–1.01) | 0.053 | – | – |
| Baseline HBeAg Reactived | 70 | 8 (18.6%) | 6 (22.2%) | 0.71 (0.24–2.63) | 0.713 | ||
| Baseline log10 HIV RNA, copies/ml | 72 | 4.8 (4.2–5.4) | 4.3 (4.1–4.8) | 1.87 (0.99–3.58) | 0.058 | – | – |
| Baseline CD4+T-cells <200 cells/mm3 | 72 | 16 (37.2%) | 3 (10.3%) | 5.14 (1.34–19.7) | 0.017 | 0.99 (0.98–0.99) | 0.003 |
| BCP A1762T/G1764A | 28 | 6 (37.5%) | 8 (66.7%) | 0.30 (0.07–1.44) | 0.133 | – | – |
| PreS2 Indels | 14 | 1 (20%) | 4 (44.4%) | 0.31 (0.02–4.02) | 0.372 | ||
| Any Liver Disease Severity Mutatione | 29 | 9 (56.3%) | 11 (84.6%) | 0.23 (0.04–1.42) | 0.114 | – | – |
| Time on Antiretroviral Therapy, monthsf | 72 | 28 (18–31) | 27 (23–31) | 1.00 (0.99–1.01) | 0.543 | ||
| Follow-up HBV DNA <20 IU/mL at 36 months | 72 | 30 (69.8%) | 20 (69%) | 1.04 (0.37–2.88) | 0.942 | ||
| Follow-up HIV RNA <200 copies/mL at 36 months | 72 | 27 (62.8%) | 24 (82.8%) | 0.43 (0.16–1.15) | 0.094 | 0.20 (0.06–0.71) | 0.013 |
Abbreviations: BMI, body-mass-index; HBV, hepatitis B virus; HIV, human immunodeficiency virus; BCP, basal core promoter
Multivariable model controlled for baseline liver stiffness. Further covariates with p-value <0.20 in UV analysis were run in MV model via backward stepwise regression. – in MV column indicates covariates that were assessed and removed from final model due to non-significance.
Total number of patients with valid data for each variable. Values < 95 are representative of missing data, or insufficient sample / inability to sequence the patient sample
For all continuous variables, ORs are measured per unit increment;
Includes 5 patients with concurrent HBeAg and anti-HBe
Includes: BCP A1762T/G1764A mutation, 1766, 1753, F22S and preS2 deletion mutations
Time between ART initiation and follow-up TE scan
Discussion
We report results from the first published study evaluating molecular characteristics of HBV in relationship to liver fibrosis before and after ART initiation in HIV/HBV co-infected patients. We identified a high frequency of preS2 deletions (6/16, 37.5%). PreS1 and preS2 mutations have been associated with more severe liver disease, including fulminant hepatitis, cirrhosis and HCC. HCC, in turn, appears to occur at high frequencies and at younger ages in certain sub-Saharan cohorts than in other populations (22, 39). PreS2 mutants may cause hepatocellular toxicity due to the overproduction and accumulation of mutated envelope proteins, leading to endoplasmic reticulum stress, DNA damage and genomic instability (13). In a large study evaluating the frequency of HBV genome mutations in pre-ART HIV/HBV and HBV mono-infected individuals, preS2 mutations were found more commonly in co-infected patients (12/82, 14.6% vs. 2/60, 3.3%, p=0.04) (40). This finding was independent of genotype, although this cohort did not include any patients with GTE infection. A molecular study involving multiple countries in West Africa found preS2 deletions in 30.6% (33/108), including 15/35 (42.8%) from Nigeria with unknown HIV status (18). Other studies including patients from SSA or with GTE have reported lower frequencies of preS2 deletions, and the effect of HIV infection on the presence of these mutations in this region is unclear (29, 32, 41, 42). In this study, preS2 indel mutations were associated with baseline liver fibrosis severity, but not fibrosis regression; liver fibrosis information was not available for the GTE patients in the prior studies.
