Abstract
Background and Aims
The biological environment varies across the colorectum and may therefore differently affect neoplastic growth in the proximal and distal colon. The aim of the study was to evaluate the risk for recurrent adenomas and their anatomic location based on adenoma location at baseline colonoscopy.
Methods
Data were extracted from three adenoma prevention trials (n= 2430). Participants had at least one adenoma at baseline colonoscopy and underwent subsequent surveillance colonoscopy, at which time metachronous adenomas could be detected. We calculated the risk ratio (RR) and the 95% confidence interval (CI) for metachronous adenomas by location of the baseline lesion and considered the impact of advanced neoplasia and multiplicity.
Results
At baseline 522 subjects (21.5%) had adenomas only in the proximal colon, 1266 subjects (52.1%) had adenomas only in the distal colorectum and 642 (26.4%) had adenomas in both regions. Overall 877 subjects (36.5%) had metachronous adenomas during the follow-up period. Those with only proximal adenomas at baseline had a higher risk of metachronous adenomas compared to subjects with only distal adenomas (RR 1.17, 95% CI 1.01–1.35). A greater proximal risk was found after restricting the analysis to subjects with multiple proximal adenomas versus multiple distal adenomas (RR 1.35, 95% CI 1.10–1.67). The risk of recurrent adenomas on the same side was 48% higher for subjects with only proximal adenomas at baseline compared to those with only distal adenomas at baseline (RR 1.48, 95% CI 1.22–1.80).
Conclusions
Patients with proximal adenomas only have a modestly greater risk of adenoma recurrence than patients with adenomas limited to the distal colon, as well as a greater likelihood of adenoma recurrence on the same side compared to subjects with distal adenomas. This observation suggests that biological factors may differentially affect neoplasia growth across the colon.
Keywords: Colonoscopy, polyps, recurrence
Introduction
It is well recognized that colorectal cancers (CRC) differ in their epidemiological and molecular characteristics in their location throughout the colorectum,1 suggesting different pathways to cancer2. For instance, the frequency of cancers with CIMP (CpG island methylator) phenotype or those with a BRAF mutation gradually increase from the distal to the proximal colorectum3. A CIMP phenotype is often associated with mismatch repair gene hypermethylation, which results in microsatellite instability. The proportion of cancers with microsatellite instability is correspondingly greater in the proximal colon4–6. A recent study suggests that proximal and distal adenomas express distinct DNA methylation patterns7 suggesting that carcinogenesis may differ by location in the colon.
The proximal colon and the distal colon differ in their embryologic development and various luminal and mucosal characteristics. These include morphological qualities (e.g. mucosal capillary network, crypt length), biochemical features (e.g. fatty acid production), physiological processes (e.g. microbiome variation, secondary bile acid metabolism, bacterial mutagenic metabolites, apoptotic index), and differences in stool consistency8–11. These characteristics reflect a different biologic environment along the large intestine and a varying milieu at the mucosal-luminal interface. Because the milieu may foster or impede carcinogenesis12, it is possible that adenomas in the proximal and distal colon may vary in their risk of recurrence.
In this study, we aimed to determine whether risk of metachronous adenomas and their anatomic location vary by the proximal versus distal location of baseline lesions. We analyzed the association between the baseline location of adenomas and subsequent risk of metachronous adenomas, the effect of multiplicity on this association, and whether the side of adenoma location is associated with the location of recurrent adenomas.
Methods
Participants
Data on participants from three multicenter randomized chemoprevention trials were used for the current analysis. Details of the study designs and principal findings have been reported previously13–15. The Anti-Oxidant Polyp Prevention Study14 examined the effect of beta-carotene, vitamins C, and E on adenoma recurrence. The trial randomized 864 subjects into four treatment arms in a factorial design from 1985 to 1988. The Calcium Polyp Prevention Study was a similar trial of the effect of Calcium supplementation15. It randomized 930 subjects into two treatment arms from 1988 to 1992. The Aspirin-Folate Polyp Prevention Study examined two different doses of Aspirin (81 mg and 325 mg) and Folate (1 mg). This factorial trial randomized 1121 subjects into three Aspirin treatment arms and 1021 subjects into two Folate treatment arms from 1994 to 1998.
