Abstract
The treatment of elderly patients with advanced hematological malignancies has expanded to include reduced-intensity conditioning (RIC) allogeneic hematopoietic cell transplantation (alloHCT) as a potentially curative option. We studied the association between disease risk index (DRI) and clinical outcomes of 196 elderly patients (median age: 64.8 [60-75] years) with hematological malignancies receiving RIC alloHCT (2000-2014). Donors were adult related and unrelated (RD, URD; n = 100, 51.1%) or umbilical cord blood (UCB) (n = 96, 48.9%). DRI classified 12 patients (6.1%) as low risk (LR), 146 patients (74.5%) as intermediate risk (IR) and 38 patients (19.4%) as high-risk (HR). Two-year overall survival (OS) was 47% (52% for LR/IR vs. 29% for HR; p<0.01) and two-year disease-free survival (DFS) was 39% (44% for LR/IR vs. 21% for HR; p<0.01). Relapse incidence was 30% (26% for LR/IR vs. 44% for HR; p<0.01). Treatment-related mortality (TRM) was 29% at 2 years; this was similar for all DRI groups. In multiple regression analysis, HR DRI was associated with increased risk of relapse (HR=2.07; 95% CI 1.34-3.33; p=0.02) and treatment failure (HR=2.07; 95% CI 1.35-3.18; p<0.01), and decreased OS (HR=2.11; 95% CI 1.34-3.33; p<0.01). In elderly patients, DRI is a significant prognostic factor for post-transplant relapse, treatment failure, and mortality. Due to increased risk of relapse leading to poor survival in HR DRI, participation in clinical trials offering relapse prevention strategies after RIC alloHCT should be encouraged when available.
Keywords: Disease Risk Index, Elderly
Introduction
The incidence and prevalence of most hematologic malignancies increase with age. Elderly patients often have more comorbidities and worse performance status that makes optimal treatment challenging, particularly when more intensive treatment is indicated. Allogeneic hematopoietic stem cell transplantation (alloHCT) is a potentially curative therapy for hematologic malignancies;[1][2][3] however, up to 60% of elderly patients with acute myeloid leukemia (AML) are not treated following diagnosis due to perceived poor tolerance to intensive therapies including alloHCT and subsequently have very poor survival. [4] [5] Several studies have shown that elderly patients ≥ 60 years of age have similar treatment-related mortality (TRM) and overall survival (OS) post-alloHCT compared to younger patients, with the majority of transplants utilizing reduced-intensity conditioning (RIC) regimens.[6][7][8][9]
The ability of RIC to harness graft-versus-leukemia (GVL) effect with fewer transplant-related toxicities compared to myeloablative (MA) conditioning has made it an attractive and increasingly popular choice for elderly patients that are not candidates for MA transplant.[10] In order to better select patients for alloHCT, clinical risk tools including the HCT-specific comorbidity index (HCT-CI) [11] and frailty index [12] can help clinicians assess patient-specific factors that influence TRM and OS after alloHCT. Additionally, a disease-specific predictive tool, the disease risk index (DRI), [13] was developed and later revised to improve the ability to estimate OS after alloHCT across various hematologic malignancies regardless of age, conditioning regimen, graft source, or donor type.
We examined the impact of the revised DRI on clinical outcomes in elderly patients (≥60 years old) with hematologic malignancies receiving RIC alloHCT at the University of Minnesota.
Methods
Study Design
We identified 196 patients ≥60 years of age that received RIC alloHCT for hematologic malignancies between 2000 and 2014. Data was acquired from the University of Minnesota Blood and Marrow Transplant database, supplemented as needed by individual medical record review. Criteria for transplantation was disease specific, with acute leukemia and MDS requiring <5% blasts by morphology prior to transplantation, non-blast phase of chronic myeloid leukemia (CML), and chemotherapy-sensitive lymphoma or multiple myeloma patients. DRI, Karnofsky Performance Score (KPS), and HCT-CI were assessed prior to alloHCT.
The majority of patients received conditioning consisting of cyclophosphamide 50 mg/kg IV on day -6, fludarabine 30 mg/m2 daily on day -5 to day -2, and total body irradiation (TBI) 200cGy on day -1. Equine ATG at 15 mg/kg was given twice daily in patients with a remotely preceding (> 12 months) autologous transplant or no immunosuppressive chemotherapy within 3 months. GVHD prophylaxis consisted of cyclosporine A (CSA) and mycophenolate mofetil (MMF) starting on day -3 in matched related donor (RD) and matched unrelated donor (URD) transplants. For UCB transplants, CSA/MMF was used prior to 2012 and CSA was replaced with sirolimus thereafter. All patients provided written consent and the protocol was approved by the institutional IRB.
