Abstract
Introduction
Although Indian Health Service, tribally-operated, and urban Indian (I/T/U) healthcare facilities have higher human papillomavirus (HPV) vaccine series initiation and completion rates among adolescent patients aged 13–17 years than the general U.S. population, challenges remain. I/T/U facilities have lower coverage for HPV vaccine first dose compared with coverage for other adolescent vaccines, and HPV vaccine series completion rates are lower than initiation rates. Researchers aimed to assist I/T/U facilities in identifying interventions to increase HPV vaccination series initiation and completion rates.
Study design
Best practice and intervention I/T/U healthcare facilities were identified based on baseline adolescent HPV vaccine coverage data. Healthcare professionals were interviewed about barriers and facilitators to HPV vaccination. Researchers used responses and evidence-based practices to identify and assist facilities in implementing interventions to increase adolescent HPV vaccine series initiation and completion. Coverage and interview data were collected from June 2013 to June 2015; data were analyzed in 2015.
Setting/participants
I/T/U healthcare facilities located within five Indian Health Service regions.
Intervention
Interventions included analyzing and providing feedback on facility vaccine coverage data, educating providers about HPV vaccine, expanding access to HPV vaccine, and establishing or expanding reminder recall and education efforts.
Main outcome measures
Impact of evidence-based strategies and best practices to support HPV vaccination.
Results
Mean baseline first dose coverage with HPV vaccine at best practice facilities was 78% compared with 46% at intervention facilities. Mean third dose coverage was 48% at best practice facilities versus 19% at intervention facilities. Intervention facilities implemented multiple low-cost, evidence-based strategies and best practices to increase vaccine coverage. At baseline, most facilities used electronic provider reminders, had standing orders in place for administering HPV vaccine, and administered tetanus, diphtheria, and acellular pertussis and HPV vaccines during the same visit. At intervention sites, mean coverage for HPV initiation and completion increased by 24% and 22%, respectively.
Conclusions
A tailored multifaceted approach addressing vaccine delivery processes and patient and provider education may increase HPV vaccine coverage.
INTRODUCTION
The Indian Health Service (IHS) is the U.S. federal agency responsible for providing health care to 2.2 million American Indian and Alaska Native (AI/AN) people from 566 federally recognized tribes. IHS is divided into 12 administrative regions, and supports a network of IHS, tribally-operated, and urban Indian (I/T/U) healthcare facilities in 35 states (Figure 1).1
Figure 1.
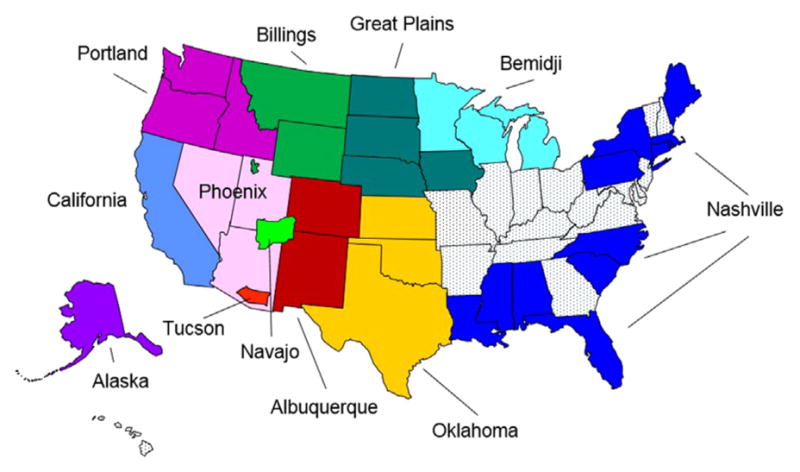
Indian Health Service Administrative Regions.
Note: Some Indian Health Service Administrative Regions are named for the city or state in which the regional office is located, but encompass a broader geographic region.