We found a relatively high number of mutations in the ‘a’ determinant region in this population (12/42, 26.7%). The ‘a’ determinant is critical for antibody recognition. HBsAg variants not recognized by antibodies can cause infection despite vaccination or treatment with hepatitis B immunoglobulin (HBIG); additionally commercial assays may fail to detect certain HBsAg mutants (43–45). Interestingly, a study from Nigeria published in 2001 only identified ‘a’ determinant mutations in 1/20 (5%) patients (16). Studies in other parts of the world have demonstrated increasing ‘a’ determinant mutations over time, likely related to the expansion of hepatitis B vaccination (45). In Nigeria the hepatitis B vaccine was introduced in 1995, however not widely available until 2004 (46, 47). Patients in this study were all born prior to 2004, although vaccination history was not available in our study patients.
More recent studies from Nigeria have reported immune escape mutants in pregnant women, asymptomatic patients, and HBsAg detection failures related to ‘a’ mutations (19, 20). In this cohort we found two patients with the well-described G145R mutation, as well as several patients with other mutations potentially leading to immune escape (including ‘a’ determinant sites 126, 129, 130, 131 and 144). The most common S region mutants in our cohort occurred outside the ‘a’ determinant, at amino acids 110 and 164. The clinical significance of these mutations is unknown, though s164 mutants have been found in association with negative HBsAg testing (45, 48). Similar frequencies of these mutations have been seen in other GTE cohorts (42, 49). Additionally, two of our patients had sA184V, which was shown to impede detection by commercial assays of HBsAg in GTE infected Nigerian patients (20). As HBV is hyperendemic in western SSA and prevalence is rising, knowledge of the genotype and predominant HBV molecular variants circulating in this region is critical given the potential impact on diagnostic capabilities and vaccine effectiveness (18, 21).
We found a high frequency of the “TA” mutation, (15/35, 43%) and G1896A mutation (20/35, 57%); mutations in these regions affect the production of HBeAg. The double A1762T/G1764A (“TA” mutation) in the BCP region affects mRNA transcription and decreases HBeAg production (50). In the precore region, the G1896A mutation creates a stop codon in the precore gene abolishing HBeAg expression (51). The majority of BCP/PC mutations in this cohort occurred in e-antigen negative patients or e-antigen positive patients who subsequently seroconverted to anti-HBe, a phenomenon previously reported (52, 53). The TA mutation frequency in this study was higher than that observed in other studies from Western Africa, although the frequency of G1896A was similar (42, 54–56). Interestingly, rates of HBeAg seropositivity [19 (20.2%)] were significantly lower in this cohort than those reported in two large European and multinational HIV/HBV co-infected cohorts (Lacombe, et al: 161/301, 53.5%; Thio, et al: 57/113, 50.4%), although these studies included few patients with GTE infection (9, 57). HBeAg positivity in predominantly HIV-negative GTE West African cohorts appears quite variable, ranging from 6.8% to 34% in three studies (54, 56, 58). While data from HIV/HBV co-infected cohorts in West Africa are scarce, a previous study from Jos found a significantly higher HBeAg seropositivity in co-infected patients than mono-infected (28/100 vs. 15/100, p=0.03). It is uncertain whether HIV has any effect on BCP/PC mutations, although two (non-GTE) comparative studies found more frequent BCP mutants in HIV-negative patients in certain genotypes (40, 59). A novel precore -1G mutant has been identified in HIV/HBV patients with GTA and associated with higher HBV DNA levels, which we did not identify in this cohort (40, 60).
BCP, and to a lesser degree PC, mutations have also been associated with hepatocellular carcinoma and liver cirrhosis, but also isolated in patients with mild or asymptomatic disease (10, 11, 61). The effects of these mutations may differ depending HBeAg status: eAg (+) patients with BCP/PC mutants have been associated with more liver inflammation, but also lower HBV DNA levels than their eAg (−) counterparts (61, 62). We found no association between BCP, PC, or combined mutations with baseline HBV DNA, liver cirrhosis or lack of liver fibrosis regression.
Overall, we did not find any significant associations between specific HBV mutants and liver disease progression or HBV virological suppression after the initiation of ART. HBV virologic suppression after ART initiation did not impact fibrosis regression, whereas HIV virologic suppression (RNA <200 copies/ml) was significantly associated with fibrosis regression.