Study End Points and Definitions
Primary study endpoint was OS. Secondary endpoints included disease-free survival (DFS; defined as survival without death or relapse) and relapse incidence at 2 years, neutrophil recovery (defined as ANC ≥ 500 for 3 consecutive days) at post-HCT day 42, platelet recovery (defined as platelet count ≥ 20 × 109 /L) at 6 months, grade II-IV and III-IV acute GVHD at day 180, chronic GVHD at 2 years, TRM (defined as death in the first 28 days after transplantation or any death after day 28 in continuous remission), and GVHD-free (acute III-IV or chronic) relapse-free survival (GRFS) at 2 years. [14]
DRI was defined as specified by Armand et al [13] and classified for analysis as low or intermediate risk (LR/IR) vs. high or very high risk (HR/VHR). Acute and chronic GVHD were graded per consensus criteria. [15][16] GRFS was defined as absence of grade III-IV acute GVHD, chronic GVHD requiring systemic immunosuppression, disease relapse, or death from any cause. [14] RIC was defined by Center for Blood and Marrow Transplant Research (CIBMTR) functional definitions [17]
Statistical Analysis
The Kaplan-Meier method was used to estimate OS, DFS and GRFS with 95% confidence intervals (CI) derived from the standard errors. Log-rank test was used for univariate comparisons. Cumulative incidence estimator was used to calculate the probabilities of neutrophil and platelet engraftment, relapse, and acute and chronic GVHD reflecting the nonevent deaths as competing risks. The cumulative incidence of NRM was also calculated reflecting relapse as a competing risk. Fine and Gray analysis was used to compare the differences between cumulative incidence curves for the endpoints of neutrophil and platelet engraftment, relapse, NRM, and GVHD.[18] Factors considered in the analysis included DRI group LR/IR vs. HR, year of transplant ≥2007 or ≤2006, KPS <80% vs. ≥80%, HCT-CI score of 0, 1 to 2, or ≥3, age <65 or ≥65, gender, recipient CMV status, and donor source. Prognostic factor models for all clinical outcomes were built by backward selection method, and a p value significance of ≤0.05 was required for remaining in the model. The study data were analyzed using SAS version 9.3 (SAS Institute, Cary, NC).
Results
Patient Characteristics
We identified 104 patients age 60-64 (53%) and 92 patients age ≥65 (47%) who received alloHCT for hematological malignancies (Table 1). AML, MDS, NHL, and ALL comprised 90.3% of all diagnoses. DRI classified 12 patients (6.1%) as low risk (LR), 146 patients (74.5%) as intermediate risk (IR), and 38 patients (19.4%) as high risk (HR). There were no patients in our analysis with VHR DRI.
Table 1. Patient Characteristics.
| All Groups | LR/IR | HR | P-value | |
|---|---|---|---|---|
| N=196 | N=158 | N=38 | ||
| Sex | <0.01 | |||
| Male | 123(62.8%) | 92(58.2%) | 31(81.6%) | |
| Female | 73(37.2%) | 66(41.8%) | 7(18.4%) | |
| Age | 0.76 | |||
| 60-64 | 104(53.1%) | 83(52.5%) | 21(55.3%) | |
| ≥65 | 92(46.9%) | 75(47.5%) | 17(44.7%) | |
| Diagnosis | ||||
| AML | 101 (51.5%) | 83 (52.5%) | 18 (47.4%) | |