For many vaccines, the IHS patient population has higher coverage than the general U.S. population.2,3 IHS supports a comprehensive electronic health record (EHR) system used by the majority of I/T/U facilities that includes provider reminders for routinely recommended vaccines. Data reside at the facility and are not shared between facilities. The EHR and programs like Vaccines for Children, which provides free vaccines for all AI/AN children, likely contribute to higher vaccine coverage.2–4
The human papillomavirus (HPV) vaccine is routinely recommended for male and female adolescents to prevent HPV-associated genital warts and cancers of the cervix, anus, oropharynx, vagina, vulva, and penis.5 The Advisory Committee on Immunization Practices recommends adolescents receive tetanus, diphtheria, and acellular pertussis (Tdap), meningococcal conjugate (MenACWY), and HPV vaccines at age 11 or 12 years. Despite this recommendation, nationwide, HPV vaccine coverage falls short of coverage with other adolescent vaccines.6
The HPV vaccine is particularly important for AI/AN women, as they experience higher rates of cervical cancer and cervical cancer–related incidence and mortality compared with white women, and in some geographic regions, higher rates of HPV infection than white women.7–9 In IHS, HPV vaccine coverage is higher than coverage in the general U.S. population, but consistently lags behind coverage with Tdap and MenACWY vaccines.10 For 2013, IHS first dose HPV vaccine coverage among male and female adolescents aged 13–17 years was 72%, and third dose coverage was 57%. Coverage for Tdap and MenACWY vaccines for this age group was 92% and 89%, respectively.10
Previous research among providers, including those serving tribal communities, identified multiple HPV vaccination barriers such as infrequent adolescent visits to clinics, limited time with patients, and parental concerns about HPV vaccine.11–13 In the present study, researchers collaborated with a selection of I/T/U healthcare facilities with comparatively low HPV vaccine coverage to identify and implement interventions to overcome barriers and increase HPV vaccine coverage.
METHODS
Study Sample
Using IHS quarterly vaccine coverage data, baseline adolescent (female and male adolescents aged 13–17 years) coverage with Tdap and HPV vaccines were determined as of June 30, 2013, at I/T/U facilities within each of the 12 IHS administrative regions. Owing to resource limitations and practical considerations, the study was limited to five regions. Researchers considered overall adolescent vaccine coverage, geography, and existing relationships with regional immunization coordinators and targeted the following regions: Great Plains, Nashville, Navajo, Oklahoma, and Portland.
Within the five regions, all 97 I/T/U facilities were ranked by three measures: (1) the difference between Tdap and HPV first dose vaccine coverage; (2) HPV first dose coverage (i.e., series initiation); and (3) HPV third dose coverage (i.e., series completion). Across these measures, the three highest-ranked and three lowest-ranked I/T/U facilities in each region were chosen as potential best practice facilities and intervention facilities, respectively, totaling 30 potential study facilities. Regional immunization coordinators reviewed the six facilities in their regions and considering practical issues, such as facility staffing levels and competing priorities, selected a total of 14 intervention and 12 best practice facilities representing the five regions.
Potential best practice and intervention facilities were invited to participate in the study. If a response was not received after two emails, two phone calls, and contact from the immunization coordinator, the facility was not included.
At each participating facility, snowball sampling was used to identify providers involved in adolescent care or vaccination activities. Researchers interviewed staff in 2014–2015, either individually or as a group, using a structured interview guide from previously published provider surveys about HPV vaccination that was modified for the I/T/U setting.13 All present study team members compared notes for accuracy. Researchers collected facility characteristics, current vaccination procedures, and perceived barriers to and facilitators of administering HPV vaccine to adolescents. In addition, each facility’s baseline vaccine coverage data were shared. Assessments for intervention facilities and best practice facilities were identical, save for an additional open-ended item for intervention facilities regarding changes needed to increase HPV vaccine coverage. Researchers compiled assessment responses from all sites and analyzed data using Microsoft Access in 2015. Researchers performed a descriptive data analysis of responses, given the sample size was too small to detect statistically significant differences between groups.
Data Collection
Researchers compiled possible interventions to increase HPV vaccine coverage from the Guide to Community Preventive Services and other published literature as well as strategies gathered during interviews with best practice facilities (Table 1).14–16 Researchers then worked with each intervention facility to identify feasible interventions, and assisted the facility in implementing its self-selected interventions.
Table 1.