To our knowledge, this study is one of first to assess HBV mutations in HIV/HBV co-infected patients in West Africa and examine the effect of specific mutations on long-term clinical and virological outcomes. It provides information on the prevalence of mutations within one of the most important and least studied HBV genotypes, adding to the knowledge required to optimize detection and treatment of HBV in this population. Important limitations of this study include its observational design and exclusion of HBV mono-infected patients. Also, LSM is an imperfect measure of liver fibrosis, and its interpretation may differ based on disease, particularly for fibrosis stages <F4 (63). This may impact the use of TE in multifactorial liver disease. Along those lines, we were not able to examine other potential contributors to liver disease, including hepatitis D virus (HDV), quantitative alcohol intake, other hepatotoxic medications or herbs or aflatoxin. The small number of sequenced plasma samples and issue of multiple comparisons also limit the strength of our conclusions. It is possible that the relationship between preS2 mutations and liver fibrosis severity is due to chance alone, however these mutations have previously been associated with severe liver disease with a biologically plausible mechanism (12, 13). Lastly, we did not assess HBV quasispecies populations, which may impact the clinical manifestations of viral mutations (52, 64).
In conclusion, a high frequency of BCP, PC, ‘a’ determinant, and preS2 mutations were observed in this ART-naïve GTE-infected HIV/HBV cohort. While preS2 mutations were associated with advanced fibrosis and cirrhosis at baseline, none of the mutations were associated with fibrosis progression or failure to suppress HBV after ART initiation. This suggests that the presence of these viral mutations at baseline does not negatively impact liver fibrosis regression after initiation of ART.
Supplementary Material
Acknowledgments
We would like to acknowledge Richard Longnecker, PhD, and Trevor Bell, PhD for their technical expertise and assistance in completing this study. Also, we would like to thank the patients and staff of the HIV clinic at JUTH for their support. This work was funded by: the Northwestern University AIDS International Training and Research Program Fogarty International Center / National Institutes of Health grant # 5D43TW007995–03S1; the Program for African Studies; in part by the US Department of Health and Human Services, Health Resources and Services Administration (U51HA02522) and the Centers for Disease Control and Prevention (CDC) through a cooperative agreement with APIN (PS 001058). The contents are solely the responsibility of the authors and do not represent the official views of the funding institutions.
References
- 1.World Health Organization WHO. Hepatitis B: Fact Sheet N 204. 2015 [updated July 2015 Available from: http://www.who.int/mediacentre/factsheets/fs204/en/
- 2.Lai CL, Ratziu V, Yuen M-F, Poynard T. Viral hepatitis B. The Lancet. 2003;362(9401):2089–94. doi: 10.1016/S0140-6736(03)15108-2. [DOI] [PubMed] [Google Scholar]
- 3.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Munoz A, et al. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet (London, England) 2002;360(9349):1921–6. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 4.Hoffmann CJ, Seaberg EC, Young S, Witt MD, D'Acunto K, Phair J, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. Aids. 2009;23(14):1881–9. doi: 10.1097/QAD.0b013e32832e463a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burnett RJ, François G, Kew MC, Leroux-Roels G, Meheus A, Hoosen AA, et al. Hepatitis B virus and human immunodeficiency virus co-infection in sub-Saharan Africa: a call for further investigation. Liver International. 2005;25(2):201–13. doi: 10.1111/j.1478-3231.2005.01054.x. [DOI] [PubMed] [Google Scholar]
- 6.Kew MC, Kramvis A, Yu MC, Arakawa K, Hodkinson J. Increased hepatocarcinogenic potential of hepatitis B virus genotype A in Bantu-speaking sub-saharan Africans. Journal of medical virology. 2005;75(4):513–21. doi: 10.1002/jmv.20311. [DOI] [PubMed] [Google Scholar]