The trials enrolled adults between the ages of 21 and 80 years, who had at least one adenomatous polyp detected within three months prior to enrollment into the first two trials13, 14 and up to sixteen months prior to enrollment into the Aspirin/Folate trial15. Information regarding the size, location and histology of these polyps was obtained from endoscopy and pathology reports. All participants were considered clear of adenomas on the basis of a complete colonoscopy within 3 months prior to study entry. Subjects with a history of colorectal cancer or Familial Adenomatous Polyposis, or with a detected cancer at the entry examination were excluded. An additional clearing colonoscopy was performed after one year in the first two trials.
We defined the study entry colonoscopy and the one-year clearing examination together as the “baseline colonoscopy” in the first two trials13, 14 and the entry colonoscopy as the baseline colonoscopy in the third trial15. Follow-up colonoscopy examinations in the first two trials were performed after four years, and in the third trial after three years following the baseline clearing exams. All polyps identified during follow up were removed. Information about the size and location of each lesion was abstracted from the endoscopy and pathology reports at the time of each trial. In addition, during each trial, all removed tissue underwent central review by a single study pathologist. Polyps were judged as adenomatous or non-adenomatous (e.g. hyperplastic). At the time of the three polyp prevention trials, non-dysplastic serrated polyps were typically considered to be hyperplastic. Therefore, all serrated polyps at baseline or follow-up examination were considered non-neoplastic and not included in the analysis.
Analysis
Our primary objective was to determine the risk of metachronous adenomas based on the location of adenomas at baseline colonoscopy. Proximal adenomas were defined as adenomas occurring proximal to the splenic flexure, and distal adenomas were defined as those at or distal to the splenic flexure. We defined metachronous adenomas as all adenomas that were detected during follow-up – up to and including the four-year colonoscopy in the first two trials and the three-year colonoscopy in the third trial. Advanced adenomas were defined conventionally as adenomas ≥10mm or with advanced histological characteristics (tubulovillous or villous adenomas, high grade dysplasia) or cancer.
The occurrence of metachronous adenomas was assessed both as absolute and relative risks. Adjusted risk ratios and 95% confidence intervals (95% CI) were obtained from generalized linear models with age, sex, study, clinical center, treatment assignment, length of follow-up, and number of baseline adenomas as covariates. These models used a natural-logarithm link function with Poisson-distributed errors, and were adjusted for over/under-dispersion.
We first assessed the risk of any metachronous adenomas for subjects with only proximal adenomas and for subjects with only distal adenomas at baseline. We repeated the analysis to determine the association between adenoma location at baseline and the risk for any metachronous advanced adenomas.
Recognizing that the presence of multiple adenomas is a known risk factor for recurrence16, we then restricted our analysis to patients with at two or more adenomas. We calculated the risk ratios for metachronous adenomas in patients with multiple proximal adenomas at baseline and those with bilateral adenomas at baseline using subjects with multiple adenomas located only distally as the reference. This analysis was repeated for metachronous advanced adenomas in patients with multiple adenomas at baseline.
Next, we examined whether the site of the baseline lesion was associated with the site of metachronous adenomas. For this analysis we included all subjects with metachronous adenomas. We first obtained the absolute risks of metachronous adenomas on the same side (ipsilateral), on the other side (contralateral) or on both sides (bilateral) by adenoma location at baseline. Absolute risks were compared using chi-squared test contingency table analyses.
Finally, we then computed adjusted risk ratios for patients with only proximal adenomas at baseline to have ipsilateral metachronous adenomas in comparison to patients with only distal adenomas at baseline to have ipsilateral adenomas, and similarly for metachronous contralateral adenomas. The analysis was repeated using metachronous advanced adenomas as the outcome.
Results
Of 2915 participants in the three polyp prevention trials, 2430 (83.4%) were included in this analysis. Excluded were subjects without a follow-up colonoscopy (n=248), missing information on adenoma location at baseline (n=104), at follow-up (n=110) or at both examinations (n=12) or subjects who only had sessile serrated adenomas at baseline by current diagnostic criteria (n=11). Characteristics of study participants differed moderately between the trials (Table 1). The mean age of participants was 59.5 years, 70.3% were men, and 85.6% were white. At baseline 21.5% of subjects had adenomas only in the proximal colon, and 52.1% only in the distal colon.