| MDS | 41 (20.9%) | 29 (18.4%) | 12 (31.6%) | |
| NHL | 20 (10.2%) | 17 (10.8%) | 3 (7.9%) | |
| ALL | 15 (7.7%) | 11 (7.0%) | 4 (10.5%) | |
| Myeloma | 8 (4.1%) | 7 (4.4%) | 1 (2.6%) | |
| Myeloproliferative Disorder | 6 (3.1%) | 6 (3.8%) | 0 | |
| CML | 5 (2.6%) | 5 (3.2%) | 0 | |
| Graft source | 0.08 | |||
| Matched Related Donor | 83 (42.3%) | 72 (45.6%) | 11 (29.0%) | |
| Adult Unrelated Donor | 17 (8.7%) | 11 (7.0%) | 6 (15.8%) | |
| UCB | 96(49.0%) | 75(47.5%) | 21(55.3%) | |
| HCT-CI | 0.09 | |||
| 0 | 63(32.1%) | 56(35.4%) | 7(18.4%) | |
| 1 to 2 | 56(28.6%) | 41(25.9%) | 15(39.5%) | |
| 3+ | 70(35.7%) | 57(36.1%) | 13(34.2%) | |
| Missing | 7(3.6%) | 4(2.5%) | 3(7.9%) | |
| KPS | 0.76 | |||
| ≤80 | 34(17.3%) | 27(17.1%) | 7(18.4%) | |
| >80 | 153(78.1%) | 125(79.1%) | 28(73.7%) | |
| Missing | 9(4.6%) | 6(3.8%) | 3(7.9%) | |
| Recipient CMV status | 0.4 | |||
| Positive | 120(61.2%) | 99(62.7%) | 21(55.3%) | |
| Negative | 76(38.8%) | 59(37.3%) | 17(44.7%) | |
| Treatment Year | 0.16 | |||
| ≤2006 | 45(23.0%) | 33(20.9%) | 12(31.6%) | |
| ≥2007 | 151(77.0%) | 125(79.1%) | 26(68.4%) | |
Median age at transplant was 64.8 years (range, 60-75 years): 53% age 60-64 and 47% age ≥65 (≥70 years, n=15). KPS was < 80% in 34 patients (17.3%) prior to transplant. Higher HCT-CI score (HCT-CI ≥3) was present in 70 patients (35.7%), with approximately 1/3 of patients with pre-transplant HCT-CI of 0, 1 to 2, and ≥3, respectively. UCB was donor source in 96 patients (49%), matched RD in 83 patients (42.3%), and URD in 17 patients (8.7%). Patient characteristics were similar between LR/IR and HR DRI groups, except more males than females in HR DRI group (81% in HR vs 58% in LR/IR, p < 0.01). AML and MDS were the most common diseases undergoing allogeneic transplantation in elderly patients, as these diseases comprised 72% of all transplants (71% of LR/IR DRI and 79% of HR DRI). NHL (n = 3), ALL (n = 4) and MM (n = 1) were remainder of HR DRI diseases receiving transplant. Majority of transplants were performed ≥2007 compared to ≤2006 (77% vs. 23%), similar between LR/IR and HR DRI groups.
Overall Survival, Cause of Death and DFS
OS probability at 2 years in the entire cohort was 47% (95% CI 40% - 54%). OS was significantly lower for HR DRI group (52% for LR/IR vs. 29% for HR; p<0.01) (Figure 1a). Median OS in HR DRI group was 5 months (range 3-21 months) compared to 29 months (range 13-47 months) in LR/IR group. In multiple regression analysis, after adjusting for graft type, HCT-CI, and treatment year, OS was significantly lower for HR DRI group (HR=2.03; 95% CI 1.28-3.23; p<0.01) than LR/IR DRI (Table 2). HCT-CI score, graft type, and treatment year did not significantly influence the risk of mortality after alloHCT in multivariate analysis. The most common cause of death was relapse (n = 51), followed by infection (n = 18), GVHD (n = 15), neurotoxicity (n = 8), and respiratory failure (n = 7).
Figure 1.
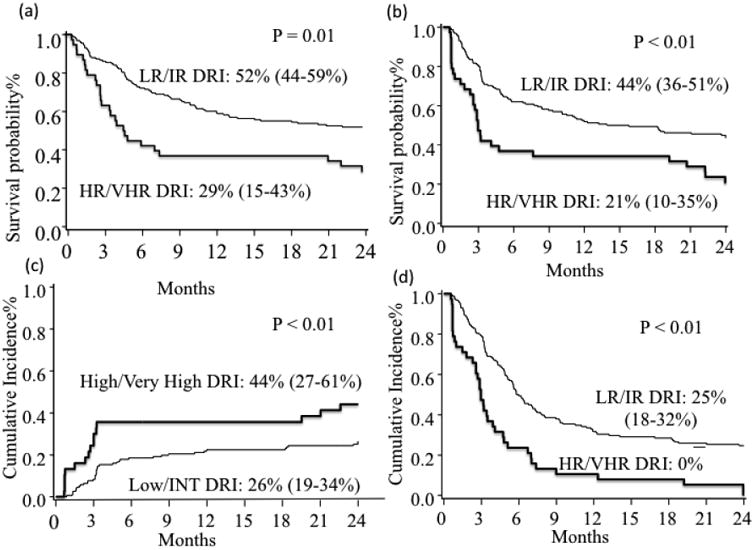
(a) 2-year OS by DRI group. (b) 2-year DFS by DRI group. (c) 2-year Relapse by DRI group. (d) 2-year TRM by DRI group.
