Abstract
Aims:
This study is aimed at determining the prognostic factors influencing successful pregnancy following intrauterine insemination (IUI).
Settings and design:
Retrospective analysis.
Materials and Methods:
A total of 2123 cycles undergone by 871 couples during the period of 5 years (2011–2015) were retrospectively studied.
Statistical Analysis Used:
Each of the factors was compared with pregnancy outcome (PO) using statistical analysis with a confidence interval of 95% in SPSS software version 19. Chi-square test and logistic regression analysis method were used to determine the significance of each factor with the PO.
Results:
Among the various factors included in our study population, male habits (P = 0.004), male occupational environment (P = 0.025), male age (P = 0.002), and female age (P = 0.001) were found to significantly influence the PO following IUI.
Conclusion:
Our results indicate that avoiding smoking and alcohol consuming prior and during the IUI treatment along with working in low-heat-generating environment might lead to better success following the treatment.
KEYWORDS: IUI, pregnancy outcome, prognostic factors
INTRODUCTION
Despite various modern treatment options which show higher success rates, it would be economical to consider the least expensive treatment for subfertile couples before undergoing expensive and invasive techniques like in vitro fertilization (IVF) and intracytoplasmic sperm injection.[1,2] Intrauterine insemination (IUI) is a simple noninvasive technique used as a first-line treatment for many subfertile couples. Subfertile couples with indications like male factor subfertility, unexplained subfertility, endometriosis related complications, and others are widely treated with IUI to enhance the probability of conception using either the partner’s sperm or donor sperm.[3,4] In case of unexplained infertility, IUI is usually accompanied with controlled ovarian hyperstimulation using clomiphene citrate/human chorionic gondatotropi (HCG)/human menopausal gonadotropin (HMG), to increase the probability of fertilization.[5]
It is difficult to decide on either the seminal or the female parameters having a higher conception potential due to the heterogeneity of the patient sample population.[6] Previous researches have analyzed the relationship among environmental, socioeconomic and lifestyle factors, and pregnancy outcome (PO) following treatment in subfertile couples with unexplained subfertility.[7,8] Many of these studies are based on retrospective data analysis, which either failed to include the PO details or have focused on research in an IVF setup and not in IUI.[8,9,10,11]
This study focuses on identifying significant clinical parameters of the patients influencing positive outcome after IUI. We considered both female and male parameters like female age, type of infertility, hormonal levels, number of IUI cycles undergone, male seminal parameters, occupational conditions, social habits, and others. Knowledge of the relationship between these factors and IUI outcome will help clinicians counsel the patients, customize the treatment for individuals and predict the outcome based on these factors.
MATERIALS AND METHODS
In this study, a total of 2123 cycles of IUI performed with a standard IUI technique using the partner’s spermatozoa were analyzed. The cycles were carried out between January 2011 and December 2015 in private fertility clinic. The study was approved by the Institutional Human Ethics Committee.
At the time of first examination and treatment, the couples had a minimum of 5 months of subfertility and had undergone a basic subfertility evaluation consisting of the basal hormone level evaluation in female partner like follicle-stimulating hormone (FSH), luteinizing hormone (LH), thyroid stimulating hormone (TSH), prolactin, antimullerian hormone (AMH), and semen analysis of the male partner was performed. Other details of the couples, including previous surgical history, presence of any ovarian disorders like polycystic ovarian syndrome (PCOS), other diseases, type of infertility (primary or secondary), female and male occupation, male smoking and alcoholic habits, and male bathing conditions (hot or cold water), were collected. Patients having a maximum of 5 months of infertility period were excluded from the analysis.
All the patients were stimulated with a combination of clomifene citrate (100 mg from third to seventh day of the menstrual cycle) and HMG (75 IU IM) beginning at 8th day of menstruation, which was adjusted with the follicular development monitored by transvaginal ultrasound. When at least one follicular diameter was 17 to 18 mm, 10,000 IU intra muscular (IM) HCG was administered. Approximately 36 to 38 h after HCG injection, IUI procedure was performed.
All semen samples were collected after 3 to 5 days of sexual abstinence, after liquefaction for 30 to 60 min at 37°C, volume, pH, sperm count, progressive motility, concentration, and morphology were evaluated according to World Health Organization (WHO) manual 2010 (5th edition). Samples were proceeded with density gradient method or swim-up method. Swim-up method is usually a method of choice for normal semen samples. The semen was prepared by swim-up technique in which equal volume of sperm wash medium (Quinns Sperm Wash Medium; Cooper Surgical Company, Trumbull, Connecticut, USA) was used then centrifuged at 1500 rpm for 10 min was done. The resultant supernatant was discarded and the pellets were dislodged and over layered with 0.5 ml of sperm wash medium then incubated at 37°C for 30 to 45 min to allow the most active motile sperms to swim-up into the medium which is carefully removed. Then sperm count and motility was examined and sample is ready for insemination.