Evidence-based Strategies and Best Practices to Support HPV Vaccination
| Strategy | Best practice facilities already using strategy at baseline (N=9)a | Intervention facilities already using strategy at baseline (N=10)a | Intervention facilities implementing new strategy as part of intervention (N=8)a |
|---|---|---|---|
| Electronic provider reminders | 9 (100) | 10 (100) | 0 (0) |
| Co-administer HPV with Tdap and meningococcal vaccines | 8 (89) | 9 (90) | 0 (0) |
| Educate patients or parents about HPV vaccine outside clinical visits | 8 (89) | 4 (40) | 1 (13) |
| Standing orders | 8 (89) | 8 (80) | 2 (25) |
| Walk-in or nurse immunization clinics | 8 (89) | 6 (60) | 3 (38) |
| Access state immunization information system | 7 (78) | 7 (70) | 2 (25) |
| Letter or postcard patient reminders | 6 (67) | 5 (50) | 2 (25) |
| Record dates of patient’s appointment for subsequent doses on paper given to patients or parents | 4 (44) | 6 (60) | 1 (13) |
| Distribute patient reminder magnets provided by vaccine manufacturer | 3 (33) | 4 (40) | 3 (38) |
| Vaccinate 9- and 10-year-olds | 3 (33) | 1 (10) | 1 (13) |
| Administer HPV vaccine outside the healthcare facility (schools, community health fairs) | 2 (22) | 3 (30) | 3 (38) |
| Phone call patient reminders (initiated or enhanced existing) | 1 (11) | 7 (70) | 4 (50) |
| Perform missing data analysisb | N/A | N/A | 4 (50) |
| Provider educationa | N/A | N/A | 3 (38) |
| Increase patient education during clinical visitsb | N/A | N/A | 2 (25) |
| Perform missed opportunities analysisb | N/A | N/A | 2 (25) |
| Staffing changesb | N/A | N/A | 1 (13) |
Data are shown as n (%).
Strategy not captured as part of baseline assessment.
HPV, human papillomavirus; N/A, not applicable; Tdap, tetanus, diphtheria, and acellular pertussis.
For facilities requesting assistance examining missing data, researchers compared the completeness of the facility’s EHR vaccination records (for patients missing at least one dose of the recommended adolescent vaccines) with the state immunization information system (IIS) vaccination records, and provided a one-time update to the facility’s EHR. For facilities requesting assistance analyzing missed HPV vaccination opportunities (defined as an in-person healthcare visit when a patient was due for, but did not receive, the HPV vaccine), researchers reviewed the last ten healthcare visits of patients missing an adolescent vaccination.
Researchers collected baseline HPV and Tdap vaccine coverage data as of June 30, 2013, for each facility. To monitor the effects of interventions on vaccine coverage, researchers collected quarterly vaccine coverage data from June 30, 2013 through June 30, 2015 from intervention facilities and compared those with baseline data. Researchers also compared coverage for intervention facilities with coverage for best practice facilities and coverage for facilities in the same regions that were not targeted for the project (non-participating facilities). Researchers performed cross-sectional analyses of coverage data in 2015.
The study protocol was reviewed by both the Centers for Disease Control and Prevention and the IHS IRBs. Both determined this project constituted public health practice and not research; therefore, no formal IRB approvals were required.
RESULTS
Of the 12 best practice facilities and 14 intervention facilities invited to participate, nine best practice and ten intervention facilities agreed to participate, representing the five IHS regions. Researchers conducted 35 interviews among staff.
Best practice and intervention facilities, at baseline, had a total of 6,294 and 6,239 adolescents aged 13–17 years, respectively. Best practice facilities, compared with intervention facilities, had higher mean first dose HPV vaccine coverage (78% [range, 54%–92%] vs 46% [range, 29%–71%]) and higher mean third dose HPV vaccine coverage (48% [range, 31%–67%] vs 19% [range, 6%–33%]). Owing to regional variability, intervention facilities in some regions had similar coverage to best practice facilities in other regions.
All facilities had an electronic provider reminder for both HPV vaccine initiation and series completion. In addition, most facilities in both groups (89% among best practice and 90% among intervention facilities) reported co-administering HPV with Tdap and MenACWY vaccines (Table 1).
