Dear Editor
The acute respiratory distress syndrome (ARDS) is the leading cause of respiratory-related death disease in both intensive care unit (ICU) and hospital-wide, with a mortality rate of up to 40% (1). Despite a decreasing mortality rate of ARDS over time due to improved management (2), this syndrome is under-diagnosed and insufficiently treated, and as a result remains highly deadly (1).
MicroRNAs (miRNAs) are small non-coding RNAs, usually ∼22 nucleotides in length. They regulate gene expression by binding to specific target sites on messenger RNAs to either repress the translation of or degrade the transcript. MiRNAs play important role in inflammation and infection (3), both of which are common manifestations in ARDS (4). In addition, miRNAs are used to construct prognostic classifier for early prediction of disease outcomes, including cancer (5).
Here we report a survival analysis as part of the Molecular Epidemiology Study of ARDS (MEARDS) from the ICU at Massachusetts General Hospital and Beth Israel Deaconess Medical Center. We collected 78 whole blood RNA samples from MEARDS. Expression of 754 human miRNAs identified by TaqMan OpenArray Human MicroRNA Panel was measured. After quality control screening (supplement), we selected 294 miRNAs for data analysis. Imputation was used to handle missing miRNA data (supplement). We used multi-variate Cox proportional regression analysis to estimate the hazards ratio (HR) of miRNA for ARDS 28-day mortality. The Kaplan-Meier and log-rank method was performed to test the equality for survival distributions in different groups. All analyses were performed with R software (version 3.2.3) and Statistical Analysis System software (v.9.4, SAS Institute).
Demographic characteristics can be found in the supplement (Table s1). We identified nineteen miRNAs potentially associated with ARDS survival in patients with moderate to severe ARDS (Table s2). Among them, five miRNAs were most differentially expressed, miR-628.3p (HR=1.70, p<0.01), miR-922 (HR=1.05, p<0.01), miR-505* (HR=1.65, p<0.01), miR-130b* (HR=1.44, p<0.01), miR-624 (HR=1.38, p<0.01). In addition, based on all statistical significant miRNAs, we used backwards elimination methods with Akaike information criterion to select miRNAs that have potential to predict ARDS 28-day mortality (miR-628.3p, miR-922, miR-766, miR-194 and miR-7). The final miRNA classifier was obtained by both most differential expression and backwards elimination. Expression of miRNA classifier larger than median was assigned as high expression, lower than median was assigned as low expression. The Kaplan–Meier curves for 28-day mortality groups, using the eight-miRNA classifier, are shown in Figure 1. Time to death is shorter in patients with higher eight-miRNA classifier expression (p=0.04), which is comparable to APACHE III (supplement).
Figure 1.
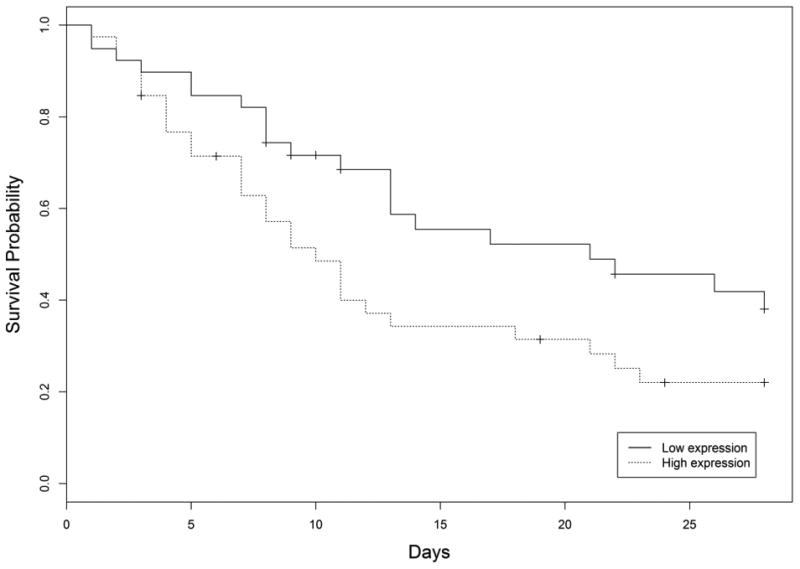
Kaplan–Meier survival analysis according to the miRNA classifier grouped by expression level.
To our knowledge, this is the first study of miRNA as a prognostic classifier from whole blood for ARDS 28-day mortality. Whole blood contains both immune cell- and tissue-specific miRNAs and thus offers a major advantage for miRNA profiling compared with other tissue types. While our study confidence is limited by sample size and the mortality rate in this small cohort is high and may not be representative of general ARDS cohort, the classifier containing miRNAs discovered in this study offers a potentially valuable, novel biomarker signature in the clinical practice to better ARDS 28-day mortality prognosis.
Supplementary Material
Acknowledgments
The authors thank Andrea Shafer and Sean Levy from Massachusetts General Hospital and Beth Israel Deaconess Medical Center for assistance in clinical data retrieval missing clinical data.
Funding: This study was supported by grants R01 HL060710 and P30 ES000002 (DCC) from National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health.
Footnotes
Authors' contributions: Z.Z., D.C.C, L.L., A.B., Q.L. designed the study. D.C.C, E.B., B.T., L.S. established the MEARDS cohort and collected the sample. Z.Z. and L.S. performed the experiment and data collection. Z.Z. and R.Z. did the data analysis and interpretation. Z.Z. drafted the manuscript.
Competing interests: The authors declare no competing interests.
Ethical standards statement: The institutional review boards of the MGH, BIDMC, and Harvard T.H. Chan School of Public Health approved this study.
References
- 1.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A Investigators LS, Group ET. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA : the journal of the American Medical Association. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Villar J, Blanco J, Anon JM, Santos-Bouza A, Blanch L, Ambros A, Gandia F, Carriedo D, Mosteiro F, Basaldua S, Fernandez RL, Kacmarek RM Network A. The ALIEN study: incidence and outcome of acute respiratory distress syndrome in the era of lung protective ventilation. Intensive care medicine. 2011;37:1932–1941. doi: 10.1007/s00134-011-2380-4. [DOI] [PubMed] [Google Scholar]
- 3.O'Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annual review of immunology. 2012;30:295–312. doi: 10.1146/annurev-immunol-020711-075013. [DOI] [PubMed] [Google Scholar]
- 4.Sheu CC, Gong MN, Zhai R, Bajwa EK, Chen F, Thompson BT, Christiani DC. The influence of infection sites on development and mortality of ARDS. Intensive care medicine. 2010;36:963–970. doi: 10.1007/s00134-010-1851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Volinia S, Croce CM. Prognostic microRNA/mRNA signature from the integrated analysis of patients with invasive breast cancer. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:7413–7417. doi: 10.1073/pnas.1304977110. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