Table 2. Multivariable Analysis of Clinical Outcomes.
| Variable | Total N | Multivariable | ||
|---|---|---|---|---|
| HR | 95% CI | P-value | ||
| OS at 2 years | ||||
| DRI LR/IR | 158 | 1 | ||
| DRI HR | 38 | 2.03 | 1.28-3.23 | <0.01 |
| Adult Donor | 100 | 1 | ||
| UCB | 96 | 1.32 | 0.88-1.96 | 0.18 |
| HCT CI 0-2 | 119 | 1 | ||
| HCT-CI ≥3 | 70 | 1.47 | 0.98-2.19 | 0.06 |
| TX year ≤ 2006 | 1 | |||
| TX year ≥ 2007 | 0.76 | 0.48-1.2 | 0.23 | |
| DFS at 2 years | ||||
| DRI LR/IR | 158 | 1 | 1 | |
| DRI HR | 38 | 2.07 | 1.35-3.18 | <0.01 |
| Adult Donor | 100 | 1 | 1 | |
| UCB | 96 | 1.18 | 0.81-1.7 | 0.39 |
| HCT CI 0-2 | 119 | 1 | 1 | |
| HCT-CI ≥3 | 70 | 1.42 | 0.98-2.07 | 0.07 |
| TX year ≤ 2006 | 1 | 0.04 | ||
| TX year ≥ 2007 | 0.63 | 0.41-0.97 | ||
| Relapse at 2 years | ||||
| DRI LR/IR | 158 | 1 | ||
| DRI HR | 38 | 2.07 | 1.14-3.71 | 0.02 |
| TRM at 2 years | ||||
| DRI LR/IR | 158 | 1 | ||
| DRI HR | 38 | 1.26 | 0.66-2.53 | 0.46 |
| HCT-CI 0-2 | 119 | 1 | ||
| HCT-CI ≥3 | 70 | 1.45 | 0.84-2.52 | 0.18 |
| TX year ≤ 2006 | 1 | |||
| TX year ≥ 2007 | 0.75 | 0.4-1.42 | 0.37 | |
DFS at 2 years was 39%, (95% CI 0.32–0.46) and was significantly lower for HR DRI group (44% for LR/IR vs. 21% for HR; p<0.01)(Figure 1b). In HR DRI group, median DFS was 3 months (range 2-8 months), while it was 15 months (range 9-24 months) for the LR/IR DRI group. Patient age, KPS and HCT-CI had no significant impact on DFS. In multiple regression analysis, treatment failure (inverse of DFS; HR=1.93; 95% CI 1.25-2.99; p<0.01) was significantly increased for HR DRI group after adjusting for graft type, HCT-CI score, and treatment year. Treatment year ≥ 2007 was significantly associated with increased DFS (HR 0.66, 95% CI 0.43-1; p = 0.05) in multivariate analysis.
Hematopoietic Recovery, TRM and Relapse
Neutrophil engraftment at day 42 was 96% (95% CI 92-98%) (95% for LR/IR vs. 100% for HR; p=0.40) and platelet engraftment was 72% (95% CI 64-81%) at 6 months (76% for LR/IR vs. 58% for HR; p=0.08). UCB stem cell source was associated with lower rates of neutrophil engraftment (93% vs. 98%; p< 0.01) and platelet engraftment (65% vs. 80%, p<0.01) as compared to adult donor grafts. In multiple regression analysis, after adjusting for graft type, platelet recovery was significantly worse for HR DRI group (HR=0.56; 95% CI 35-91%; p=0.02), while neutrophil recovery was not influenced by DRI. The use of UCB as compared to adult donor grafts was independently associated with lower recovery of both neutrophils (HR=0.38, 95% CI 0.27-0.52; p<0.01) and platelets (HR=0.39, 95% CI 0.28-0.56; p<0.01).
TRM at 2 years was 29% (95% CI 23-36%): 28% for LR/IR DRI group and 34% for HR DRI group (p=0.12). In multiple regression analysis, after adjusting for HCT-CI and year of transplant, TRM was not significantly affected by DRI. None of the factors analyzed, including patient age, KPS, HCT-CI, year of transplant, or graft source influenced the risk of TRM.
Relapse rate in entire cohort was 30% (95% CI 23-37%), and was significantly higher for HR DRI group (26% for LR/IR vs. 44% for HR; p<0.01). HR DRI remained the only significant predictor of increased risk of relapse (HR=2.07; 95% CI 1.14-3.71; p=0.02).