Density gradient method technique was used in samples with less count and motility, when there are more noncellular elements. Here we take equal volume of 80% gradient and layer a 40% gradient (PURESPERM; Nidacon International, Mölndal, Sweden) over 80% and layer equal volume of semen over it and centrifuge at 1800 rpm for 10 min. The supernatant was removed and resuspended the pellet with 0.5 ml media and checked for motility and count. This was further used for insemination.
After processing IUI was done using 0.3 to 05 ml of sperm suspension using IUI catheter attached to a 1 ml of BD syringe. Slow injection of the suspension into uterine cavity after exposure of the cervix with cuscos speculum and cleaning of the cervix with saline. Patients remained in a lithotomy position for 30 min after injection. Luteal phase support was given in the form of micronized progesterone (MIPROGEN) 400 mg daily for 3 weeks till pregnancy test, that is, 20 days post-IUI. Clinical pregnancy rate was defined as the presence of gestational sac using transvaginal ultrasound done after 5 weeks after IUI.
Statistical Analysis
Logistic regression analysis and chi-square analysis were used to identify significant variables that contributed to the success of IUI treatment. The variables selected for initial screening were female age, type of infertility, basal female hormones levels—FSH, LH, TSH, and prolactin, total motile sperm count (TMSC), number of IUI cycles undergone, male occupational conditions, male bathing conditions, male smoking and alcoholic habits, semen volume, prewash sperm count and presence, or absence of menstrual irregularities. AMH levels were not considered due to insufficient availability of data. PO was considered as the categorical dependant variable and the rest of the parameters as independent variables.
The following are the parameters used for statistical analyses: menstrual irregularity—presence or absence; type of infertility—primary or secondary; male habits—nil, smoking, alcoholic, and both; bathing condition of male—hot water or cold water; work environment—low-heat-generating, moderate-heat-generating, and high-heat-generating environment; prewash sperm count—1 to 10, 10 to 15, and >15 million/ml; number of IUI cycle—first cycle or more than one; TMSC—less than 1, 1 to 5, 5 to 10, 10 to 20, and more than 20 million/ml were used as categorical independent variables. Female age, male age, concentrations of FSH, LH, TSH, prolactin, and semen volume were used as continuous independent variables. Missing and insignificant data generated due to manual errors while data collection were removed and the corrected data was used for analysis.
Logistic regression analysis was performed to determine the significance of continuous independent variable with the PO and chi-square analysis was performed for categorical independent variable with the PO. A P value of less than 0.05 was considered to be significant. All the statistical tests were performed using SPSS software version 19 (SPSS Inc., Chicago, Illinois, USA).
RESULTS
Analysis was done on 2123 cycles of IUI undergone by 871 patients over a period of 5 years in a private fertility clinic. Detailed patient characteristics are listed in Table 1. Each couple underwent 2.4 ± 1.9 IUI cycles on an average. The clinical pregnancy rates were 18% per patient and 7% per cycle. The mean age was 34.2 ± 4.7 years for men and 28.5 ± 4.4 years for women and the mean period of infertility was 4.7 ± 3.4 years
Table 1.