Table 1.
Characteristics of Participant from the 3 Clinical Trials.
| Polyp Prevention Study | ||||
|---|---|---|---|---|
| Antioxidants N (%) |
Calcium N (%) |
Aspirin/Folate N (%) |
Total N (%) |
|
| Entry characteristics | ||||
| N | 724 | 780 | 926 | 2430 |
| Age, mean years (SD) | 61.0 (8.3) | 60.6 (9.0) | 57.3 (9.7) | 59.5 (9.2) |
| Sex (male) | 574 (79.3) | 555 (71.2) | 580 (62.6) | 1709 (70.3) |
| Race (non-white) | 105 (14.6) | 118 (15.1) | 127 (13.7) | 350 (14.4) |
| Location of baseline adenomas | ||||
| Distal only | 383 (52.9) | 392 (50.3) | 491 (53.0) | 1266 (52.1) |
| Proximal only | 108 (14.9) | 141 (18.1) | 273 (29.5) | 522 (21.5) |
| Both sides | 233 (32.2) | 247 (31.7) | 162 (17.5) | 642 (26.4) |
| Subjects with ≥2 adenomas, distal only | 128 (17.7) | 128 (16.4) | 97 (10.5) | 353 (14.5) |
| Subjects with ≥2 adenomas, proximal only | 44 (6.1) | 61 (7.8) | 70 (7.6) | 175 (7.2) |
| Location of baseline advanced adenomas | ||||
| Distal only | 306 (42.3) | 220 (28.2) | 193 (20.8) | 719 (29.6) |
| Proximal only | 56 (7.7) | 44 (5.6) | 55 (5.9) | 155 (6.4) |
| Both sides | 45 (6.2) | 34 (4.4) | 15 (1.6) | 94 (3.9) |
| Follow-up events | ||||
| Mean follow-up, mean months (SD) | 36.6 (2.7) | 36.7 (3.5) | 37.6 (4.1) | 37.0 (3.6) |
| Subjects with at least one adenoma | 268 (37.0) | 264 (33.8) | 355 (38.3) | 877 (36.5) |
| Distal only | 105 (14.5) | 77 (9.9) | 140 (15.1) | 322 (13.3) |
| Proximal only | 110 (15.2) | 131 (16.8) | 151 (16.3) | 392 (16.1) |
| Both sides | 53 (7.3) | 56 (7.2) | 64 (6.9) | 173 (7.1) |
| Subjects with advanced adenomas (%) | 64 (8.8) | 64 (8.2) | 82 (8.5) | 210 (8.5) |
| Distal only | 30 (4.1) | 20 (2.6) | 34 (3.7) | 84 (3.5) |
| Proximal only | 30 (4.1) | 41 (5.3) | 45 (4.9) | 116 (4.8) |
| Both sides | 4 (0.6) | 3 (0.4) | 3 (0.3) | 10 (0.4) |
After a mean follow-up of 3.1 years, metachronous adenomas were found in 36.5% of subjects; 16.1% with adenomas only in the proximal colon and 13.3% only in the distal colon and 7.1% with adenomas in both regions. Metachronous advanced adenomas were found in 8.5% of subjects; in 4.8% they were located only in the proximal colon, in 3.5% only in the distal colon and in 0.4% in both regions.
Adenoma location and risk of metachronous adenomas
Among subjects with only proximal adenomas at baseline, 37.7% had recurrent adenomas compared to 28.9% for those with only distal adenomas (RR 1.17, 95% CI 1.01–1.35) (Figure 1). The results were similar when the analysis was restricted to subjects with multiple adenomas at baseline. 50.3% of subjects with multiple and only proximal adenomas had metachronous adenomas, compared to 34.0% with multiple and only distal adenomas (RR 1.35, 95% CI 1.10–1.67). The risk for metachronous adenomas was also increased for subjects with adenomas on both sides, as compared to subjects with multiple adenomas only in the distal colon (RR 1.28, 95% CI 1.08–1.51).
Figure 1.