Acute and Chronic GVHD
Cumulative incidence of grade II-IV acute GVHD at day 180 was 42% (95% CI 35-49%) for the entire cohort (41% for LR/IR vs. 47% for HR, p=0.32). Similarly, the cumulative incidence of chronic GVHD at 2 years was 34% (95% CI 26-41%) for the entire cohort (35% for LR/IR vs. 31% for HR, p=0.07). UCB donor type was associated with significantly lower risk of chronic GVHD (22% vs. 46% for adult donor, p<0.01). In multiple regression analysis, UCB source was associated with significantly lower risks of chronic GVHD (HR 0.42, 95% CI 0.25-0.72 p<0.01), while there was no risk factor associated with acute GVHD.
Discussion
We identified that revised DRI is the most powerful prognostic tool for OS after RIC alloHCT in the elderly. While encouraging 2-year OS (52%) and DFS (44%) rates were observed for patients in LR/IR DRI group, corresponding outcomes were lower for those in HR group (29% for OS and 21% for DFS). In HR DRI, graft-versus-tumor effect can control disease long-term in a proportion of patients with otherwise poor outcome with chemotherapy alone, thus the role of transplantation remains vital in the current era. It has been previously shown that patients with intermediate or high-risk AML and MDS, which comprise the majority of allogeneic transplants in the elderly in our study, have better outcomes with HCT consolidation than without. [19] [20] Due to increased risk of relapse leading to poor survival in HR DRI, participation in clinical trials offering relapse prevention strategies after RIC alloHCT should be encouraged when available.
There have been multiple studies assessing risk factors for outcomes of RIC transplantation in elderly patients, [6][7][21] but DRI was generally not considered. Similar to our observation, a recent study by Pohlen et al. showed 3 year OS of 43-54% for LR/IR DRI and 18-32% for HR/VHR DRI in 187 elderly patients (≥ 60 years) with AML or MDS. [8] Our study broadens Pohlen's findings to a larger range of hematologic malignancies and includes umbilical cord donor source for elderly patients, a population not included in their analysis. Although our study did not include large enough numbers of each DRI subgroup to make generalizations, particularly in high-risk and very-high risk DRI subgroups, our findings are consistent with a large CIBMTR registry report showing 2-year OS rates ranging from 64% for LR DRI group to 24% for VHR DRI group in adults of all ages with hematologic malignancies. [13]
While prior large studies have reported results of predominantly matched sibling and unrelated donor alloHCT, approximately half of patients in our study received UCB transplant. Although we observed delayed ANC and platelet engraftment in UCB transplant recipients, UCB source did not negatively impact OS or DFS in this population. Lower incidence of chronic GVHD in UCB transplants remains one of the major advantages of UCB transplant, and is particularly relevant to elderly patients who have poor tolerance to prolonged immunosuppressive therapy. [22] Here, we show that incidence of chronic GVHD is lower in elderly patients who receive UCB transplant than adult donors.
While several prior studies found lower OS rates with increasing age [23][24], DRI was the only factor that significantly influenced OS in our analysis. We did not observe any difference in secondary outcomes of DFS, TRM, relapse, GVHD, GRFS or hematopoietic recovery for age 60-64 compared to age ≥65. Our institution has previously published an analysis on outcomes of elderly AML and MDS patients ≥ age 70 receiving alloHCT with sibling and umbilical cord donors (n = 19), with 2 year OS rates of 55-60%, similar to patients aged 60-69 (n = 60) with 2 year OS rate of 42-43%.[25] Other studies looking at impact of age on outcomes similarly have not shown an association. [6][7][21]
Our study supports continued use of RIC transplantation as a potentially curative therapy for elderly patients with good performance status and comorbidity scores, however is limited by small patient sample size. Larger studies of elderly patients are needed to assess outcomes and improve disease risk stratification, particularly in real world settings where elderly patients may be frailer than those enrolled in clinical trials. Our TRM of 29% is similar to other reported TRM of RIC transplants among elderly patients [21][6], and higher than that of younger patients receiving RIC transplant (10-26% at 3 years). [26][27][28] TRM did not significantly differ between age groups of 60-64 vs ≥65 years in our study, consistent with other reports [6][21]. TRM was also not affected by DRI in our study, similar to a large validation cohort of the European Society for Blood and Marrow Transplantation (EBMT) which did not report significant differences in TRM across LR, IR, HR, and VHR DRI groups.[29] RIC transplantation is feasible, but with significant TRM risk, in this population.