Clinical details of patients who underwent IUI
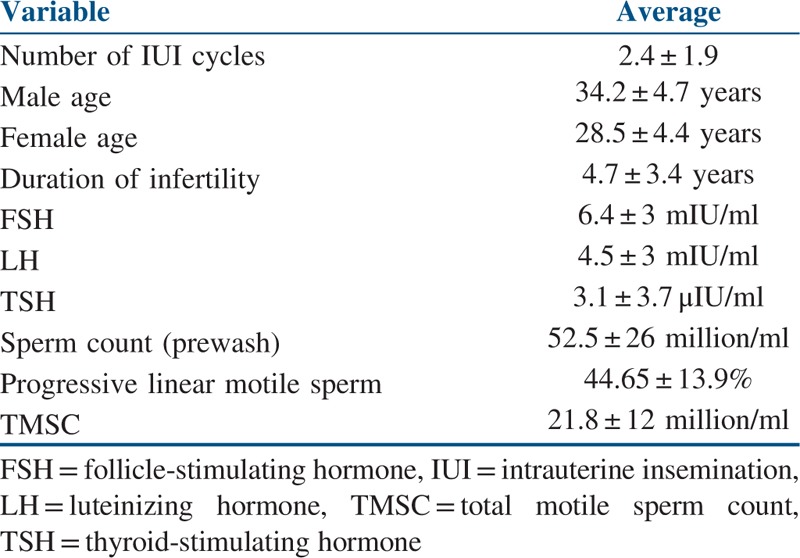
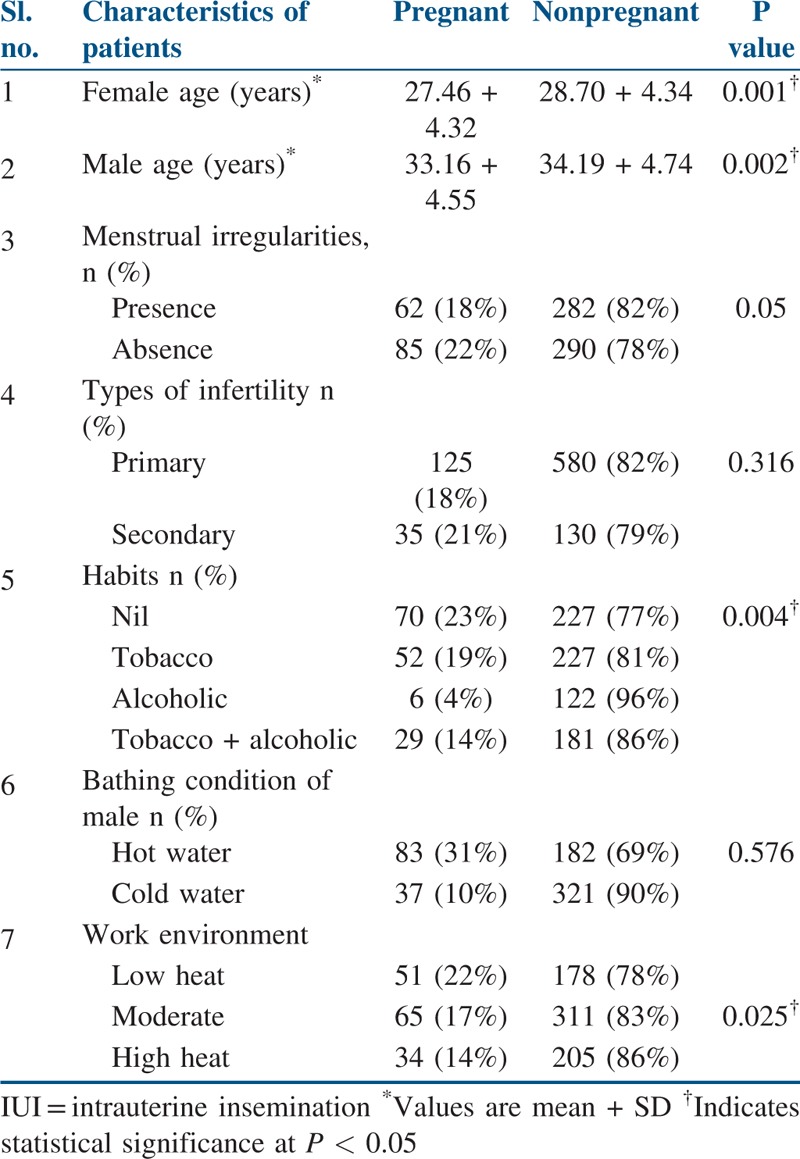
Table 2 compares the characteristics of the patients who have undergone IUI and the patients were categorized into pregnant and nonpregnant groups. Logistic regression analysis was performed to determine the significance of continuous independent variables (female and male age) and dichotomous dependant variable PO. Male age (P = 0.002) and female age (P = 0.001) were found to be significantly associated with chances of the success. Chi-square analysis was performed to determine the significant categorical variables such as male habits, male partner’s occupation, and type of infertility, menstrual irregularity presence, or absence with the PO. From the analysis, male habits (P = 0.004) and male occupational environment (P = 0.025) were found to significantly influence the PO in our study population.
Table 2.
Characteristics of patients who underwent IUI
To analyze the age-associated fall in the female fecundity, female age was categorized into four groups and chi-square analysis was performed for the patients enrolled in our retrospective study [Table 3]. The proportion of positive pregnancy rates significantly declined with the increase in age of the female partners.