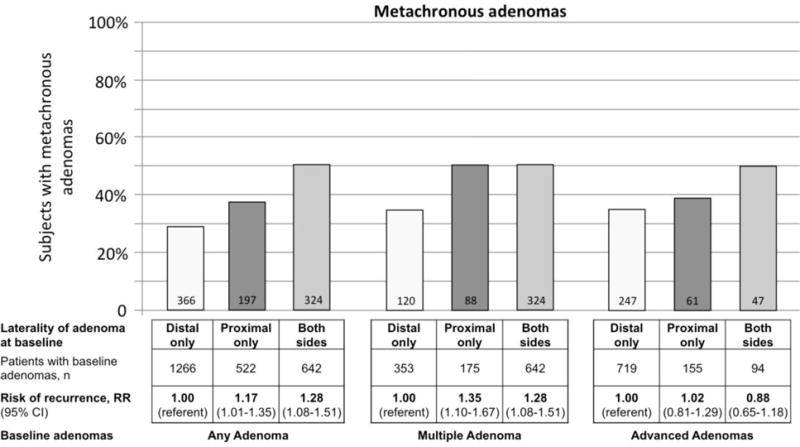
Metachronous adenoma risk in relation to adenoma location at baseline colonoscopy.
The risk of metachronous adenomas was not greater for patients with proximal location of advanced adenomas compared to those with distal advanced adenomas at baseline (RR 1.02, 95% CI 0.81–1.29) (Figure 1). Similarly, the risk of metachronous advanced adenomas was not associated with proximal location of adenomas (RR 1.12, 95% CI 0.78–1.59) or advanced adenomas (RR 0.83, 95% CI 0.50–1.39) at baseline (Figure 2).
Figure 2.
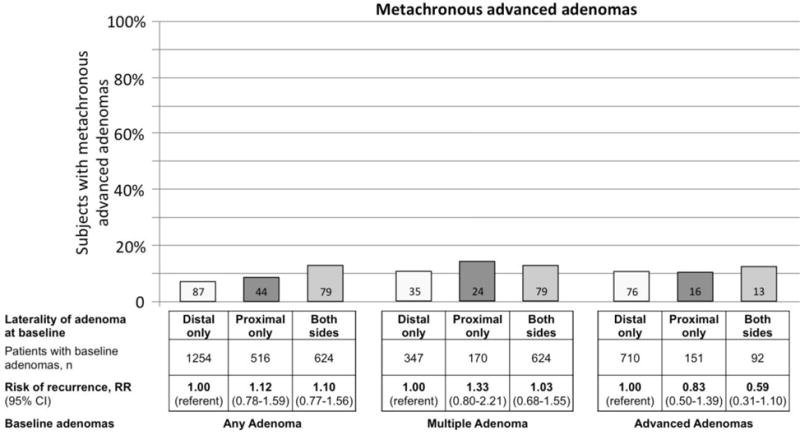
Metachronous advanced adenomas in relation to adenoma location at baseline colonoscopy.
Adenoma location and side of metachronous adenomas
Subjects with adenomas only in the proximal colon at baseline more often had metachronous adenomas in the proximal colon than in the distal colon (28.4% vs. 15.9%) (Figure 3). In contrast, subjects with adenomas only in the distal colon had similar risks of metachronous adenomas in the distal or the proximal colon (16.7% vs. 15.9% respectively). Consequently, subjects with only proximal adenomas at baseline had a greater risk of same side (i.e. ipsilateral) recurrence than subjects with only distal adenomas at baseline (RR 1.48, 95% CI 1.22–1.80). The risk ratio remained similar when restricting the analysis to patients with only ipsilateral recurrence (RR 1.49, 95% CI 1.19–1.88).
Figure 3.