Most best practice and intervention facilities (89% and 80%, respectively) had standing orders in place; however, a higher proportion of best practice facilities than intervention facilities reported providing vaccines during nurse-only visits (89% vs 60%, respectively). Few facilities reported vaccinating outside the clinic setting (22% of best practice and 30% of intervention facilities). Most best practice facilities (89%) reported educating patients and parents about the HPV vaccine outside clinical visits through schools or at community-based events such as health fairs. By contrast, less than half of intervention facilities (40%) reported educating patients and parents about the HPV vaccine outside clinical visits. Although researchers did not ask specifically about nurse involvement, discussion initiated by the open-ended questions revealed best practice facilities had greater nurse involvement in clinical visits and with reminder strategies than intervention facilities. The most notable difference between best practice and intervention facilities was their use of phone call reminders: Seventy percent of intervention facilities used this strategy at baseline compared with only 11% of best practice facilities. Table 1 includes other strategies both best practice and intervention facilities used.
Overall, the top five barriers to HPV vaccination at intervention facilities were reported by providers to include the lack of patient awareness about vaccine benefits, missing vaccination data, clinic access issues, vaccine safety concerns, and parent beliefs that HPV vaccine increases sexual activity. More intervention facilities than best practice facilities (60% vs 33%, respectively) reported that a major barrier to HPV vaccine series completion was lack of awareness among patients and their parents of the recommendation for three doses (data not shown).
After the assessments, eight of ten intervention facilities implemented strategies to increase HPV vaccine coverage (Table 1). All eight facilities implementing interventions selected more than one intervention.
All intervention facilities implementing strategies started or enhanced reminder recall activities using phone calls, patient reminder magnets, and postcard/letter reminders (Table 1), with some facilities implementing more than one reminder recall strategy.
Three intervention facilities requested provider education sessions (Table 1). Researchers performed five staff education sessions (three in person and two virtual) incorporating the Centers for Disease Control and Prevention’s “You Are the Key to HPV Cancer Prevention” campaign.16 Researchers presented facility-specific information on HPV vaccine coverage and shared barriers the facility identified in the baseline assessments and data on missed opportunities and missing data (for facilities opting for these analyses).
Several facilities increased HPV vaccine access by vaccinating outside the clinic (n=3), establishing walk-in nurse immunization clinics (n=3), or through implementing standing orders more consistently (n=2). Two facilities requested assistance conducting a missing data analysis and a missed opportunities analysis (Table 1). Two additional facilities conducted their own review of missing data, updating patient records with data from their state IIS. Table 1 includes other interventions that facilities implemented.
Initiation and completion of HPV vaccine increased at intervention facilities throughout the study. Among intervention facilities from June 30, 2013 to June 30, 2015, HPV vaccine initiation increased from 47% to 71% (mean, 24%; range, 8%–38%) (Figure 2 and Appendix Figure 1, available online), and completion increased from 20% to 42% (mean, 22%; range, 4%–35%) (Figure 3 and Appendix Figure 1, available online). The mean increase in HPV vaccine initiation and completion at intervention facilities surpassed the mean increase at best practice and non-participating facilities (Appendix Figure 1, available online).
Figure 2.
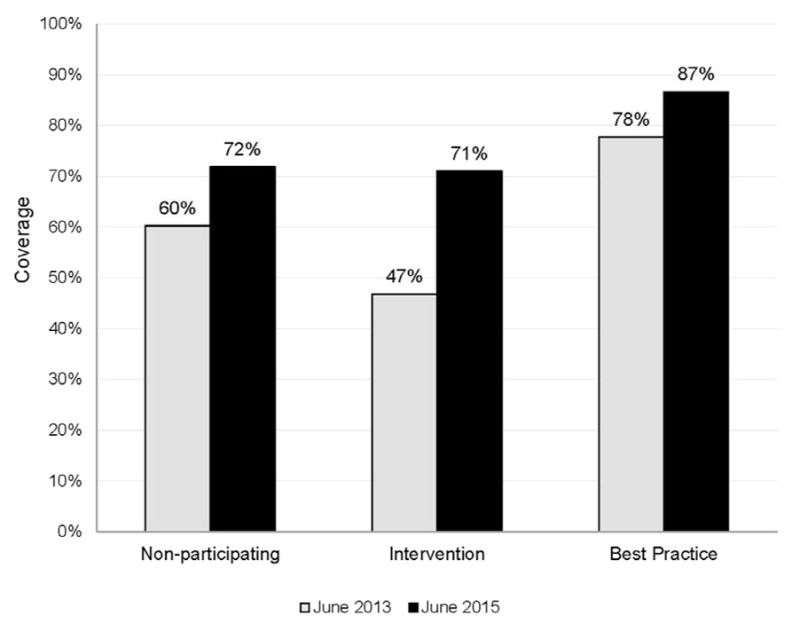
HPV vaccine initiation coverage at non-participating, intervention, and best practice facilities, June 2013 versus June 2015.