Higher relapse rates following RIC transplantation compared to MA conditioning have been well described. [30] Strategies to overcome increased risk of relapse, particularly those that can enhance GVL, are needed in elderly patients who are usually only candidates for RIC. Donor lymphocyte infusions (DLI) can be used successfully as a prophylactic measure [31] or at time of relapse [32], however the increased risk of GVHD and DLI availability are limiting factors for wide utilization of this strategy. Hypomethylating agents have been used for post-transplant maintenance in MDS and AML. [33] Immunotherapy using inhibition of CTLA-4 and PD-L1 pathways has been encouraging in early phase studies of hematologic malignancies with acceptable rates of GVHD. [34] [35] For FLT3/ITD-mutated AML, sorafenib as maintenance therapy post-transplant is being adopted into practice after showing promising results in early phase clinical trials. [36][37]
DRI risk group is an important prognostic tool in elderly patients with hematologic malignancies undergoing alloHCT. However, limitations of DRI include lack of incorporation of minimal residual disease (MRD) status and molecular subtype of disease. Pre-transplant MRD status by flow cytometry [38] or next-generation sequencing has been reported to be prognostic in AML, and may be even more important in setting of reduced intensity conditioning. [39][40] Molecular mutations have been shown to have independent prognostic implications in patients undergoing allogeneic transplant for MDS. [41] Larger studies, particularly involving non-AML and MDS disease subtypes, are needed to further validate DRI in a real-world setting with elderly patients.
Highlights.
DRI is important prognostic factor for elderly patients ≥60 years
High risk DRI group has reduced overall and disease-free survival post-transplant
Age group 60-64 vs ≥65 does not affect survival or treatment-related mortality
Novel or alternative therapies are needed for high risk and very high risk disease
Acknowledgments
This work was supported in part by grants from the National Cancer Institute P01 CA65493.
Footnotes
The authors have no conflicts of interest to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cornelissen JJ, van Putten WLJ, Verdonck LF, Theobald M, Jacky E, Daenen SMG, et al. Results of a HOVON/SAKK donor versus no-donor analysis of myeloablative HLA-identical sibling stem cell transplantation in first remission acute myeloid leukemia in young and middle-aged adults: benefits for whom? Blood. 2007;109:3658–66. doi: 10.1182/blood-2006-06-025627. [DOI] [PubMed] [Google Scholar]
- 2.Gupta V, Richards S, Rowe J Acute Leukemia Stem Cell Transplantation Trialists' Collaborative Group. Allogeneic, but not autologous, hematopoietic cell transplantation improves survival only among younger adults with acute lymphoblastic leukemia in first remission: an individual patient data meta-analysis. Blood. 2013;121:339–50. doi: 10.1182/blood-2012-07-445098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freytes CO, Loberiza FR, Rizzo JD, Bashey A, Bredeson CN, Cairo MS, et al. Myeloablative allogeneic hematopoietic stem cell transplantation in patients who experience relapse after autologous stem cell transplantation for lymphoma: a report of the International Bone Marrow Transplant Registry. Blood. 2004;104:3797–803. doi: 10.1182/blood-2004-01-0231. [DOI] [PubMed] [Google Scholar]
- 4.Oran B, Weisdorf DJ. Survival for older patients with acute myeloid leukemia: a population-based study. Haematologica. 2012;97:1916–24. doi: 10.3324/haematol.2012.066100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127–38. doi: 10.1007/s00277-015-2351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McClune BL, Weisdorf DJ, Pedersen TL, Tunes da Silva G, Tallman MS, Sierra J, et al. Effect of age on outcome of reduced-intensity hematopoietic cell transplantation for older patients with acute myeloid leukemia in first complete remission or with myelodysplastic syndrome. J Clin Oncol. 2010;28:1878–87. doi: 10.1200/JCO.2009.25.4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Cheikh J, Sfumato P, Sobh M, Fegueux N, Mohty M, Vigouroux S, et al. Allogeneic hematopoietic stem cell transplantation after reduced intensity conditioning regimen for elderly patients (60 years and older) with hematologic malignancies using unrelated donors: a retrospective study from the French society for stem cell tra. Haematologica. 2016;101:e262–5. doi: 10.3324/haematol.2015.139345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pohlen M, Groth C, Sauer T, Görlich D, Mesters R, Schliemann C, et al. Outcome of allogeneic stem cell transplantation for AML and myelodysplastic syndrome in elderly patients (≥60 years) Bone Marrow Transplant. 2016 doi: 10.1038/bmt.2016.156. [DOI] [PubMed] [Google Scholar]