- 7.Kramvis A. Genotypes and genetic variability of hepatitis B virus. Intervirology. 2014;57(3–4):141–50. doi: 10.1159/000360947. [DOI] [PubMed] [Google Scholar]
- 8.Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. Journal of gastroenterology and hepatology. 2011;26(Suppl 1):123–30. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- 9.Lacombe K, Massari V, Girard PM, Serfaty L, Gozlan J, Pialoux G, et al. Major role of hepatitis B genotypes in liver fibrosis during coinfection with HIV. Aids. 2006;20(3):419–27. doi: 10.1097/01.aids.0000200537.86984.0e. [DOI] [PubMed] [Google Scholar]
- 10.Kramvis A, Kew MC, Bukofzer S. Hepatitis B virus precore mutants in serum and liver of Southern African Blacks with hepatocellular carcinoma. Journal of hepatology. 1998;28(1):132–41. doi: 10.1016/s0168-8278(98)80212-2. [DOI] [PubMed] [Google Scholar]
- 11.Chen CH, Lee CM, Hung CH, Hu TH, Wang JH, Wang JC, et al. Clinical significance and evolution of core promoter and precore mutations in HBeAg-positive patients with HBV genotype B and C: a longitudinal study. Liver international : official journal of the International Association for the Study of the Liver. 2007;27(6):806–15. doi: 10.1111/j.1478-3231.2007.01505.x. [DOI] [PubMed] [Google Scholar]
- 12.Yeung P, Wong DK, Lai CL, Fung J, Seto WK, Yuen MF. Association of hepatitis B virus pre-S deletions with the development of hepatocellular carcinoma in chronic hepatitis B. The Journal of infectious diseases. 2011;203(5):646–54. doi: 10.1093/infdis/jiq096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollicino T, Cacciola I, Saffioti F, Raimondo G. Hepatitis B virus PreS/S gene variants: pathobiology and clinical implications. Journal of hepatology. 2014;61(2):408–17. doi: 10.1016/j.jhep.2014.04.041. [DOI] [PubMed] [Google Scholar]
- 14.Ganne-Carrie N, Williams V, Kaddouri H, Trinchet JC, Dziri-Mendil S, Alloui C, et al. Significance of hepatitis B virus genotypes A to E in a cohort of patients with chronic hepatitis B in the Seine Saint Denis District of Paris (France) Journal of medical virology. 2006;78(3):335–40. doi: 10.1002/jmv.20545. [DOI] [PubMed] [Google Scholar]
- 15.Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. The Lancet. 386(10003):1546–55. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 16.Odemuyiwa SO, Mulders MN, Oyedele OI, Ola SO, Odaibo GN, Olaleye DO, et al. Phylogenetic analysis of new hepatitis B virus isolates from Nigeria supports endemicity of genotype E in West Africa*. Journal of medical virology. 2001;65(3):463–9. [PubMed] [Google Scholar]
- 17.Forbi JC, Vaughan G, Purdy MA, Campo DS, Xia GL, Ganova-Raeva LM, et al. Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PloS one. 2010;5(7):e11615. doi: 10.1371/journal.pone.0011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mulders MN, Venard V, Njayou M, Edorh AP, Bola Oyefolu AO, Kehinde MO, et al. Low genetic diversity despite hyperendemicity of hepatitis B virus genotype E throughout West Africa. The Journal of infectious diseases. 2004;190(2):400–8. doi: 10.1086/421502. [DOI] [PubMed] [Google Scholar]
- 19.Faleye TO, Adewumi OM, Ifeorah IM, Akere A, Bakarey AS, Omoruyi EC, et al. Detection and circulation of hepatitis B virus immune escape mutants among asymptomatic community dwellers in Ibadan, southwestern Nigeria. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2015;39:102–9. doi: 10.1016/j.ijid.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 20.Olinger CM, Weber B, Otegbayo JA, Ammerlaan W, van der Taelem-Brule N, Muller CP. Hepatitis B virus genotype E surface antigen detection with different immunoassays and diagnostic impact of mutations in the preS/S gene. Med Microbiol Immunol. 2007;196(4):247–52. doi: 10.1007/s00430-007-0050-5. [DOI] [PubMed] [Google Scholar]