Note: “Non-participating facilities” refers to Indian Health Service, tribally-operated, and urban Indian healthcare facilities within the Great Plains, Portland, Nashville, Navajo, and Oklahoma Indian Health Service regions, that were neither considered intervention facilities nor best practice facilities. Facilities missing data from either June 2013 or June 2015 were omitted from the analysis. Mean 13–17-year-old male and female population size from 2013 and 2015 for: 68 non-participating facilities=25,259 persons; nine intervention facilities=5,943 persons; and nine best practice facilities=6,026 persons.
HPV, human papillomavirus.
Figure 3.
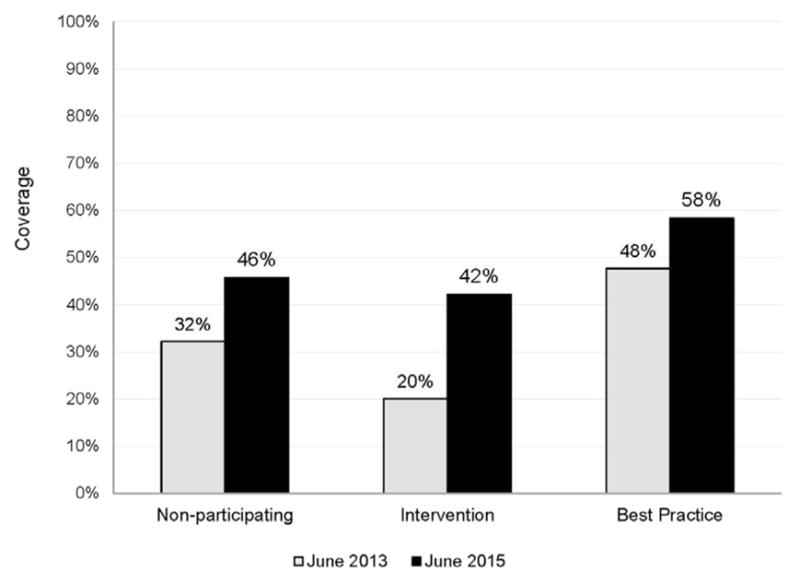
HPV vaccine series completion coverage at non-participating, intervention, and best practice facilities, June 2013 versus June 2015.
Note: “Non-participating facilities” refers to Indian Health Service, tribally-operated, and urban Indian healthcare facilities within the Great Plains, Portland, Nashville, Navajo, and Oklahoma Indian Health Service regions, that were neither considered intervention facilities nor best practice facilities. Facilities missing data from either June 2013 or June 2015 were omitted from the analysis. Mean 13–17-year-old male and female population size from 2013 and 2015 for: 68 non-participating facilities=25,259 persons; nine intervention facilities=5,943 persons; and nine best practice facilities=6,026 persons.
HPV, human papillomavirus.
DISCUSSION
The diversity among intervention facilities, both at baseline and with regard to the implemented interventions, does not allow for direct comparisons to establish the most effective interventions, and was not this study’s intention. Despite many differences, all intervention facilities shared one characteristic: They had lower HPV vaccine coverage compared with other I/T/U facilities in their region. This study recognizes the diversity among healthcare facilities and highlights the importance of multifaceted, tailored interventions to increase HPV vaccine coverage. As this study demonstrated, healthcare facilities can implement facility-specific strategies at minimal cost to increase adolescent HPV vaccine uptake.
The baseline assessment, which provided an opportunity to share coverage data with facilities, may have served as an intervention in and of itself. Coverage with the first and third dose of HPV vaccine increased at two intervention sites that only participated in the initial assessment (data not shown).
Provider reminders in EHR systems have been shown to increase both HPV initiation and series completion.17 During the baseline assessment, all facilities in the study were already employing electronic provider reminders. Also, most facilities were already using standing orders and reminder recall, both of which the Community Preventive Services Task Force recommends as evidenced-based strategies for increasing vaccine coverage. 18 These strategies may help explain the higher HPV vaccine coverage among patients seen at I/T/U facilities compared with the general U.S. population.