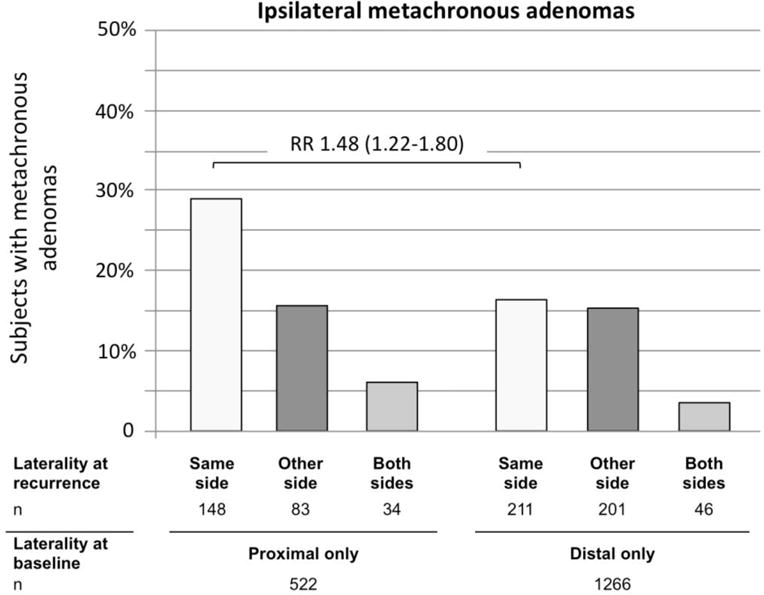
Side of metachronous adenomas by adenoma location at baseline.
The risk ratio expresses the risk of ipsilateral (same side) recurrence for subjects with only proximal adenomas at baseline to have any proximal metachronous adenoma in comparison to subjects with only distal adenomas at baseline to have any distal metachronous adenomas.
When examining location and risk of metachronous advanced adenomas, we found a similar association. Metachronous advanced adenomas were more often detected on the same side as the baseline lesion. Among subjects with baseline adenomas only in the proximal colon, 7.1% had an advanced metachronous lesion in the proximal colon, and 1.9% in the distal colon (Figure 4). Conversely, among subject with baseline adenomas only in the distal colon, 4.1% had metachronous advanced adenomas in the distal colon compared to 2.8% in the proximal colon. The risk for metachronous advanced adenomas to occur on the same side (i.e. ipsilateral) was non-significantly greater for subjects with proximal adenomas only at baseline as compared to those with only distal adenomas at baseline (RR 1.45, 95% CI 0.96–2.21). The risk ratio was similar if only ipsilateral advanced adenomas were considered (RR 1.39, 95% CI 0.90–2.15).
Figure 4.
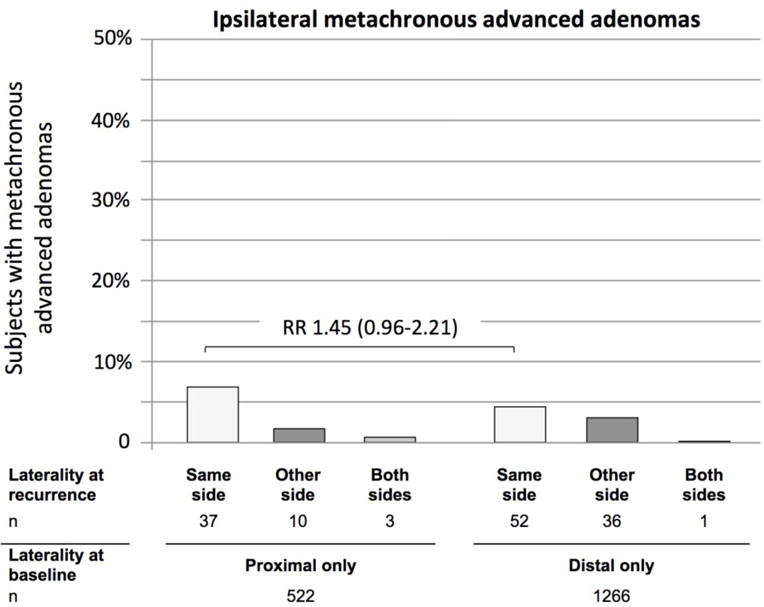
Side of metachronous advanced adenomas by adenoma location at baseline.
The risk ratio expresses the risk of ipsilateral (same side) recurrence for subjects with only proximal adenomas at baseline to have any proximal metachronous advanced adenoma in comparison to subjects with only distal sided adenomas at baseline to have any distal metachronous advanced adenomas.