- 9.Devine SM, Owzar K, Blum W, Mulkey F, Stone RM, Hsu JW, et al. Phase II Study of Allogeneic Transplantation for Older Patients With Acute Myeloid Leukemia in First Complete Remission Using a Reduced-Intensity Conditioning Regimen: Results From Cancer and Leukemia Group B 100103 (Alliance for Clinical Trials in Oncolo. J Clin Oncol. 2015;33:4167–75. doi: 10.1200/JCO.2015.62.7273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Storb R, Sandmaier BM. Nonmyeloablative allogeneic hematopoietic cell transplantation. Haematologica. 2016;101:521–30. doi: 10.3324/haematol.2015.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sorror ML, Maris MB, Storb R, Baron F, Sandmaier BM, Maloney DG, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arora M, Sun CL, Ness KK, Teh JB, Wu J, Francisco L, et al. Physiologic Frailty in Nonelderly Hematopoietic Cell Transplantation Patients: Results From the Bone Marrow Transplant Survivor Study. JAMA Oncol. 2016;2:1277–86. doi: 10.1001/jamaoncol.2016.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Armand P, Kim HT, Logan BR, Wang Z, Alyea EP, Kalaycio ME, et al. Validation and refinement of the Disease Risk Index for allogeneic stem cell transplantation. Blood. 2014;123:3664–71. doi: 10.1182/blood-2014-01-552984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holtan SG, DeFor TE, Lazaryan A, Bejanyan N, Arora M, Brunstein CG, et al. Composite endpoint of graft-versus-host disease-free, relapse-free survival after allogeneic hematopoietic cell transplantation. Blood. 2015;125:1333–9. doi: 10.1182/blood-2014-10-609032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 16.Jagasia MH, Greinix HT, Arora M, Williams KM, Wolff D, Cowen EW, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21:389–401.e1. doi: 10.1016/j.bbmt.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biol Blood Marrow Transplant. 2009;15:367–9. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fine JP, G R. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 19.Versluis J, Hazenberg CLE, Passweg JR, van Putten WLJ, Maertens J, Biemond BJ, et al. Post-remission treatment with allogeneic stem cell transplantation in patients aged 60 years and older with acute myeloid leukaemia: a time-dependent analysis. Lancet Haematol. 2015;2:e427–36. doi: 10.1016/S2352-3026(15)00148-9. [DOI] [PubMed] [Google Scholar]
- 20.Koreth J, Schlenk R, Kopecky KJ, Honda S, Sierra J, Djulbegovic BJ, et al. Allogeneic stem cell transplantation for acute myeloid leukemia in first complete remission: systematic review and meta-analysis of prospective clinical trials. JAMA. 2009;301:2349–61. doi: 10.1001/jama.2009.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorror ML, Sandmaier BM, Storer BE, Franke GN, Laport GG, Chauncey TR, et al. Long-term outcomes among older patients following nonmyeloablative conditioning and allogeneic hematopoietic cell transplantation for advanced hematologic malignancies. JAMA. 2011;306:1874–83. doi: 10.1001/jama.2011.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brunstein CG, Gutman JA, Weisdorf DJ, Woolfrey AE, Defor TE, Gooley TA, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–9. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guo RJ, Atenafu EG, Craddock K, Chang H. Allogeneic hematopoietic cell transplantation may alleviate the negative prognostic impact of monosomal and complex karyotypes on patients with acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:690–5. doi: 10.1016/j.bbmt.2014.01.027. [DOI] [PubMed] [Google Scholar]
- 24.Michelis FV, Messner HA, Atenafu EG, Kim DD, Kuruvilla J, Lipton JH, et al. Benefit of allogeneic transplantation in patients age ≥ 60 years with acute myeloid leukemia is limited to those in first complete remission at time of transplant. Biol Blood Marrow Transplant. 2014;20:474–9. doi: 10.1016/j.bbmt.2013.12.560. [DOI] [PubMed] [Google Scholar]