The baseline assessment, however, revealed variation in the consistency with which facilities implemented evidence-based strategies. Although the presence of standing orders between best practice and intervention facilities was similar at baseline, there were differences in implementation; respondents at some intervention facilities indicated that not all clinicians follow existing standing orders. Consistent implementation of standing orders can increase access to HPV vaccine within the clinic. Several intervention facilities chose to promote better adherence to existing standing orders as an intervention, and some used them to establish nurse-only walk-in immunizations.
Use of reminder recall similarly varied by facility. Intervention facilities reported implementing reminder recall sporadically, whereas best practice facilities had dedicated staff to carry out routine reminder recall. All intervention facilities implemented strategies to improve reminder recall efforts. At baseline, the use of patient phone call reminders differed, with more intervention facilities than best practice facilities using phone call reminders. Though calling patients when they are due for HPV vaccine should, in theory, help increase HPV vaccine coverage, phone call reminders may not be as effective as other strategies given inaccurate or frequently changing phone numbers, which may explain why best practice facilities were less likely to use this method.19 Some intervention facilities chose to explore reminder recall methods such as distributing postcards, letters, and reminder magnets or focusing phone call reminders to certain patients. As this study demonstrated, the mere presence of reminder recall strategies is not sufficient; the method and the consistency with which facilities implement reminder recall is critical.
The extent of community outreach healthcare facilities performed also differed between best practice and intervention facilities. In the baseline assessment, only 40% of intervention facilities reported educating about HPV vaccine outside the clinic compared with 89% of best practice facilities. Although neither clinic-based education nor community-wide education alone have been found to effectively increase vaccine coverage, using these strategies in conjunction with other strategies has been shown to increase coverage.14 Barriers intervention facilities reported, such as patient concerns about safety, parental concerns about the link between HPV vaccine and sexual activity, and lack of awareness about the recommendation for three doses, suggest more education and community outreach could be important to increase HPV vaccine coverage. After reviewing the assessment findings and learning about strategies best practice facilities employed (e.g., educating in non-clinical settings), some intervention facilities opted to provide HPV vaccine, information about HPV, or both outside the facility at health fairs or other community settings. Though providing HPV vaccine outside the clinic is not feasible for all facilities, more could be done to increase awareness in the community.
Finally, ensuring providers can access complete immunization data for their patients to support clinical decision making is important. Facilities that opted to improve data completeness using the state IIS saw an increase in HPV vaccine coverage, highlighting the need to continue supporting automated, bidirectional immunization data exchange between EHRs and state IISs.
This study highlights the success of targeted, multifaceted approaches to increase HPV vaccine coverage. As previous studies demonstrated, increasing vaccine coverage requires a combination of strategies tailored to fit the needs of individual facilities.14 All intervention facilities in the study implemented multiple strategies to address local issues affecting HPV vaccination, including making systems-level changes to the vaccine delivery process, increasing both provider and patient knowledge about HPV vaccine, and leveraging the state IIS. HPV vaccine series initiation and series completion coverage increased at intervention facilities once interventions were implemented (data not shown). Increases in coverage at intervention facilities surpassed those at non-participating facilities. Best practice facilities, which already had relatively high rates of HPV vaccine coverage, also saw smaller increases compared with intervention facilities, likely due to high baseline coverage levels.
Importantly, interventions did not require substantial monetary resources. For example, facilities took advantage of free reminder recall resources, such as magnets and postcards the vaccine manufacturer made available. Facilities accomplished all interventions using existing staff resources and by adjusting existing systems and work flow.
Limitations
This study includes multiple limitations. I/T/U facilities invited to participate were selected from just five IHS regions, and not all facilities invited to participate chose to do so. Although most I/T/U facilities use the same EHR provider reminders and serve AI/AN communities, differences in the facilities and patient populations may exist.
Second, researchers cannot infer a causal relationship between interventions and changes in HPV vaccine coverage. Temporal relationships cannot be distinguished because facilities implemented multiple interventions at different times. State health departments and other local organizations’ activities may have also contributed to the noted increases.
Third, researchers designated facilities as best practice or intervention facilities using vaccination data from one fiscal quarter. These data did not vary substantially from adjacent quarters, suggesting data accurately represent baseline coverage at each selected facility.