- 21.Stanaway JD, Flaxman AD, Naghavi M, Fitzmaurice C, Vos T, Abubakar I, et al. The global burden of viral hepatitis from 1990 to 2013: findings from the Global Burden of Disease Study 2013. The Lancet. doi: 10.1016/S0140-6736(16)30579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tognarelli J, Ladep NG, Crossey MME, Okeke E, Duguru M, Banwat E, et al. Reasons why West Africa continues to be a hotbed for hepatocellular carcinoma. Nigerian Medical Journal : Journal of the Nigeria Medical Association. 2015;56(4):231–5. doi: 10.4103/0300-1652.165032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladep NG, Lesi OA, Mark P, Lemoine M, Onyekwere C, Afihene M, et al. Problem of hepatocellular carcinoma in West Africa. World journal of hepatology. 2014;6(11):783–92. doi: 10.4254/wjh.v6.i11.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hawkins C, Agbaji O, Ugoagwu P, Thio CL, Auwal MM, Ani C, et al. Assessment of liver fibrosis by transient elastography in patients with HIV and hepatitis B virus coinfection in Nigeria. Clin Infect Dis. 2013;57(12):e189–92. doi: 10.1093/cid/cit564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.WHO Guidelines Review Committee. Antiretroviral Therapy for HIV Infection in Adults and Adolescents: Recommendations for a Public Health Approach: 2010 Revision. Geneva: World Health Organization; 2010. [PubMed] [Google Scholar]
- 26.Federal Ministry of Health. National guidelines for HIV and AIDS treatment and care in adolescents and adults. Abuja, Nigeria: 2010. [Google Scholar]
- 27.Zahn A, Li C, Danso K, Candotti D, Owusu-Ofori S, Temple J, et al. Molecular characterization of occult hepatitis B virus in genotype E-infected subjects. The Journal of general virology. 2008;89(Pt 2):409–18. doi: 10.1099/vir.0.83347-0. [DOI] [PubMed] [Google Scholar]
- 28.Ochwoto M, Chauhan R, Gopalakrishnan D, Chen CY, Ng’ang’a Z, Okoth F, et al. Genotyping and molecular characterization of hepatitis B virus in liver disease patients in Kenya. Infect Genet Evol. 2013;20:103–10. doi: 10.1016/j.meegid.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 29.Makondo E, Bell TG, Kramvis A. Genotyping and molecular characterization of hepatitis B virus from human immunodeficiency virus-infected individuals in southern Africa. PloS one. 2012;7(9):e46345. doi: 10.1371/journal.pone.0046345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vermeulen M, Dickens C, Lelie N, Walker E, Coleman C, Keyter M, et al. Hepatitis B virus transmission by blood transfusion during 4 years of individual-donation nucleic acid testing in South Africa: estimated and observed window period risk. Transfusion. 2012;52(4):880–92. doi: 10.1111/j.1537-2995.2011.03355.x. [DOI] [PubMed] [Google Scholar]
- 31.Yousif M, Mudawi H, Bakhiet S, Glebe D, Kramvis A. Molecular characterization of hepatitis B virus in liver disease patients and asymptomatic carriers of the virus in Sudan. BMC infectious diseases. 2013;13:328. doi: 10.1186/1471-2334-13-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yousif M, Mudawi H, Hussein W, Mukhtar M, Nemeri O, Glebe D, et al. Genotyping and virological characteristics of hepatitis B virus in HIV-infected individuals in Sudan. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2014;29:125–32. doi: 10.1016/j.ijid.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 33.Bell TG, Kramvis A. Bioinformatics tools for small genomes, such as hepatitis B virus. Viruses. 2015;7(2):781–97. doi: 10.3390/v7020781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell TG, Kramvis A. Fragment merger: an online tool to merge overlapping long sequence fragments. Viruses. 2013;5(3):824–33. doi: 10.3390/v5030824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nicholas KB, Nicholas H, Deerfield D. GeneDoc: analysis and visualization of genetic variation. Embnew news. 1997;4(1) [Google Scholar]