- 25.Sandhu KS, Brunstein C, DeFor T, Bejanyan N, Arora M, Warlick E, et al. Umbilical Cord Blood Transplantation Outcomes in Acute Myelogenous Leukemia/Myelodysplastic Syndrome Patients Aged ≥70 Years. Biol Blood Marrow Transplant. 2016;22:390–3. doi: 10.1016/j.bbmt.2015.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Majhail NS, Brunstein CG, Tomblyn M, Thomas AJ, Miller JS, Arora M, et al. Reduced-Intensity Allogeneic Transplant in Patients Older Than 55 Years: Unrelated Umbilical Cord Blood Is Safe and Effective for Patients without a Matched Related Donor. Biol Blood Marrow Transplant. 2008;14:282–9. doi: 10.1016/j.bbmt.2007.12.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen Y Bin, Aldridge J, Kim HT, Ballen KK, Cutler C, Kao G, et al. Reduced-Intensity Conditioning Stem Cell Transplantation: Comparison of Double Umbilical Cord Blood and Unrelated Donor Grafts. Biol Blood Marrow Transplant. 2012;18:805–12. doi: 10.1016/j.bbmt.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 28.Brunstein CG, Barker JN, Weisdorf DJ, DeFor TE, Miller JS, Blazar BR, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: Impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–70. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shouval R. The Disease Risk Index is a Robust Tool for Allogeneic Hematopoietic Stem Cell Transplantation Risk Stratification: An Independent Validation Study on a Large Cohort of the European Society for Blood and Marrow Transplantation (EBMT) Am Soc Hematol. 2016 [Google Scholar]
- 30.Storb R, Sandmaier BM. Nonmyeloablative allogeneic hematopoietic cell transplantation. Haematologica. 2016;101:521–30. doi: 10.3324/haematol.2015.132860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jedlickova Z, Schmid C, Koenecke C, Hertenstein B, Baurmann H, Schwerdtfeger R, et al. Long-term results of adjuvant donor lymphocyte transfusion in AML after allogeneic stem cell transplantation. Bone Marrow Transplant. 2016;51:1–5. doi: 10.1038/bmt.2015.234. [DOI] [PubMed] [Google Scholar]
- 32.He F, Warlick E, Miller JS, MacMillan M, Verneris MR, Cao Q, et al. Lymphodepleting chemotherapy with donor lymphocyte infusion post-allogeneic HCT for hematological malignancies is associated with severe, but therapy-responsive aGvHD. Bone Marrow Transplant. 2016:1–6. doi: 10.1038/bmt.2016.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Craddock C, Jilani N, Siddique S, Yap C, Khan J, Nagra S, et al. Tolerability and Clinical Activity of Post-Transplantation Azacitidine in Patients Allografted for Acute Myeloid Leukemia Treated on the RICAZA Trial. Biol Blood Marrow Transplant. 2016;22:385–90. doi: 10.1016/j.bbmt.2015.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients with Relapse after Allogeneic Transplantation. N Engl J Med. 2016;375:143–53. doi: 10.1056/NEJMoa1601202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Merryman RW, Kim HT, Zinzani PL, Carlo-stella C, Ansell SM, Perales M, et al. Safety and ef fi cacy of allogeneic hematopoietic stem cell transplant after PD-1 blockade in relapsed / refractory lymphoma. 2017;129:1380–9. doi: 10.1182/blood-2016-09-738385.Presented. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunner AM, Li S, Fathi AT, et al. Hematopoietic cell transplantation with or without sorafenib maintenance for patients with FLT3-ITD acute myeloid leukemia in CR1. Blood. 2015;126 Abstract 864. [Google Scholar]
- 37.Chen YB, Li S, Lane AA, Connolly C, Del Rio C, Valles B, et al. Phase I trial of maintenance sorafenib after allogeneic hematopoietic stem cell transplantation for fms-like tyrosine kinase 3 internal tandem duplication acute myeloid leukemia. Biol Blood Marrow Transplant. 2014;20:2042–8. doi: 10.1016/j.bbmt.2014.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Y, Othus M, Araki D, Wood BL, Radich JP, Halpern AB, et al. Pre- and post-transplant quantification of measurable (“minimal”) residual disease via multiparameter flow cytometry in adult acute myeloid leukemia. Leukemia. 2016:1456–64. doi: 10.1038/leu.2016.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ustun C, Wiseman AC, Defor TE, Yohe S, Linden MA, Oran B, et al. Achieving stringent CR is essential before reduced-intensity conditioning allogeneic hematopoietic cell transplantation in AML. Bone Marrow Transplant. 2013;48:1415–20. doi: 10.1038/bmt.2013.124. [DOI] [PubMed] [Google Scholar]
- 40.Gaballa S, Saliba R, Oran B, Brammer JE, Chen J, Rondon G, et al. Relapse risk and survival in patients with FLT3 mutated acute myeloid leukemia undergoing stem cell transplantation. Am J Hematol. 2017;92:331–7. doi: 10.1002/ajh.24632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lindsley RC, Saber W, Mar BG, Redd R, Wang T, Haagenson MD, et al. Prognostic Mutations in Myelodysplastic Syndrome after Stem-Cell Transplantation. N Engl J Med. 2017;376:536–47. doi: 10.1056/NEJMoa1611604. [DOI] [PMC free article] [PubMed] [Google Scholar]