Fourth, owing to snowball sampling, qualitative data collected through interviews were subject to selection bias and may not fully represent actual barriers and facilitators to HPV vaccination.20 Data were also subject to interview bias because researchers knew beforehand whether respondents represented a best practice or intervention facility.21 To reduce interview bias, researchers compared notes after each interview and asked respondents to review the summary for accuracy.
Finally, certain themes emerged from the baseline interviews, such as more proactive nursing staff among best practice facilities, which the assessment tool did not systematically assess or quantify. These themes speak to the need for further exploration of how to encourage proactive approaches supporting vaccination efforts.
CONCLUSIONS
Numerous and complex factors affect vaccine delivery; however, this study found that a low-cost, targeted, multipronged approach to addressing vaccine delivery, provider and patient education, and data completeness may increase HPV vaccine coverage.
Efforts to increase vaccine coverage should address the different components of the vaccination process, from patient and provider knowledge to systems increasing access, delivery, and monitoring of vaccines. Overall, the adolescent patient population I/T/U healthcare facilities serve and the general adolescent U.S. population experience similar HPV vaccination barriers.11–13,22 Healthcare providers should consider local context and multiple strategies to increase vaccine coverage. They should also note that healthcare facilities facing similar challenges have already successfully implemented numerous evidenced-based, relatively low-cost interventions.
Supplementary Material
Acknowledgments
We would like to acknowledge the Indian Health Service Area Immunization Coordinators and all staff at both the intervention and best practice facilities for their steadfast support and assistance with this project.
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention or Indian Health Service.
JL Jacobs-Wingo headed the writing, helped facilities implement interventions, analyzed data, and contributed to the revisions. CC Jim helped facilities implement interventions, analyzed data, and contributed to the revisions. AV Groom conceptualized the design, helped facilities implement interventions, and contributed substantially to the revisions.
No financial disclosures were reported by the authors of this paper.
Footnotes
Supplemental materials associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.amepre.2017.01.024.
References
- 1. [Accessed May 4, 2016];IHS Year 2015 Profile. www.ihs.gov/newsroom/factsheets/
- 2.Groom AV, Santibanez TA, Bryan RT. Vaccination coverage among American Indian and Alaska native children, 2006–2010. Pediatrics. 2012;130(6):e1592–e1599. doi: 10.1542/peds.2012-1001. http://dx.doi.org/10.1542/peds.2012-1001. [DOI] [PubMed] [Google Scholar]
- 3.Bridges CB, Hurley LP, Williams WW, Ramakrishnan A, Dean AK, Groom AV. Meeting the challenges of immunizing adults. Am J Prev Med. 2015;49(6 Suppl 4):S455–S464. doi: 10.1016/j.amepre.2015.08.014. http://dx.doi.org/10.1016/j.amepre.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 4. [Accessed May 4, 2016];Vaccines for Children Program. www.cdc.gov/vaccines/programs/vfc/index.html.
- 5.Petrosky E, Bocchini, Hariri S, et al. Use of 9-valent human papillomavirus (HPV) vaccine: updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2015;64(11):300–304. [PMC free article] [PubMed] [Google Scholar]
- 6.Elam-Evans LD, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years–United States, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(29):625–633. [PMC free article] [PubMed] [Google Scholar]
- 7.Schmidt-Grimminger DC, Bell MC, Muller CJ, Maher DM, Chauhan SC, Buchwald DS. HPV infection among rural American Indian women and urban white women in South Dakota: an HPV prevalence study. BMC Infect Dis. 2011;11:252. doi: 10.1186/1471-2334-11-252. http://dx.doi.org/10.1186/1471-2334-11-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell MC, Schmidt-Grimminger D, Patrick S, Ryschon T, Linz L, Chauhan SC. There is a high prevalence of human papillomavirus infection in American Indian women of the Northern Plains. Gynecol Oncol. 2007;107(2):236–241. doi: 10.1016/j.ygyno.2007.06.007. http://dx.doi.org/10.1016/j.ygyno.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson M, Benard V, Thomas C, Brayboy A, Paisano R, Becker T. Cervical cancer incidence and mortality among American Indian and Alaska Native women, 1999–2009. Am J Public Health. 2014;104(Suppl 3):S415–S422. doi: 10.2105/AJPH.2013.301681. http://dx.doi.org/10.2105/AJPH.2013.301681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. [Accessed May 5, 2016];National Immunization Reporting System Reports. 2015 www.ihs.gov/NonMedicalPrograms/ihpes/immunizations/index.cfm?module=immunizations&option=reports.