- 36.Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol Biol Evol. 2016;33(7):1870–4. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia X. DAMBE5: a comprehensive software package for data analysis in molecular biology and evolution. Mol Biol Evol. 2013;30(7):1720–8. doi: 10.1093/molbev/mst064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miailhes P, Pradat P, Chevallier M, Lacombe K, Bailly F, Cotte L, et al. Proficiency of transient elastography compared to liver biopsy for the assessment of fibrosis in HIV/HBV-coinfected patients. Journal of viral hepatitis. 2011;18(1):61–9. doi: 10.1111/j.1365-2893.2010.01275.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang JD, Gyedu A, Afihene MY, Duduyemi BM, Micah E, Kingham TP, et al. Hepatocellular Carcinoma Occurs at an Earlier Age in Africans, Particularly in Association With Chronic Hepatitis B. The American journal of gastroenterology. 2015;110(11):1629–31. doi: 10.1038/ajg.2015.289. [DOI] [PubMed] [Google Scholar]
- 40.Audsley J, Littlejohn M, Yuen L, Sasadeusz J, Ayres A, Desmond C, et al. HBV mutations in untreated HIV-HBV co-infection using genomic length sequencing. Virology. 2010;405(2):539–47. doi: 10.1016/j.virol.2010.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huy TTT, Ushijima H, Win KM, Luengrojanakul P, Shrestha PK, Zhong ZH, et al. High Prevalence of Hepatitis B Virus Pre-S Mutant in Countries Where It Is Endemic and Its Relationship with Genotype and Chronicity. Journal of clinical microbiology. 2003;41(12):5449–55. doi: 10.1128/JCM.41.12.5449-5455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lago BV, Mello FC, Ribas FS, Valente F, Soares CC, Niel C, et al. Analysis of Complete Nucleotide Sequences of Angolan Hepatitis B Virus Isolates Reveals the Existence of a Separate Lineage within Genotype E. PloS one. 2014;9(3):e92223. doi: 10.1371/journal.pone.0092223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carman WF, Zanetti AR, Karayiannis P, Waters J, Manzillo G, Tanzi E, et al. Vaccine-induced escape mutant of hepatitis B virus. Lancet (London, England) 1990;336(8711):325–9. doi: 10.1016/0140-6736(90)91874-a. [DOI] [PubMed] [Google Scholar]
- 44.Seddigh-Tonekaboni S, Waters JA, Jeffers S, Gehrke R, Ofenloch B, Horsch A, et al. Effect of variation in the common “a” determinant on the antigenicity of hepatitis B surface antigen. Journal of medical virology. 2000;60(2):113–21. doi: 10.1002/(sici)1096-9071(200002)60:2<113::aid-jmv2>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 45.Zuckerman AJ. Effect of hepatitis B virus mutants on efficacy of vaccination. The Lancet. 2000;355(9213):1382–4. doi: 10.1016/S0140-6736(00)02132-2. [DOI] [PubMed] [Google Scholar]
- 46.Musa BM, Bussell S, Borodo MM, Samaila AA, Femi OL. Prevalence of hepatitis B virus infection in Nigeria, 2000–2013: a systematic review and meta-analysis. Nigerian journal of clinical practice. 2015;18(2):163–72. doi: 10.4103/1119-3077.151035. [DOI] [PubMed] [Google Scholar]
- 47.Ikobah J, Okpara H, Elemi I, Ogarepe Y, Udoh E, Ekanem E. The prevalence of hepatitis B virus infection in Nigerian children prior to vaccine introduction into the National Programme on Immunization schedule. The Pan African medical journal. 2016;23:128. doi: 10.11604/pamj.2016.23.128.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Datta S, Banerjee A, Chandra PK, Chowdhury A, Chakravarty R. Genotype, phylogenetic analysis, and transmission pattern of occult hepatitis B virus (HBV) infection in families of asymptomatic HBsAg carriers. Journal of medical virology. 2006;78(1):53–9. doi: 10.1002/jmv.20503. [DOI] [PubMed] [Google Scholar]
- 49.Pourkarim MR, Lemey P, Amini-Bavil-Olyaee S, Houspie L, Verbeeck J, Rahman M, et al. Molecular characterization of hepatitis B virus strains circulating in Belgian patients co-infected with HIV and HBV: overt and occult infection. Journal of medical virology. 2011;83(11):1876–84. doi: 10.1002/jmv.22174. [DOI] [PubMed] [Google Scholar]
- 50.Takahashi K, Aoyama K, Ohno N, Iwata K, Akahane Y, Baba K, et al. The precore/core promoter mutant (T1762A1764) of hepatitis B virus: clinical significance and an easy method for detection. The Journal of general virology. 1995;76(Pt 12):3159–64. doi: 10.1099/0022-1317-76-12-3159. [DOI] [PubMed] [Google Scholar]
- 51.Carman WF, Jacyna MR, Hadziyannis S, Karayiannis P, McGarvey MJ, Makris A, et al. Mutation preventing formation of hepatitis B e antigen in patients with chronic hepatitis B infection. Lancet (London, England) 1989;2(8663):588–91. doi: 10.1016/s0140-6736(89)90713-7. [DOI] [PubMed] [Google Scholar]