Discussion
Using data from three polyp prevention trials we examined patterns of adenoma recurrence based on adenoma location at baseline. The study has three main findings. First, subjects with only proximal adenomas have a greater risk of metachronous adenomas compared to subjects with only distal adenomas. Second, the risk appeared to be greater if subjects had multiple adenomas in the proximal colon. Third, subjects with proximal adenomas were more likely to have metachronous adenomas on the same side (i.e. in the proximal colon) than subjects with distal adenomas (i.e. in the distal colon). These results support the idea that large bowel carcinogenesis differs by anatomic location and suggest that proximal adenomas portend a higher recurrence risk than distal adenomas.
Studies have repeatedly shown that adenomas detected at a surveillance colonoscopy tend to be more proximal than on the baseline exam17,18–20,21. Few studies assessed factors associated with adenoma recurrence dependent on the location of the baseline lesion. Martinez et al. analyzed 9,167 subjects from eight polyp prevention trials, including the three trials used in the current analysis. The authors found a moderately increased risk of future non-advanced adenomas (RR 1.29; 95% CI 1.16–1.44) or advanced adenomas (RR 1.68; 95% CI 1.43–1.98) in subjects with any proximal adenoma at baseline compared to subjects with adenomas only in the distal colon20. In the study by Laiyemo et al. – one of the eight polyp prevention trials included in the Martinez study – patients with only proximal adenomas were more likely to have any adenoma recurrence as compared to those with only distal adenomas (RR 1.14; 95% CI 1.00–1.31). Our study found a similarly small increase in the risk of future non-advanced adenomas for patients with proximal adenomas at baseline (RR 1.17, 95% CI 1.01–1.35). This effect was more pronounced if multiple proximal adenomas were found (relative to those with distal sided adenomas) at the baseline examination (RR 1.35, 95% CI 1.10–1.67). We did not find that proximal location was significantly associated with future advanced adenomas. This may be due to lack of power given the small number of advanced adenomas in our study. Alternatively, one might consider that initiation of adenoma growth may be associated with location, but adenoma growth is not.
In contrast to prior studies, we examined the strength of same side recurrence. We found that patients with only proximal adenomas have a greater risk of ipsilateral recurrence than patients with only distal adenomas at baseline. This was true for any adenoma recurrence (RR 1.56) and for advanced adenoma recurrence (RR=1.66). Prior observations support the assertion that characteristics of colorectal neoplasia differ by anatomic location. For instance, the serrated pathway of carcinogenesis is associated with proximal colorectal cancer. Proximal cancers more often express microsatellite instability, the CIMP phenotype, or BRAF mutations3–5 than those that are located more distally. However, our analysis excluded sessile serrated adenomas/polyps. The obtained results therefore suggest that risk for adenomatous polyps may also vary by location in the colon. This idea is supported by a recent study that found distinct patterns of DNA hypermethylation, independent of CIMP status, in proximal and distal adenomas7.
Differences in the microenvironment at the luminal-mucosal interface along the large intestine may contribute to varying neoplasia recurrence risk in different subsites of the colon. For example, from the proximal to the distal colon crypts increase in length and the capillary network becomes multilayered, the apoptotic index decreases, and mucin becomes more acidic9. Physiologically, the microbiome varies, the metabolism of secondary bile acid changes, the fermentation of short chain fatty acids decreases, and mutagenic metabolites vary8, 9. If we consider neoplasia development a complex process of interaction between host factors, the innate immune system, and environmental factors, changes in the microenvironment at the mucosal-luminal interface likely affect neoplasia risk12. Identifying factors that are responsible for these regional variations in adenoma risk should be the subject of future studies.
An alternative explanation for the observed relative risk is a higher rate of missed adenomas in the proximal colon. Adenomas that were missed and not removed at index exam may add to adenomas found at a later colonoscopy. This may result in a higher proximal adenoma burden at follow-up examination. However, studies have variably reported higher miss rates both in the proximal colon and the distal colorectum21–23. Finally, incomplete polyp resection may also contribute to the observed risk difference. However, incomplete resection appears to be similar throughout the large bowel24.