- 11.Schmidt-Grimminger D, Frerichs L, Black Bird AE, Workman K, Dobberpuhl M, Watanabe-Galloway S. HPV knowledge, attitudes, and beliefs among Northern Plains American Indian adolescents, parents, young adults, and health professionals. J Cancer Educ. 2013;28(2):357–366. doi: 10.1007/s13187-013-0468-y. http://dx.doi.org/10.1007/s13187-013-0468-y. [DOI] [PubMed] [Google Scholar]
- 12.Jim CC, Lee JW, Groom AV, et al. Human papillomavirus vaccination practices among providers in Indian Health Service, tribal and urban Indian healthcare facilities. J Womens Health (Larchmt) 2012;21(4):372–378. doi: 10.1089/jwh.2011.3417. http://dx.doi.org/10.1089/jwh.2011.3417. [DOI] [PubMed] [Google Scholar]
- 13.Daley MF, Crane LA, Markowitz LE, et al. Human papillomavirus vaccination practices: a survey of U.S. physicians 18 months after licensure. Pediatrics. 2010;126(3):425–433. doi: 10.1542/peds.2009-3500. http://dx.doi.org/10.1542/peds.2009-3500. [DOI] [PubMed] [Google Scholar]
- 14.Guide to Community Preventive Services. [Accessed April 16, 2015];Increasing appropriate vaccination: health care system-based interventions implemented in combination. www.thecommunityguide.org/findings/vaccinationprograms-health-care-system-based-interventions-implemented-combination.
- 15.Rosenthal SL, Weiss TW, Zimet GD, Ma L, Good MB, Vichnin MD. Predictors of HPV vaccine uptake among women aged 19–26: importance of a physician’s recommendation. Vaccine. 2011;29(5):890–895. doi: 10.1016/j.vaccine.2009.12.063. http://dx.doi.org/10.1016/j.vaccine.2009.12.063. [DOI] [PubMed] [Google Scholar]
- 16.Dunne EF, Markowitz LE, Saraiya M, et al. CDC grand rounds: reducing the burden of HPV-associated cancer and disease. MMWR Morb Mortal Wkly Rep. 2014;63(4):69–72. [PMC free article] [PubMed] [Google Scholar]
- 17.Ruffin MT, Plegue MA, Rockwell PG, Young AP, Patel DA, Yeazel MW. Impact of an electronic health record (EHR) reminder on human papillomavirus (HPV) vaccine initiation and timely completion. J Am Board Fam Med. 2015;28(3):324–333. doi: 10.3122/jabfm.2015.03.140082. http://dx.doi.org/10.3122/jabfm.2015.03.140082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Briss PA, Rodewald LE, Hinman AR, et al. Reviews of evidence regarding interventions to improve vaccination coverage in children, adolescents, and adults. The Task Force on Community Preventive Services. Am J Prev Med. 2000;18(1 Suppl):97–140. doi: 10.1016/s0749-3797(99)00118-x. http://dx.doi.org/10.1016/S0749-3797(99)00118-X. [DOI] [PubMed] [Google Scholar]
- 19.Szilagyi PG, Schaffer S, Barth R, et al. Effect of telephone reminder/recall on adolescent immunization and preventive visits: results from a randomized clinical trial. Arch Pediatr Adolesc Med. 2006;160(2):157–163. doi: 10.1001/archpedi.160.2.157. http://dx.doi.org/10.1001/archpedi.160.2.157. [DOI] [PubMed] [Google Scholar]
- 20.Last JM. A Dictionary of Epidemiology. 4. New York: Oxford University Press; 2001. International Epidemiological Association. [Google Scholar]
- 21.Aschengrau A, Seage GR. Essentials of Epidemiology in Public Health. 2. Sudbury, MA: Jones and Bartlett Publishers; 2008. [Google Scholar]
- 22.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to human papillomavirus vaccination among U.S. adolescents: a systematic review of the literature. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752. http://dx.doi.org/10.1001/jamapediatrics.2013.2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