Table 3.
Different female age groups affecting pregnancy rate in intrauterine insemination
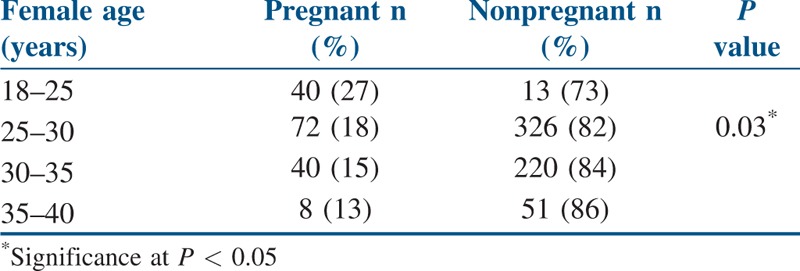
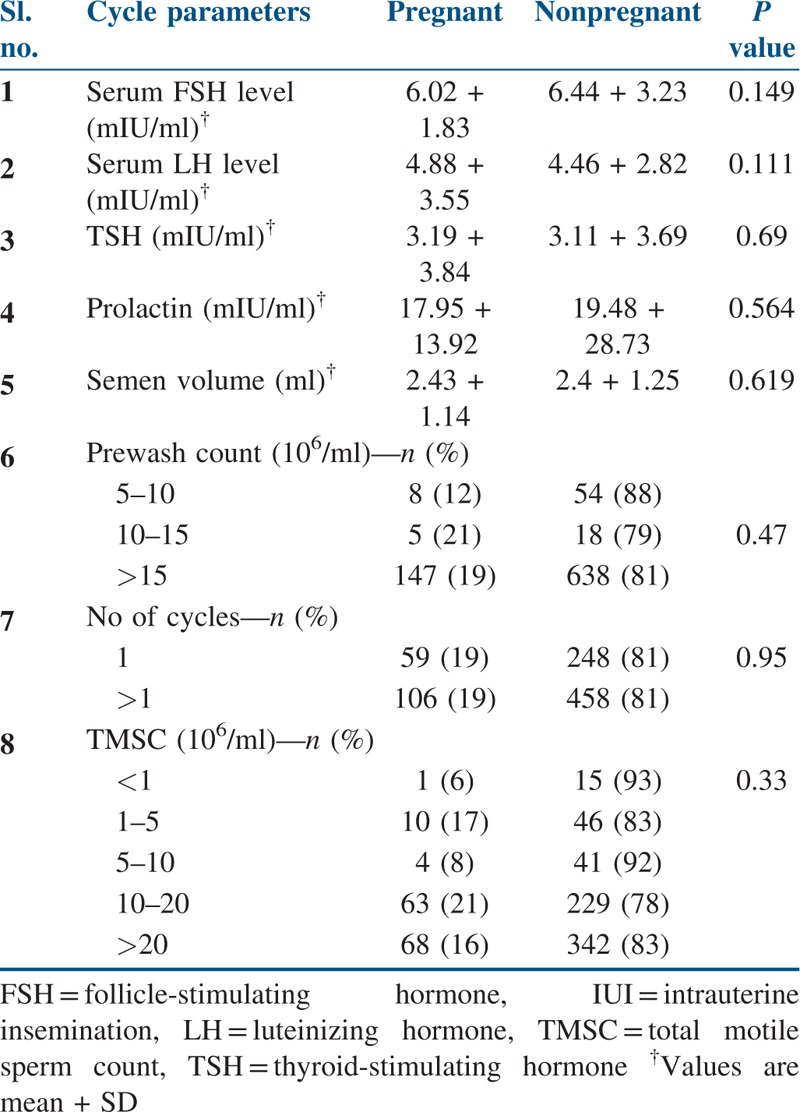
Table 4 compares the cycle parameters of patients who have undergone IUI. From the analysis, it was identified that none of these cycle parameters significantly influenced the PO in our study population.
Table 4.
Cycle parameters of patients who underwent IUI
Our retrospective data analysis showed four significant prognostic factors: female age, male age, male habits, and male working environment positively influencing PO in the analyzed study population.
DISCUSSION
Our analysis showed that the pregnancy rates in younger aged females were significantly higher than that in the older aged females. Similarly, younger aged males had a significantly higher success rates in IUI as compared to older aged males. Various studies have reported that a higher pregnancy rate was seen in the younger women than those of the older women.[12,13] In agreement to the previous studies, our study population also showed least pregnancy rate among females of age group 40 years and above. Women in the age groups of less than 25 years had a higher chance of pregnancy. This could be due to the lowered uterine receptivity as the age of the female increases.[14] The presence of any menstrual irregularities in the female partners significantly reduced the success of IUI. Types of infertility and bathing conditions in male did not significantly affect the outcome of IUI in our study.