- 52.Yang H-C, Chen C-L, Shen Y-C, Peng C-Y, Liu C-J, Tseng T-C, et al. Distinct evolution and predictive value of hepatitis B virus precore and basal core promoter mutations in interferon-induced hepatitis B e antigen seroconversion. Hepatology. 2013;57(3):934–43. doi: 10.1002/hep.26121. [DOI] [PubMed] [Google Scholar]
- 53.Bernstein D, Mangia A, Brau N, Yang JC, Ma J, Hyland R, et al., editors. Concordance Between SVR4, SVR12, and SVR24 in Genotype I HCV-Infected Patients Who Received All Oral Fixed-Dose Combination Ledipasvir/Sofosbuvir With or Without Ribavirin in Phase 3 Clinical Trials. Abstract 1947 Program and Abstracts of the 65th Annual Meeting of the American Association for the Study of Liver Diseases; November 7–11, 2014; Boston, Massachusetts. [Google Scholar]
- 54.Vray M, Debonne JM, Sire JM, Tran N, Chevalier B, Plantier JC, et al. Molecular epidemiology of hepatitis B virus in Dakar, Senegal. Journal of medical virology. 2006;78(3):329–34. doi: 10.1002/jmv.20544. [DOI] [PubMed] [Google Scholar]
- 55.Suzuki S, Sugauchi F, Orito E, Kato H, Usuda S, Siransy L, et al. Distribution of hepatitis B virus (HBV) genotypes among HBV carriers in the Cote d'Ivoire: complete genome sequence and phylogenetic relatedness of HBV genotype E. Journal of medical virology. 2003;69(4):459–65. doi: 10.1002/jmv.10331. [DOI] [PubMed] [Google Scholar]
- 56.Candotti D, Opare-Sem O, Rezvan H, Sarkodie F, Allain JP. Molecular and serological characterization of hepatitis B virus in deferred Ghanaian blood donors with and without elevated alanine aminotransferase. Journal of viral hepatitis. 2006;13(11):715–24. doi: 10.1111/j.1365-2893.2006.00741.x. [DOI] [PubMed] [Google Scholar]
- 57.Thio CL, Smeaton L, Saulynas M, Hwang H, Saravanan S, Kulkarni S, et al. Characterization of HIV-HBV coinfection in a multinational HIV-infected cohort. AIDS. 2013;27(2):191–201. doi: 10.1097/QAD.0b013e32835a9984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Traore F, Gormally E, Villar S, Friesen MD, Groopman JD, Vernet G, et al. Molecular characteristics of Hepatitis B and chronic liver disease in a cohort of HB carriers from Bamako, Mali. BMC infectious diseases. 2015;15:180. doi: 10.1186/s12879-015-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cassino L, Laufer N, Salomon H, Campos R, Quarleri J. Hepatitis B precore/core promoter mutations in isolates from HBV-monoinfected and HBV-HIV coinfected patients: a 3-yr prospective study. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2009;46(4):354–9. doi: 10.1016/j.jcv.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 60.Revill PA, Littlejohn M, Ayres A, Yuen L, Colledge D, Bartholomeusz A, et al. Identification of a novel hepatitis B virus precore/core deletion mutant in HIV/hepatitis B virus co-infected individuals. Aids. 2007;21(13):1701–10. doi: 10.1097/QAD.0b013e32826fb305. [DOI] [PubMed] [Google Scholar]
- 61.Lindh M, Horal P, Dhillon AP, Furuta Y, Norkrans G. Hepatitis B virus carriers without precore mutations in hepatitis B e antigen-negative stage show more severe liver damage. Hepatology. 1996;24(3):494–501. doi: 10.1002/hep.510240305. [DOI] [PubMed] [Google Scholar]
- 62.Chu CJ, Keeffe EB, Han SH, Perrillo RP, Min AD, Soldevila-Pico C, et al. Prevalence of HBV precore/core promoter variants in the United States. Hepatology. 2003;38(3):619–28. doi: 10.1053/jhep.2003.50352. [DOI] [PubMed] [Google Scholar]
- 63.Friedrich-Rust M, Ong MF, Martens S, Sarrazin C, Bojunga J, Zeuzem S, et al. Performance of transient elastography for the staging of liver fibrosis: a meta-analysis. Gastroenterology. 2008;134(4):960–74. doi: 10.1053/j.gastro.2008.01.034. [DOI] [PubMed] [Google Scholar]
- 64.Tseng T-C, Liu C-J, Yang H-C, Chen C-L, Yang W-T, Tsai C-S, et al. Higher proportion of viral basal core promoter mutant increases the risk of liver cirrhosis in hepatitis B carriers. Gut. 2015;64(2):292–302. doi: 10.1136/gutjnl-2014-306977. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