Several limitations of our study need to be considered. First, not all risk factors for adenoma recurrence were considered, including smoking, obesity, and diabetes. We were unable to assess SSA/P at index colonoscopy, and it is now suspected that the presence of SSA/P might be associated with risk of adenoma recurrence25. Second, data were collected before the introduction of colonoscopy quality standards. Results therefore need to be confirmed using current standards and technology26. Third, it is conceivable that a biological change over time may also account for the observed changes in adenoma recurrence by location. However, it seems unlikely that neither advances in technology nor changes in tumor biology over time would have affected adenoma detection during a fairly short 3 to 4 year follow-up period within each trial.
The categorization of the colon into proximal and distal colorectum is based on embryonic origin and anatomic landmarks. Changes at the mucosal-luminal interface (morphological, biochemical, physiological) occur gradually along the large intestine9. Recent studies show that molecular characteristics of CRCs (e.g. CIMP, BRAF, MSI) also change more gradually from proximal to distal5, 28. The categorization into distal and proximal colon is therefore a simplification, and future studies should consider examining outcome measures by subsites of the large intestine.
In summary, proximal adenoma location was associated with a small increased risk of adenoma recurrence, and of adenoma recurrence on the same side as compared to distal adenoma location. The results may suggest different biology and disease process early in the adenoma-carcinoma sequence depending on anatomic location in the colon, which through a varying microenvironment at the luminal-mucosal interface may exert a different neoplasia risk. Future work more directly evaluating the contribution of specific molecular factors to adenoma recurrence by location within the colon is warranted.
Acknowledgments
The findings, statements, and views expressed are those of the authors and do not necessarily represent those of the Commission, the Department of Veterans Affairs or the United States Government.
The study was funded through NCI grants related to the polyp prevention trials (CA037287, CA023108, CA046927, CA059005).
References
- 1.Lindblom A. Different mechanisms in the tumorigenesis of proximal and distal colon cancers. Curr Opin Oncol. 2001;13:63–9. doi: 10.1097/00001622-200101000-00013. [DOI] [PubMed] [Google Scholar]
- 2.Ahnen DJ. The American College of Gastroenterology Emily Couric Lecture–the adenoma-carcinoma sequence revisited: has the era of genetic tailoring finally arrived? Am J Gastroenterol. 2011;106:190–8. doi: 10.1038/ajg.2010.423. [DOI] [PubMed] [Google Scholar]
- 3.Yamauchi M, Lochhead P, Morikawa T, et al. Colorectal cancer: a tale of two sides or a continuum? Gut. 2012;61:794–7. doi: 10.1136/gutjnl-2012-302014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Markowitz SD, Bertagnolli MM. Molecular origins of cancer: Molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60. doi: 10.1056/NEJMra0804588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamauchi M, Morikawa T, Kuchiba A, et al. Assessment of colorectal cancer molecular features along bowel subsites challenges the conception of distinct dichotomy of proximal versus distal colorectum. Gut. 2012;61:847–54. doi: 10.1136/gutjnl-2011-300865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boland CR, Goel A. Microsatellite instability in colorectal cancer. Gastroenterology. 2010;138:2073–2087 e3. doi: 10.1053/j.gastro.2009.12.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koestler DC, Li J, Baron JA, et al. Distinct patterns of DNA methylation in conventional adenomas involving the right and left colon. Mod Pathol. 2014;27:145–55. doi: 10.1038/modpathol.2013.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentley DW, Nichols RL, Condon RE, et al. The microflora of the human ileum and intrabdominal colon: results of direct needle aspiration at surgery and evaluation of the technique. J Lab Clin Med. 1972;79:421–9. [PubMed] [Google Scholar]
- 9.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101:403–8. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 10.McBain AJ, Macfarlane GT. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol. 1998;47:407–16. doi: 10.1099/00222615-47-5-407. [DOI] [PubMed] [Google Scholar]