From the previous studies, significantly higher pregnancy rate (18.2%) was observed when TMSC was in the range of 10 to 20 million. A TMSC of less than 1 million showed poor pregnancy.[15] Our current study also shows similar results. TMSC in the range 10 to 20 million showed the pregnancy rate of 21%, whereas TMSC less than 1 million showed only 6% of pregnancy rate. It was seen that this difference in the pregnancy rates due to TMSC count was not statistically significant.
There have been a very few studies that have reported the relationship between the male partner’s smoking and/or alcoholic habits and success of IUI treatment. Most of the studies have stated the detrimental impact of smoking on fecundability.[11,16] The current study also conveyed similar results, male partners who were teetotallers showed a significantly higher chances of pregnancy rate (23%) compared to the smoking male (19%), alcoholic male (4%), and smoking and alcohol consuming (14%) male partners. Interestingly, men who had a habit of consuming alcohol showed higher rates of positive pregnancy as compared to men who were practising both smoking and alcohol consumption. This could be because of the difference in the sample size. There were very few men who had the habit of consuming alcohol alone and not smoking in the group and hence, could be the discrepancy.
Spermatogenesis and many important testicular functions occur in a temperature range which is a couple of degrees lower than the core body temperature. Such low temperatures are necessary for the synthesis of good quality sperm and hence contributing to the reproductive health of an individual. Very few studies have concentrated on the study of relating occupational conditions of the male, scrotal heat generation, and male fertility. Earlier researches have supported the view that increased male scrotal temperatures due to occupational conditions like baking, working with computers on the laptop for a longer duration, driving for long hours, and others have deteriorating effects on sperm quality and hence male fertility.[17,18] Bathing in hot water regularly and also working in high temperature conditions is considered even more damaging to the male reproductive health. The results of our study, in accordance with the previous findings report that female patients with male partners who work in high scrotal heat generating involving occupational conditions have significantly lower rates (14%) of positive PO compared to those patients with male partners working in no-heat-generating (22%) and moderate-heat-generating occupations (17%).
CONCLUSION
This study was carried out to identify the possible predictive factors for post-IUI PO. From this study it was evident that the success of IUI is determined by the following factors: females with age lesser than 40 years, male age, male partner’s smoking/alcoholic habits, and male partner’s working environment. Smoking and alcohol consumption were also found to be linked with low success rates in this study, which implies that avoiding alcohol consumption and smoking prior and during IUI treatment might lead to a better success following treatment. These findings could assist clinicians to counsel the patients seeking infertility treatment based on their biological parameters and their lifestyle habits. This would also pave way for clinicians to provide customized treatment for their patients based on the assessment of these parameters. Although there were many studies reporting conflicting results on the predictive factors deciding success in an IUI treatment option, ours is the first study reporting a compiled data on the life style parameters as well as the clinical characteristics in this geographical location. Our study highlights that the major influencing parameter are the work environment of male and the social habits of male together with previously reported parameters like male and female age. This emphasizes that although the seminal parameters were normal in these men, the quality of the sperm would have been declined because of the continuous exposure of high heat and Deoxy ribo Nucleic Acid (DNA) damage caused due to smoking and alcoholism. Hence it is advisable to evaluate the sperm quality in these individuals using tests like DNA damage detection and the levels of free radicals in the seminal fluid before deciding upon further treatment options for these men.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Peterson CM, Hatasaka HH, Jones KP, Poulson AM, Carrell DT, Urry RL. Ovulation induction with gonadotropins and intrauterine insemination compared with in vitro fertilisation and no therapy: a prospective, non randomised, cohort study and meta-analysis. Fertil Steril. 1994;62:535–44. doi: 10.1016/s0015-0282(16)56942-8. [DOI] [PubMed] [Google Scholar]
- 2.Zayed F, Lenton EA, Cooke ID. Comparison between stimulated in-vitro fertilisation and stimulated intrauterine insemination for the treatment of unexplained and mild male factor infertility. Hum Reprod. 1997;12:2408–13. doi: 10.1093/humrep/12.11.2408. [DOI] [PubMed] [Google Scholar]
- 3.Dorjpurev U, Kuwahara A, Yano Y, Taniguchi T, Yamamoto Y, Suto A, et al. Effect of semen characteristics on pregnancy rate following intra-uterine insemination. J Med Invest. 2011;58:127–33. doi: 10.2152/jmi.58.127. [DOI] [PubMed] [Google Scholar]
- 4.Guan HT, Zheng Y, Wang JJ, Meng TQ, Xia W, Hu SH, et al. Relationship between donor sperm parameters and pregnancy outcome after intrauterine insemination: analysis of2821 cycles in 1355 couples. Andrologia. 2016;48:29–36. doi: 10.1111/and.12407. [DOI] [PubMed] [Google Scholar]
- 5.Guzick DS, Carson SA, Coutifaris C, Overstreet JW, Factor-Litvak P, Steinkampf MP, et al. Efficacy of superovulation and intrauterine insemination in the treatment of infertility. National Cooperative Reproductive Medicine Network. N Engl J Med. 1999;340:177–83. doi: 10.1056/NEJM199901213400302. [DOI] [PubMed] [Google Scholar]
- 6.Shulman A, Hauser R, Lipitz S, Frenkel Y, Dor J, Bider D, et al. Sperm motility is a major determinant of pregnancy outcome following Intrauterine Insemination. J Assist Reprod Genet. 1998;15:381–5. doi: 10.1023/A:1022585000740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dodson WC, Kunselman AR, Legro RS. Association of obesity with treatment outcomes in ovulatory infertile women undergoing super-ovulation and intra-uterine insemination. Fertil Steril. 2006;86:642–6. doi: 10.1016/j.fertnstert.2006.01.040. [DOI] [PubMed] [Google Scholar]
- 8.Hawkins LK, Rossi BV, Correia KF, Lipskind ST, Hornstein MD, Missmer SA. Perception among infertile couples of lifestyle behaviours and in-vitro fertilisation (IVF) success. J Assist Reprod Genet. 2014;31:255–60. doi: 10.1007/s10815-014-0176-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hassan MAM, Killick SR. Negative lifestyle associated with significant reduction in fecundity. Fertil Steril. 2004;81:382–92. doi: 10.1016/j.fertnstert.2003.06.027. [DOI] [PubMed] [Google Scholar]
- 10.Rossi BV, Berry KF, Homstein MD, Cramer DW, Ehrlich S, Missmer SA. Effect of alcohol consumption on in vitro fertilisation. Obstet Gynecol. 2011;117:136–42. doi: 10.1097/AOG.0b013e31820090e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taylor KC, Small CM, Dominguez CE, Murray LE, Tang W, Wilson MM, et al. Alcohol, smoking, and caffeine in relation to fecund ability with effect modification by NAT2. Ann Epidemiol. 2011;21:864–72. doi: 10.1016/j.annepidem.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kang BM, Wu TC. Effect of age on intrauterine insemination with frozen donor sperm. Obstet Gynecol. 1996;88:93–8. doi: 10.1016/0029-7844(96)00074-9. [DOI] [PubMed] [Google Scholar]
- 13.Van Noord-Zaadstra BM, Looman CW, Alsbach H, Habbema JD, te Velde ER, Karbaat J. Delaying childbearing: effect of age on fecundity and outcome of pregnancy. BMJ. 1991;302:1361–5. doi: 10.1136/bmj.302.6789.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cano F, Simon C, Remohi J, Pellicel A. Effect of aging on the female reproductive system: evidence for a role of uterine senescence in the decline in female fecundity. Fertil Steril. 1995;64:584–9. doi: 10.1016/s0015-0282(16)57797-8. [DOI] [PubMed] [Google Scholar]
- 15.Kamath MS, Bhave P, Aleyamma T, Nair R, Chandy A, Mangalaraj AM, et al. J Hum Reprod Sci. 2010;3:129–34. doi: 10.4103/0974-1208.74154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ye X, Skjaerven R, Basso O, Baird DD, Eggesbo M, Uicab LAC, et al. In utero exposure to tobacco smoke and subsequent reduced fertility in females. Hum Reprod. 2010;25:2901–6. doi: 10.1093/humrep/deq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mieusset R, Bujan L. Testicular heating and its contribution to male infertility: a review. Int J Androl. 1995;18:169–84. doi: 10.1111/j.1365-2605.1995.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 18.Thonneau P, Ducot B, Bujan L, Mieusset R, Spira A. Heat exposure as a hazard to male fertility. Lancet. 1996;347:204–5. [PubMed] [Google Scholar]