- 11.Liu LU, Holt PR, Krivosheyev V, et al. Human right and left colon differ in epithelial cell apoptosis and in expression of Bak, a pro-apoptotic Bcl-2 homologue. Gut. 1999;45:45–50. doi: 10.1136/gut.45.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ogino S, Galon J, Fuchs CS, et al. Cancer immunology–analysis of host and tumor factors for personalized medicine. Nat Rev Clin Oncol. 2011;8:711–9. doi: 10.1038/nrclinonc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenberg ER, Baron JA, Tosteson TD, et al. A clinical trial of antioxidant vitamins to prevent colorectal adenoma. Polyp Prevention Study Group. N Engl J Med. 1994;331:141–7. doi: 10.1056/NEJM199407213310301. [DOI] [PubMed] [Google Scholar]
- 14.Baron JA, Beach M, Mandel JS, et al. Calcium supplements for the prevention of colorectal adenomas. Calcium Polyp Prevention Study Group. N Engl J Med. 1999;340:101–7. doi: 10.1056/NEJM199901143400204. [DOI] [PubMed] [Google Scholar]
- 15.Baron JA, Cole BF, Sandler RS, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 16.de Jonge V, Sint Nicolaas J, van Leerdam ME, et al. Systematic literature review and pooled analyses of risk factors for finding adenomas at surveillance colonoscopy. Endoscopy. 2011:560–72. doi: 10.1055/s-0030-1256306. [DOI] [PubMed] [Google Scholar]
- 17.Martinez ME, Sampliner R, Marshall JR, et al. Adenoma characteristics as risk factors for recurrence of advanced adenomas. Gastroenterology. 2001;120:1077–83. doi: 10.1053/gast.2001.23247. [DOI] [PubMed] [Google Scholar]
- 18.Bonithon-Kopp C, Piard F, Fenger C, et al. Colorectal adenoma characteristics as predictors of recurrence. Dis Colon Rectum. 2004;47:323–33. doi: 10.1007/s10350-003-0054-1. [DOI] [PubMed] [Google Scholar]
- 19.Laiyemo AO, Murphy G, Albert PS, et al. Postpolypectomy colonoscopy surveillance guidelines: predictive accuracy for advanced adenoma at 4 years. Ann Intern Med. 2008;148:419–26. doi: 10.7326/0003-4819-148-6-200803180-00004. [DOI] [PubMed] [Google Scholar]
- 20.Martinez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009;136:832–41. doi: 10.1053/j.gastro.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laiyemo AO, Doubeni C, Sanderson AK, 2nd, et al. Likelihood of missed and recurrent adenomas in the proximal versus the distal colon. Gastrointest Endosc. 2011;74:253–61. doi: 10.1016/j.gie.2011.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Rijn JC, Reitsma JB, Stoker J, et al. Polyp miss rate determined by tandem colonoscopy: a systematic review. Am J Gastroenterol. 2006;101:343–350. doi: 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- 23.Heresbach D, Barrioz T, Lapalus MG, et al. Miss rate for colorectal neoplastic polyps: a prospective multicenter study of back-to-back video colonoscopies. Endoscopy. 2008;40:284–90. doi: 10.1055/s-2007-995618. [DOI] [PubMed] [Google Scholar]
- 24.Pohl H, Srivastava A, Bensen SP, et al. Incomplete polyp resection during colonoscopy-results of the complete adenoma resection (CARE) study. Gastroenterology. 2013;144:74–80 e1. doi: 10.1053/j.gastro.2012.09.043. [DOI] [PubMed] [Google Scholar]
- 25.Glazer E, Golla V, Forman R, et al. Serrated adenoma is a risk factor for subsequent adenomatous polyps. Dig Dis Sci. 2008;53:2204–7. doi: 10.1007/s10620-007-0143-4. [DOI] [PubMed] [Google Scholar]
- 26.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the U.S. Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. doi: 10.1111/j.1572-0241.2002.05812.x. [DOI] [PubMed] [Google Scholar]
- 27.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994. Cancer. 1999;85:1670–6. [PubMed] [Google Scholar]
- 28.Bae JM, Kim JH, Cho NY, et al. Prognostic implication of the CpG island methylator phenotype in colorectal cancers depends on tumour location. Br J Cancer. 2013;109:1004–12. doi: 10.1038/bjc.2013.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for Colonoscopy Surveillance After Screening and Polypectomy: A Consensus Update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–57. doi: 10.1053/j.gastro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Sherer EA, Ambedkar S, Perng S, et al. A predictive model of longitudinal, patient-specific colonoscopy results. Comput Methods Programs Biomed. 2013;112:563–79. doi: 10.1016/j.cmpb.2013.07.007. [DOI] [PubMed] [Google Scholar]


